-
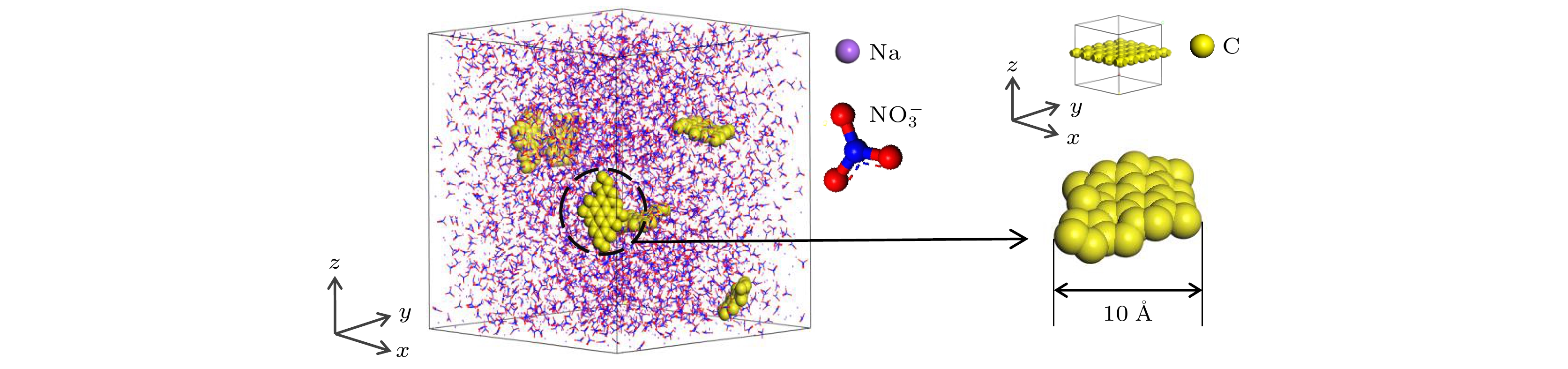
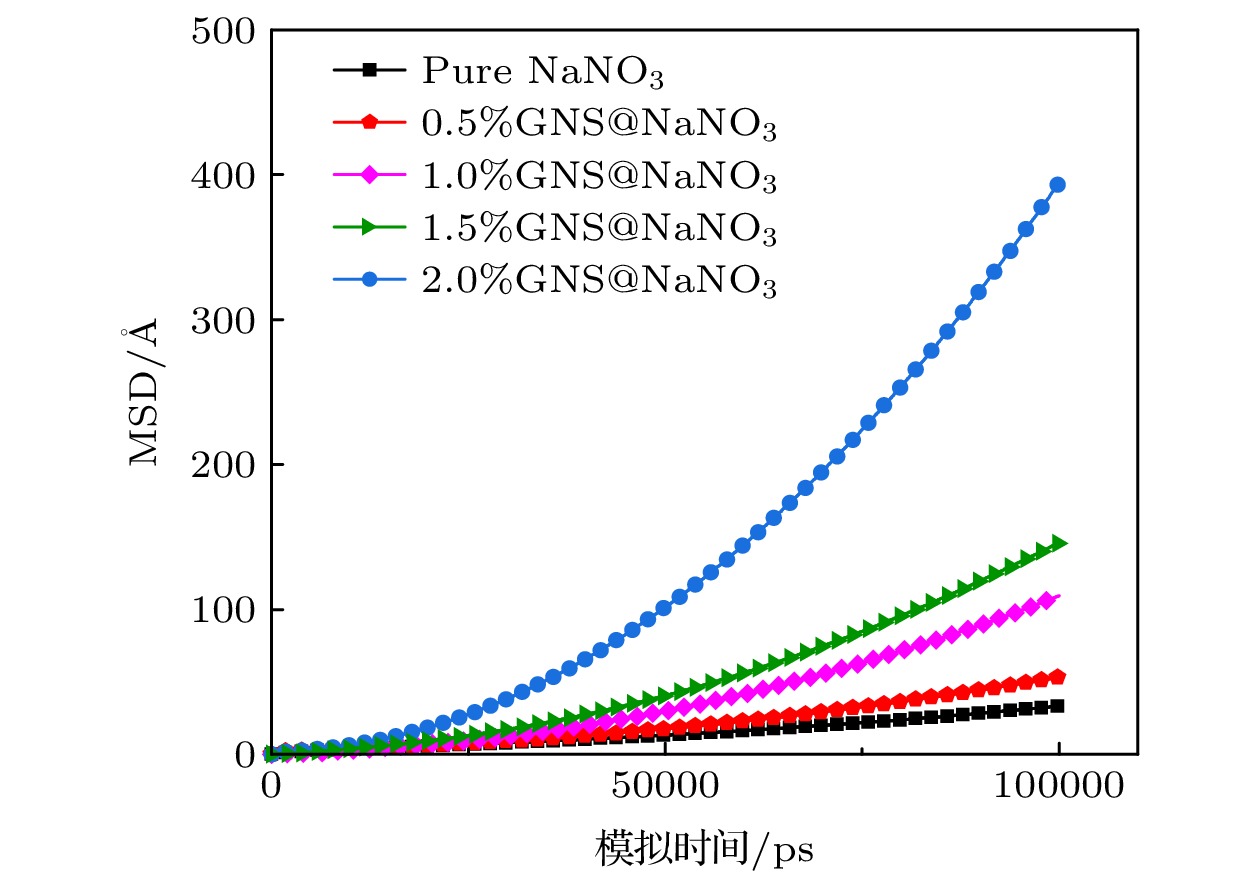
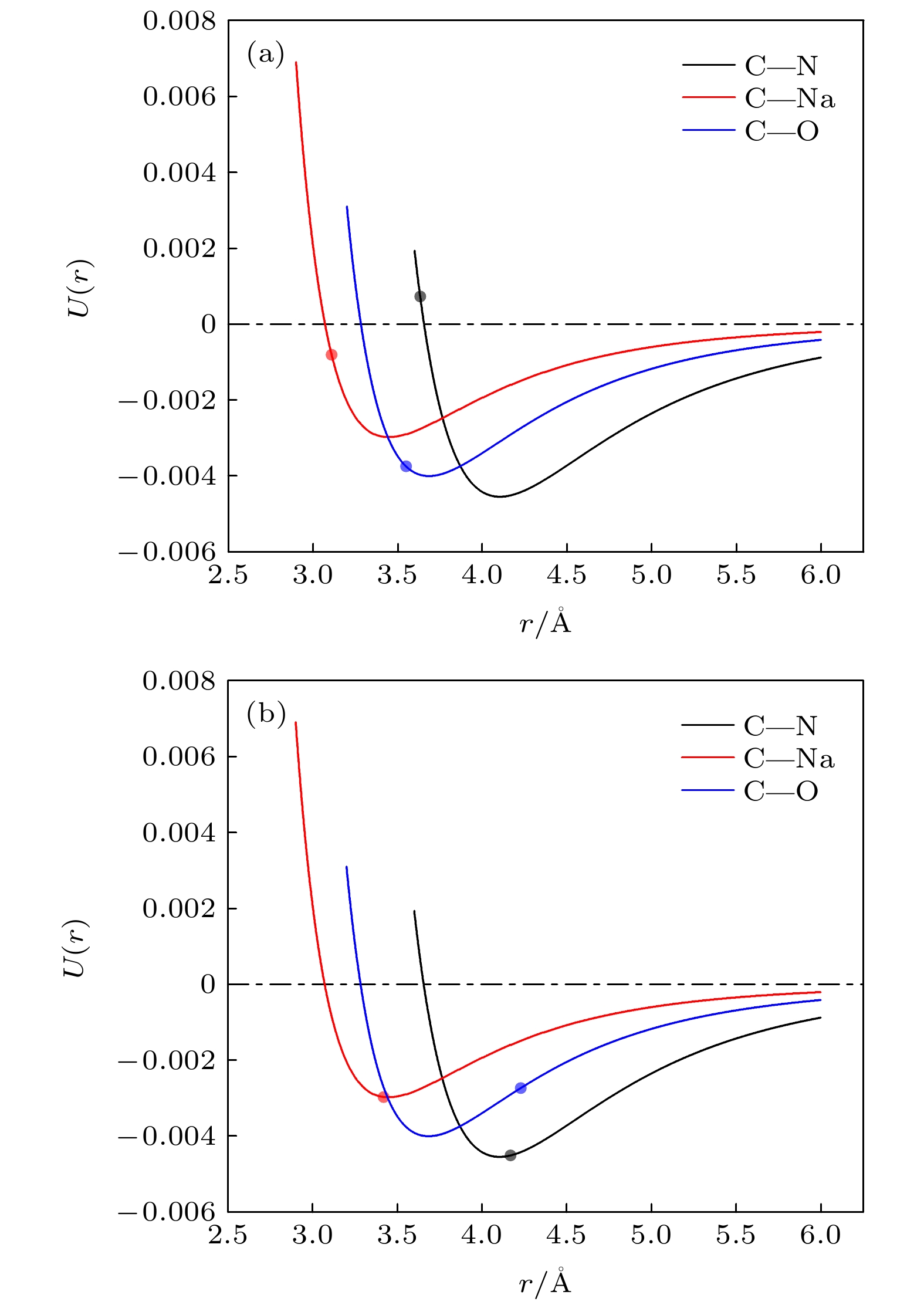
纳米增强剂通常被用来提升相变材料的导热性能, 但这种方式通常伴随着复合后材料相变焓的降低. 虽然这种降低难以避免, 但其微观机理乃至影响规律却始终未能明晰. 为深入探究纳米复合相变材料相变焓降低的机理, 本文以熔融硝酸钠(太阳盐的重要组成成分)为相变材料, 制备了石墨烯纳米片质量分数为0%, 0.5%, 1%, 1.5%, 2%的复合相变材料. 通过实验测量与分子动力学模拟的方法深入分析了石墨烯纳米片的掺杂导致熔融硝酸钠产生团簇以及复合材料熔点和相变焓非依数性降低的影响机理. 结果表明, 石墨烯纳米片质量分数为1.5%时, 硝酸钠致密层和石墨烯纳米片间的质心等效距离最接近他们相互作用势的势阱位置, 此时二者之间相互吸引作用最强, 熔盐分子的运动受限最为严重, 难以发生熔化, 从而导致相变焓降低最为显著. 为了最大限度地避免纳米复合相变材料相变焓的损失, 应根据相变材料与纳米增强剂的类型及其相互作用类型, 合理选择纳米增强剂的质量分数. 在实际应用中, 恰当的质量分数还将在一定程度上降低复合相变材料的制备成本.Molten salt is regarded as one of most promising candidates for solar energy storage due to possessing stable properties and large energy storage densities. However, the intrinsically low thermal conductivity of molten salt has become a bottleneck for rapid heat storage and transport. The addition of nanoparticles is generally considered to be a most effective way to improve the thermal conductivity of molten salt phase change materials (PCMs), while the phase change enthalpies of the nanocomposite phase change materials usually show two opposite trends of enhancement or decrement. Furthermore, the reason for the abnormal change of phase change enthalpy has not been clear in the literature so far, so the mechanism of change needs to be further explored. In this work, graphene nanosheets (GNS)@NaNO3 (sodium nitrate) nanocomposite phase change materials are prepared by the hydration ultrasonic method. The materials are characterized by scanning electron microscope, and the phase change characteristics are measured using differential scanning calorimeter. Molecular dynamics simulation is carried out to explain the mechanism for the formation of the NaNO3 dense layer and the non-collateral decrease of the enthalpy from the microscopic level. With the increase of GNS mass fraction, the melting point of the GNS@NaNO3 composite phase change material decreases slightly while the phase change enthalpy decreases significantly with a non-colligative trend. A 13.81% decrease of the theoretical phase change enthalpy is observed with a GNS doping ratio of 1.5%. The NaNO3 clusters observed on the surface of GNS are considered to have not melted, thereby resulting in a reduction in the phase change enthalpy. The mechanism is further investigated by molecular dynamics simulation, showing that the strong van der Waals attraction between GNS and NaNO3 leads the 2–4 Å-thick NaNO3 dense layer to form in the vicinity of GNS. With the increase of GNS mass fraction, the centroid equivalent distance between the dense layer and GNS gradually increases, which leads their mutual attraction to first increase and then weaken. When GNS mass fraction is 1.5%, the centroid equivalent distance reaches the position closest to the potential well, leading to a strongest mutual attraction. In other words, the phase change enthalpy decreases most obviously at this mass fraction. Thus, some conclusions can be drawn as follows. The type of interaction between molten salt and nano-enhancers and the position of the potential well are the fundamental reasons for the thickness of molten salt dense layer and the reduction of phase change enthalpy. The calculation of the interaction energy can be used to guide the selection of the mass fraction of the nano-enhancers, so as to avoid the loss of core material cluster and phase change enthalpy caused by the introduction of the nano-enhancers to a greatest extent. The preparation cost of the composite phase change material can also be reduced to a certain extent.
-
Keywords:
- nanocomposite phase change material /
- molten salt /
- phase change characteristics /
- molecular dynamics simulation
[1] Lee D, Jo B 2021 Int. J. Energy Res. 45 3231
 Google Scholar
Google Scholar
[2] Xiong Y X, Wang Z Y, Sun M Y, Wu Y T, Xu P, Qian X, Li C, Ding Y L, Ma C F 2021 Int. J. Energy Res. 45 5248
 Google Scholar
Google Scholar
[3] Kumar N, Hirschey J, LaClair T J, Gluesenkamp K R, Graham S 2019 J. Energy Storage 24 100794
 Google Scholar
Google Scholar
[4] Choi D H, Lee J, Hong H, Kang Y T 2014 Int J. Refrig. 42 112
 Google Scholar
Google Scholar
[5] Wang M R, Kang Q J, Pan N 2009 Appl. Therm. Eng. 29 418
 Google Scholar
Google Scholar
[6] Kim Y S, Kim D, Martin K J, Yu C, Grunlan J C 2010 Macromol. Mater. Eng. 295 431
 Google Scholar
Google Scholar
[7] Shin D, Banerjee D 2010 J. Heat Transfer 133 024501
 Google Scholar
Google Scholar
[8] Shin D, Banerjee D 2011 Int. J. Heat Mass Transfer 54 1064
 Google Scholar
Google Scholar
[9] Jo B, Banerjee D 2014 Acta Mater. 75 80
 Google Scholar
Google Scholar
[10] Qiao G, Alexiadis A, Ding Y L 2017 Powder Technol. 314 660
 Google Scholar
Google Scholar
[11] Xiong Y X, Wang Z Y, Xu P, Chen H B, Wu Y T 2019 Energy Procedia 158 5551
 Google Scholar
Google Scholar
[12] Li Z, Li B R, Du X Z, Wu H W 2020 Renewable Energy 146 816
 Google Scholar
Google Scholar
[13] Engelmann S, Hentschke R 2019 Sci. Rep. 9 1
 Google Scholar
Google Scholar
[14] Li J F, Lu W, Zeng Y B, Luo Z P 2014 Sol. Energy Mater. Sol. Cells 128 48
 Google Scholar
Google Scholar
[15] Liu Y S, Yang Y Z 2017 Appl. Therm. Eng. 124 533
 Google Scholar
Google Scholar
[16] Yu Q, Lu Y W, Zhang C C, Wu Y T, Sunden B 2019 Sol. Energy Mater. Sol. Cells 201 110055
 Google Scholar
Google Scholar
[17] Park S, Ruoff R S 2009 Nat. Nanotechnol. 4 217
 Google Scholar
Google Scholar
[18] Morelos-Gomez A, Terashima S, Yamanaka A, Cruz-Silva R, Ortiz-Medina J, Sánchez-Salas R, Fajardo-Díaz J L, Muñoz-Sandoval E, López-Urías F, Takeuchi K 2021 Carbon 181 118
 Google Scholar
Google Scholar
[19] Yan X X, Zhao H B, Feng Y H, Qiu L, Lin L, Zhang X X, Ohara T 2022 Compos. B Eng. 228 109435
 Google Scholar
Google Scholar
[20] D’Aguanno B, Karthik M, Grace A, Floris A 2018 Sci. Rep. 8 10485
 Google Scholar
Google Scholar
[21] Jayaraman S, Thompson A P, von Lilienfeld O A, Maginn E J 2010 Ind. Eng. Chem. Res. 49 559
 Google Scholar
Google Scholar
[22] Plimpton S 1995 J. Comput. Phys. 117 1
 Google Scholar
Google Scholar
[23] Stuart S J, Tutein A B, Harrison J A 2000 J. Chem. Phys. 112 6472
 Google Scholar
Google Scholar
[24] Jones J E 1924 Proc. R. Soc. Lond. A 106 463
 Google Scholar
Google Scholar
[25] Xiong Y H, Wu H, Gao J S, Chen W, Zhang J C, Yue Y N 2019 Acta Phys. Chim. Sin. 35 1150
 Google Scholar
Google Scholar
[26] Li Z, Cui L, Li B R, Du X Z 2020 Int. J. Heat Mass Transfer 153 119578
 Google Scholar
Google Scholar
[27] Yang M J, Stipp S S, Harding J 2008 Cryst. Growth Des. 8 4066
 Google Scholar
Google Scholar
-
图 2 石墨烯纳米片@硝酸钠复合相变材料的扫描电子显微照片 (a)石墨烯纳米片; (b) 纯硝酸钠; (c) 纳米片质量分数为0.5%的石墨烯纳米片@硝酸钠; (d) 纳米片质量分数为1.0%的石墨烯纳米片@硝酸钠; (e) 纳米片质量分数为1.5%的石墨烯纳米片@硝酸钠; (f) 纳米片质量分数为2.0%的石墨烯纳米片@硝酸钠
Fig. 2. Scanning electron micrograph of GNS@NaNO3: (a) GNS; (b) pure NaNO3; (c) GNS@NaNO3 when GNS weight concentration is 0.5%; (d) GNS@NaNO3 when GNS weight concentration is 1.0%; (e) GNS@NaNO3 when GNS weight concentration is 1.5%; (f) GNS@NaNO3 when GNS weight concentration is 2.0%.
表 1 硝酸钠和石墨烯纳米片@硝酸钠复合相变材料的相变热性能
Table 1. Phase change characteristics of pure NaNO3 and GNS@NaNO3.
样品 Tm/℃ HS,comp/(J·g–1) Theoretical HS,comp/(J·g–1) θ/% Pure NaNO3 308.00±0.57 174.00±1.62 174 100 0.5%GNS@NaNO3 306.87±0.40 153.12±1.48 173.13 88.44 1.0%GNS@NaNO3 307.91±0.55 165.24±1.68 172.26 95.92 1.5%GNS@NaNO3 307.33±0.42 147.72±1.15 171.39 86.19 2.0%GNS@NaNO3 307.46±0.60 160.08±1.53 170.52 93.88 表 2 各原子到石墨烯纳米片的质心等效距离
Table 2. Centroid equivalent distance of atoms in NaNO3 dense layer to GNS.
原子种类 Centroid equivalent distance/Å 0.5% 1.0% 1.5% 2.0% Na 3.31 3.11 3.42 3.92 N 3.23 3.63 4.17 4.53 O 3.23 3.55 4.23 5.10 -
[1] Lee D, Jo B 2021 Int. J. Energy Res. 45 3231
 Google Scholar
Google Scholar
[2] Xiong Y X, Wang Z Y, Sun M Y, Wu Y T, Xu P, Qian X, Li C, Ding Y L, Ma C F 2021 Int. J. Energy Res. 45 5248
 Google Scholar
Google Scholar
[3] Kumar N, Hirschey J, LaClair T J, Gluesenkamp K R, Graham S 2019 J. Energy Storage 24 100794
 Google Scholar
Google Scholar
[4] Choi D H, Lee J, Hong H, Kang Y T 2014 Int J. Refrig. 42 112
 Google Scholar
Google Scholar
[5] Wang M R, Kang Q J, Pan N 2009 Appl. Therm. Eng. 29 418
 Google Scholar
Google Scholar
[6] Kim Y S, Kim D, Martin K J, Yu C, Grunlan J C 2010 Macromol. Mater. Eng. 295 431
 Google Scholar
Google Scholar
[7] Shin D, Banerjee D 2010 J. Heat Transfer 133 024501
 Google Scholar
Google Scholar
[8] Shin D, Banerjee D 2011 Int. J. Heat Mass Transfer 54 1064
 Google Scholar
Google Scholar
[9] Jo B, Banerjee D 2014 Acta Mater. 75 80
 Google Scholar
Google Scholar
[10] Qiao G, Alexiadis A, Ding Y L 2017 Powder Technol. 314 660
 Google Scholar
Google Scholar
[11] Xiong Y X, Wang Z Y, Xu P, Chen H B, Wu Y T 2019 Energy Procedia 158 5551
 Google Scholar
Google Scholar
[12] Li Z, Li B R, Du X Z, Wu H W 2020 Renewable Energy 146 816
 Google Scholar
Google Scholar
[13] Engelmann S, Hentschke R 2019 Sci. Rep. 9 1
 Google Scholar
Google Scholar
[14] Li J F, Lu W, Zeng Y B, Luo Z P 2014 Sol. Energy Mater. Sol. Cells 128 48
 Google Scholar
Google Scholar
[15] Liu Y S, Yang Y Z 2017 Appl. Therm. Eng. 124 533
 Google Scholar
Google Scholar
[16] Yu Q, Lu Y W, Zhang C C, Wu Y T, Sunden B 2019 Sol. Energy Mater. Sol. Cells 201 110055
 Google Scholar
Google Scholar
[17] Park S, Ruoff R S 2009 Nat. Nanotechnol. 4 217
 Google Scholar
Google Scholar
[18] Morelos-Gomez A, Terashima S, Yamanaka A, Cruz-Silva R, Ortiz-Medina J, Sánchez-Salas R, Fajardo-Díaz J L, Muñoz-Sandoval E, López-Urías F, Takeuchi K 2021 Carbon 181 118
 Google Scholar
Google Scholar
[19] Yan X X, Zhao H B, Feng Y H, Qiu L, Lin L, Zhang X X, Ohara T 2022 Compos. B Eng. 228 109435
 Google Scholar
Google Scholar
[20] D’Aguanno B, Karthik M, Grace A, Floris A 2018 Sci. Rep. 8 10485
 Google Scholar
Google Scholar
[21] Jayaraman S, Thompson A P, von Lilienfeld O A, Maginn E J 2010 Ind. Eng. Chem. Res. 49 559
 Google Scholar
Google Scholar
[22] Plimpton S 1995 J. Comput. Phys. 117 1
 Google Scholar
Google Scholar
[23] Stuart S J, Tutein A B, Harrison J A 2000 J. Chem. Phys. 112 6472
 Google Scholar
Google Scholar
[24] Jones J E 1924 Proc. R. Soc. Lond. A 106 463
 Google Scholar
Google Scholar
[25] Xiong Y H, Wu H, Gao J S, Chen W, Zhang J C, Yue Y N 2019 Acta Phys. Chim. Sin. 35 1150
 Google Scholar
Google Scholar
[26] Li Z, Cui L, Li B R, Du X Z 2020 Int. J. Heat Mass Transfer 153 119578
 Google Scholar
Google Scholar
[27] Yang M J, Stipp S S, Harding J 2008 Cryst. Growth Des. 8 4066
 Google Scholar
Google Scholar
计量
- 文章访问数: 5472
- PDF下载量: 101
- 被引次数: 0
















 下载:
下载: