-
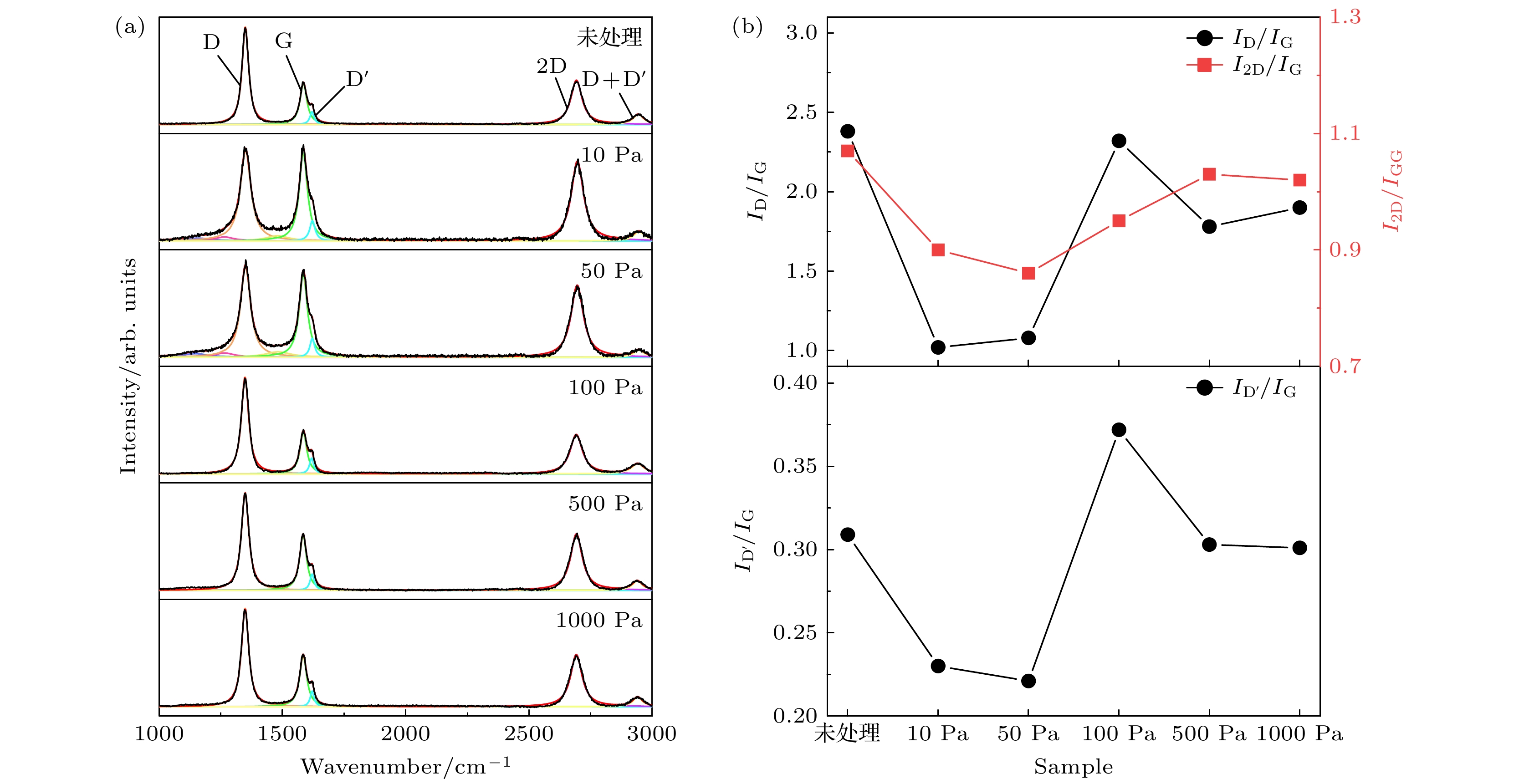
采用热丝化学气相沉积法制备了含有钽原子的石墨烯竖立片层, 并将其置于含氧环境中进行退火处理, 在常压环境中发生相变得到纳米金刚石, 并研究退火环境中氧含量变化对纳米金刚石形成的影响. 结果表明, 当退火环境气压为10 Pa和50 Pa (对应氧原子百分比为1.96%和2.04%) 时, 退火后样品形貌与结构和未处理的石墨烯片层无异; 样品100 Pa和500 Pa气压下退火后(对应氧原子百分比为2.77%和3.11%), 在其中观察到了尺寸为2—4 nm的纳米金刚石, 这些金刚石晶粒多分布于非晶碳中; 继续升高退火环境气压则发现退火后样品被大面积氧化, 石墨结构遭到严重破坏. 该研究结果为纳米金刚石的制备提供了新方法.A basic and important way to prepare diamond is to make graphite experience the phase transformation under the high-pressure high-temperature (HPHT) condition. However, this method needs stringent equipment and high investment cost. Recently, we proposed a method to prepare the diamond by phase transformation of graphite at atmospheric pressure with monodispersed Ta atoms. It is found that a phase transformation happens to H atoms under atmospheric pressure, but the role of O atoms has not been investigated. Here, we use tantalum wires as Ta source and heat the filaments to prepare vertical graphene containing Ta atoms in hot filament chemical vapor deposition (HFCVD) system. And then the vertical graphene layers are annealed in oxygen-containing environment, and nanodiamonds are obtained by phase transformation from the vertical graphene under atmospheric pressure. The results show that the sample morphologies are the same as the untreated vertical graphene’s, when the annealed ambient air pressure is at 10 Pa and 50 Pa with oxygen atom content of 1.96% and 2.04%, respectively; TEM tests reveal TaC and graphite but no diamond in these samples . Nanodiamond grains with the size range of 2–4 nm are observed in the amorphous carbon region of samples annealed at 100 Pa and 500 Pa air pressure with oxygen atom content increasing to 2.77% and 3.11%, respectively, indicating that oxidation facilitates the phase change from Ta-containing vertical graphene to diamond at atmospheric pressure. When the air pressure of the annealing environment rises to 1000 Pa with the oxygen atom content of 3.54%, the sample is extensively oxidized and the graphite structure is severely damaged,which means that a large number of oxygen atoms tend to disrupt the graphite structure rather than promote the phase change into diamond. These results supply a way to prepare nanodiamond and show the effect of O atoms in the graphite phase transition at atmospheric pressure.
-
Keywords:
- graphene /
- nanodiamond /
- phase transition under ordinary pressure
[1] Sildos I, Loot A, Kiisk V, Puust L, Hizhnyakov V, Yelisseyev A, Osvet A, Vlasov I, Kiisk V 2017 Diam. Relat. Mater. 76 27
 Google Scholar
Google Scholar
[2] Santacruz-Gomez Karla, Sarabia-Sainz Acosta-Elias M, Sarabia-Sainz M, Janetanakit Woraphong, Khosla Nathan, Melendrez R, Montero Martin Pedroza, Lal Ratnesh 2018 Nanotechnology 29 12.
 Google Scholar
Google Scholar
[3] Mochalin V N, Shenderova O, Ho D, Gogotsi Y 2012 Nat. Nanotechnol. 7 11
 Google Scholar
Google Scholar
[4] 李莲莲, 陈冠钦 2022 金刚石与磨料磨具工程 42 543
 Google Scholar
Google Scholar
Li L L, Chen G Q 2022 Diam. Abras. Eng. 42 543
 Google Scholar
Google Scholar
[5] Bulut B, Gunduz O, Baydogan M, Kayali E S 2020 Int. J. Refract. Met. H. 95 105466
 Google Scholar
Google Scholar
[6] Chen C K, He Z, Xu A C, Hu X J 2021 Funct. Diam. 1 117
 Google Scholar
Google Scholar
[7] Chen C K, Mei Y S, Cui J, Xiao L, Jiang M Y, Lu S H, Hu X J 2018 Carbon 139 982
 Google Scholar
Google Scholar
[8] Li X, Chen H, Wang C C, Chen C K, Jiang M Y, Hu X J 2023 Diam. Relat. Mater. 136 109927
 Google Scholar
Google Scholar
[9] Chen P, Huang F, Yun S 2004 Mater. Res. Bull. 39 1589
 Google Scholar
Google Scholar
[10] Jenei Z, O'bannon E F, Weir S T, Cynn H, Lipp M J, Evans W J 2018 Nat. Commun. 9 3563
 Google Scholar
Google Scholar
[11] Xu X Y, Yu Z M, Zhu Y W, Wang B C 2005 J. Solid State Chem. 178 688
 Google Scholar
Google Scholar
[12] 苗卫朋, 丁玉龙, 翟黎鹏, 包华 2019 金刚石与磨料磨具工程 39 18
 Google Scholar
Google Scholar
Miao W P, Ding Y L, Zhai L P, Bao H 2019 Diam. Abras. Eng. 39 18
 Google Scholar
Google Scholar
[13] Chen C, Fan D, Xu H, Jiang M, Li X, Lu S, Ke C, Hu X 2022 Carbon 196 466
 Google Scholar
Google Scholar
[14] Jiang M Y, Chen C K, Wang P, Guo D F, Han S J, Li X, Lu S H, Hu X J 2022 P. Natl. Acad. Sci. USA 119 e2201451119
 Google Scholar
Google Scholar
[15] Zhu Z G, Jiang C Q, Chen C K, Lu S H, Jiang M Y, Li X, Hu X J 2023 Carbon 211 118098
 Google Scholar
Google Scholar
[16] Bo Z, Mao S, Han Z J, Cen K F, Chen J H, Ostrikov K 2015 Chem. Soc. Rev. 44 2108
 Google Scholar
Google Scholar
[17] Cancado L G, Jorio A, Ferreira E H, Capaz R B, Moutinhc W V O, Ferrari A C 2012 Nano Lett. 11 3190
 Google Scholar
Google Scholar
[18] Shimada T, Sugai T, Fantini C , Souza M, Cancado L G 2005 Carbon 43 1049
 Google Scholar
Google Scholar
[19] Ferrari A C, Robertson J 2001 Phys. Rev. B 64 075414
 Google Scholar
Google Scholar
[20] Gilkes K, Sands H S, Batchelder D N, Robertson J, Milne W I 1997 Appl. Phys. Lett. 70 1980
 Google Scholar
Google Scholar
[21] Liu F B, Wang J D, Chen D R, Yan, D Y 2009 Chin. Phys. B 18 2041
 Google Scholar
Google Scholar
[22] Gnien D M 1999 Annu. Rev. Mater. Res. 29 211
-
图 3 (a) 未处理样品的低倍率HRTEM图片, 内插图为SAED图; (b) 未处理样品的高倍率HRTEM图片, 内插图为FT变换图; (c) 10 Pa样品的低倍率HRTEM图片, 内插图为SAED图; (d) 10 Pa样品的高倍率HRTEM图片, 内插图为FT变换图; (e) 50 Pa样品的HRTEM低倍率图片, 内插图为SAED图; (f) 50 Pa样品的高倍率HRTEM图片, 内插图为FT变换图
Fig. 3. (a) Low-magnification HRTEM picture of untreated sample and its inset SAED pattern; (b) high-magnification picture of untreated sample and its inset FT graph; (c) low-magnification HRTEM picture of 10 Pa sample and its inset SAED pattern; (d) high-magnification HRTEM picture of 10 Pa sample and its inset FT graph; (e) low-magnification HRTEM picture of 50 Pa sample and its inset SAED pattern; (f) high-magnification HRTEM picture of 50 Pa sample and its inset FT graph.
图 4 (a) 100 Pa样品的低倍率HRTEM图片, 内插图为SAED图; (b) 100 Pa样品的高倍率HRTEM图片, 内插图为FT变换图; (c)—(f) 图4(b)中框选区域放大图, FT-c, FT-d, FT-e和FT-f分别为其对应的FT变换图; (g) 100 Pa样品的能谱(TEM-EDS)图; (h) 100 Pa样品的HAADF和EDS-mapping图
Fig. 4. (a) Low-magnification HRTEM picture of 10 Pa sample and its inset SAED pattern; (b) high-magnification HRTEM picture of 100 Pa sample and its inset FT graph; (c)–(f) enlarged of picture high-magnification in Fig. 4(b), FT-c, FT-d, FT-e and FT-f are all FT graphs; (g) TEM-EDS of 100 Pa sample; (h) HAADF and EDS-mapping of 100 Pa sample.
图 5 (a) 500 Pa样品的低倍率HRTEM图片, 内插图为SAED图; (b) 500 Pa样品的高倍率HRTEM图片, 内插图为FT变换图; (c), (d) 图5(b)中框选区域放大图, FT-c和FT-d分别为对应的FT变换图; (e) 1000 Pa样品的低倍率HRTEM图片, 内插图为SAED图; (f) 1000 Pa样品的高倍率HRTEM图片, 内插图为FT变换图; (g) 1000 Pa样品的HAADF和EDS-mapping图
Fig. 5. (a) Low-magnification HRTEM picture of 500 Pa sample and its inset SAED pattern; (b) high-magnification HRTEM picture of 500 Pa sample and its inset FT graph; (c), (d) enlarged images of d and e in Fig.5(b), FT-c and FT-d are FT graphs; (e) low-magnification HRTEM picture of 1000 Pa sample and its inset SAED pattern; (f) high-magnification HRTEM picture of 1000 Pa sample and its inset FT graph; (g) HAADF and EDS-mapping of 1000 Pa sample.
图 6 (a) 未处理样品与不同气压下退火样品的XPS全谱图; (b) 样品的C 1s核心能级谱及其拟合曲线; (c) 样品表面C和O的原子百分比随退火气压变化图; (d), (e) 样品表面sp2 C, sp3 C, C—O键含量随退火气压变化图
Fig. 6. (a) Full range XPS spectra of untreated sample and annealed samples at different air pressures; (b) C 1s core energy level spectra and their deconvolution of samples; (c) variation of atomic content of C and O on the sample surface with annealing air pressure; (d), (e) variation of sp2-C, sp3-C, C—O and C=O content of sample surface with annealing air pressure.
-
[1] Sildos I, Loot A, Kiisk V, Puust L, Hizhnyakov V, Yelisseyev A, Osvet A, Vlasov I, Kiisk V 2017 Diam. Relat. Mater. 76 27
 Google Scholar
Google Scholar
[2] Santacruz-Gomez Karla, Sarabia-Sainz Acosta-Elias M, Sarabia-Sainz M, Janetanakit Woraphong, Khosla Nathan, Melendrez R, Montero Martin Pedroza, Lal Ratnesh 2018 Nanotechnology 29 12.
 Google Scholar
Google Scholar
[3] Mochalin V N, Shenderova O, Ho D, Gogotsi Y 2012 Nat. Nanotechnol. 7 11
 Google Scholar
Google Scholar
[4] 李莲莲, 陈冠钦 2022 金刚石与磨料磨具工程 42 543
 Google Scholar
Google Scholar
Li L L, Chen G Q 2022 Diam. Abras. Eng. 42 543
 Google Scholar
Google Scholar
[5] Bulut B, Gunduz O, Baydogan M, Kayali E S 2020 Int. J. Refract. Met. H. 95 105466
 Google Scholar
Google Scholar
[6] Chen C K, He Z, Xu A C, Hu X J 2021 Funct. Diam. 1 117
 Google Scholar
Google Scholar
[7] Chen C K, Mei Y S, Cui J, Xiao L, Jiang M Y, Lu S H, Hu X J 2018 Carbon 139 982
 Google Scholar
Google Scholar
[8] Li X, Chen H, Wang C C, Chen C K, Jiang M Y, Hu X J 2023 Diam. Relat. Mater. 136 109927
 Google Scholar
Google Scholar
[9] Chen P, Huang F, Yun S 2004 Mater. Res. Bull. 39 1589
 Google Scholar
Google Scholar
[10] Jenei Z, O'bannon E F, Weir S T, Cynn H, Lipp M J, Evans W J 2018 Nat. Commun. 9 3563
 Google Scholar
Google Scholar
[11] Xu X Y, Yu Z M, Zhu Y W, Wang B C 2005 J. Solid State Chem. 178 688
 Google Scholar
Google Scholar
[12] 苗卫朋, 丁玉龙, 翟黎鹏, 包华 2019 金刚石与磨料磨具工程 39 18
 Google Scholar
Google Scholar
Miao W P, Ding Y L, Zhai L P, Bao H 2019 Diam. Abras. Eng. 39 18
 Google Scholar
Google Scholar
[13] Chen C, Fan D, Xu H, Jiang M, Li X, Lu S, Ke C, Hu X 2022 Carbon 196 466
 Google Scholar
Google Scholar
[14] Jiang M Y, Chen C K, Wang P, Guo D F, Han S J, Li X, Lu S H, Hu X J 2022 P. Natl. Acad. Sci. USA 119 e2201451119
 Google Scholar
Google Scholar
[15] Zhu Z G, Jiang C Q, Chen C K, Lu S H, Jiang M Y, Li X, Hu X J 2023 Carbon 211 118098
 Google Scholar
Google Scholar
[16] Bo Z, Mao S, Han Z J, Cen K F, Chen J H, Ostrikov K 2015 Chem. Soc. Rev. 44 2108
 Google Scholar
Google Scholar
[17] Cancado L G, Jorio A, Ferreira E H, Capaz R B, Moutinhc W V O, Ferrari A C 2012 Nano Lett. 11 3190
 Google Scholar
Google Scholar
[18] Shimada T, Sugai T, Fantini C , Souza M, Cancado L G 2005 Carbon 43 1049
 Google Scholar
Google Scholar
[19] Ferrari A C, Robertson J 2001 Phys. Rev. B 64 075414
 Google Scholar
Google Scholar
[20] Gilkes K, Sands H S, Batchelder D N, Robertson J, Milne W I 1997 Appl. Phys. Lett. 70 1980
 Google Scholar
Google Scholar
[21] Liu F B, Wang J D, Chen D R, Yan, D Y 2009 Chin. Phys. B 18 2041
 Google Scholar
Google Scholar
[22] Gnien D M 1999 Annu. Rev. Mater. Res. 29 211
计量
- 文章访问数: 3517
- PDF下载量: 57
- 被引次数: 0














 下载:
下载: