-
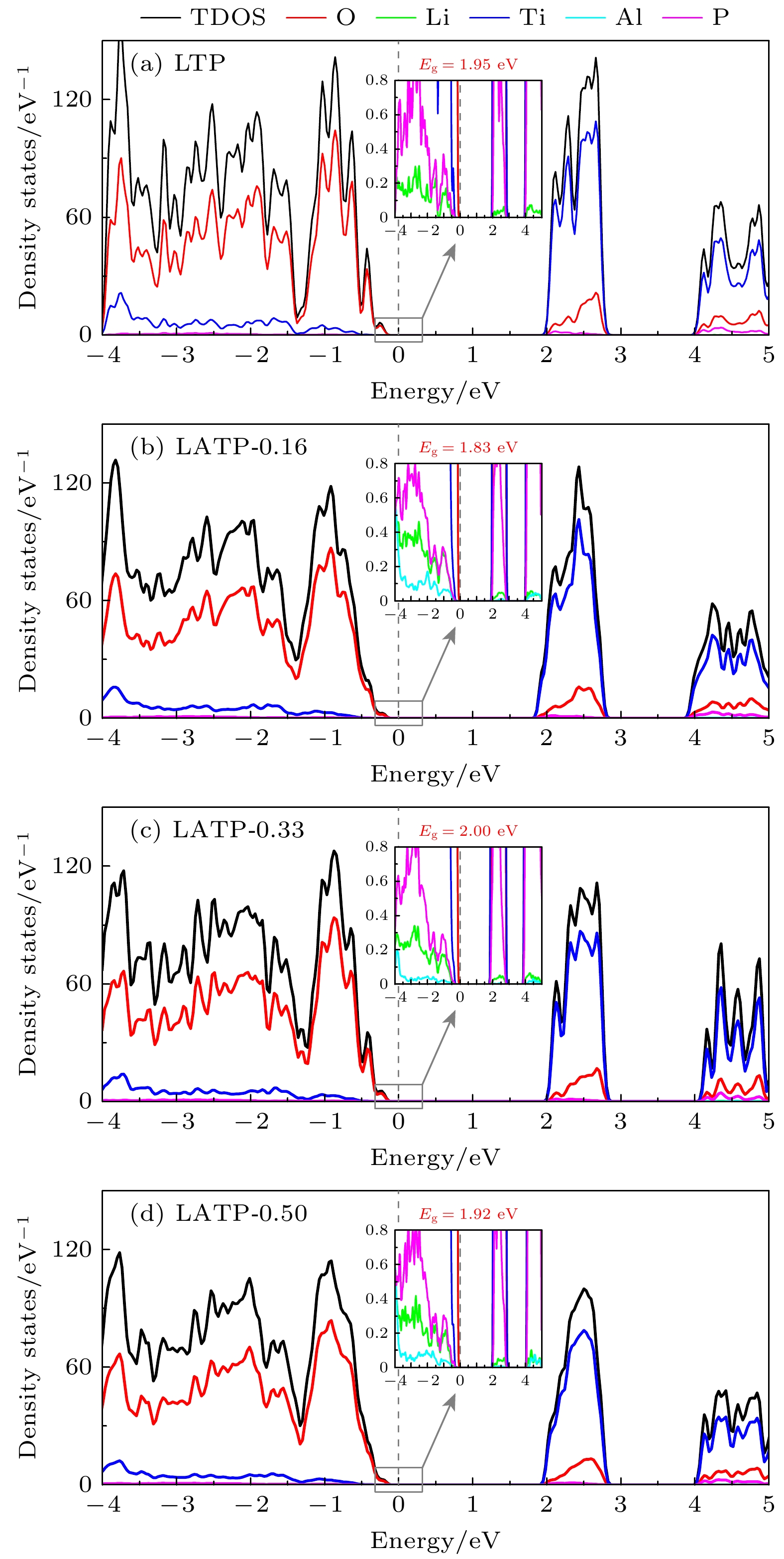
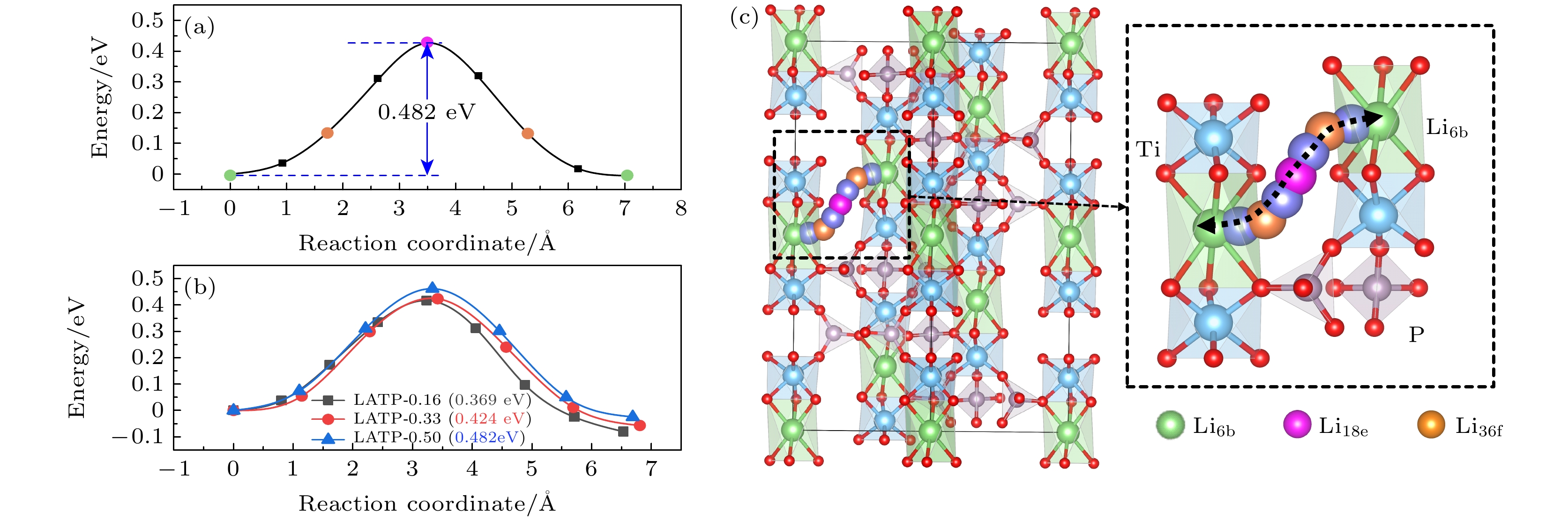
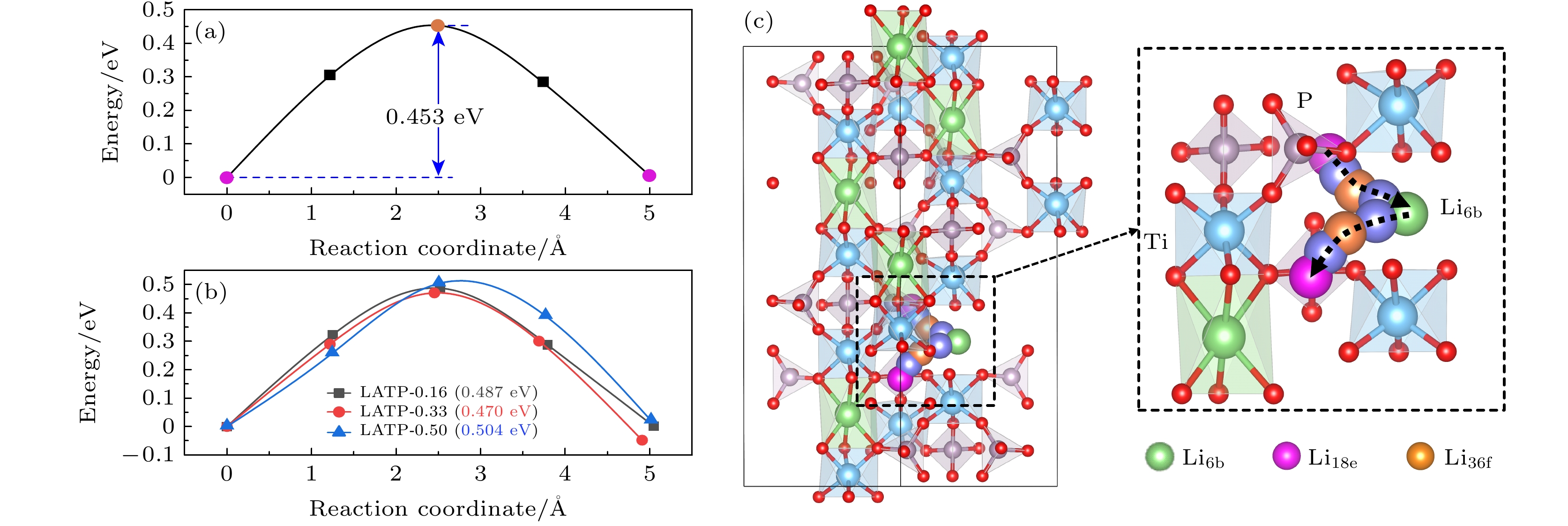
Li1+xAlxTi2–x(PO4)3 (LATP)是一种颇具前景的NASICON型锂离子固态电解质. 本文通过第一性原理计算研究了不同Al掺杂浓度(x = 0.00, 0.16, 0.33, 0.50)对LATP的结构特性、电学特性以及Li+迁移特性的影响. 结果表明, Al能够稳定掺杂进入LiTi2(PO4)3(LTP)的晶体结构当中. 当Al掺杂浓度x = 0.16时, Li—O键的平均键长最长, 成键强度最弱, 而Ti—O键强度随Al掺杂浓度变化不大. Al掺杂浓度对LATP带隙的影响不大, 但Al附近的O原子聚集了更多的负电荷, 形成AlO6极化中心. Li+不同的迁移方式(空位迁移、间隙位迁移和协同迁移)在Al掺杂浓度不同时展现出复杂的能垒变化, Li+在空位迁移中迁移势垒随Al掺杂浓度的增大而升高, 而在间隙位迁移中Li+的迁移势垒变化相反, 由于协同迁移中涉及空位和间隙位两种位点, Li+的迁移势垒表现为随Al掺杂浓度的升高先降低后升高的复杂变化. 当x = 0.50时, LATP具有最低的Li+迁移势垒0.342 eV, 这个势垒值是间隙位迁移的结果. 因此, 通过改变Al掺杂浓度, 可改变间隙Li+浓度及迁移通道结构, 进而调节Li+的迁移性能, 提高LATP中的Li+导电性能 .
-
关键词:
- 全固态Li+电池 /
- Al掺杂 /
- Li1+xAlxTi2–x(PO4)3 /
- Li+迁移
NASICON-type materials are specific skeleton structures in which ions move in three dimensions. Li1+xAlxTi2–x(PO4)3 (LATP) is a promising NASICON-type solid-state electrolyte for Li-ion batteries, due to its relatively high Li+ conductivity, chemical stability to air and moisture, and mechanical strength. Motivated by this, we study the doping and electronic properties of Li1+xAlxTi2–x(PO4)3 (x = 0.00, 0.16, 0.33, 0.50) and the transport properties of Li+ in them by using first-principles calculations based on density functional theory as implemented in Vienna ab initio Simulation Package (VASP). The results indicate that Al can substitute Ti to form a stable structure. When the Al doping concentration is x = 0.16, the average bond length of Li—O bond is longest and the bonding strength is weakest, this may lead to the expansion of channels for Li+ migration, which facilitates the diffusion of Li+. With the increase of Al doping concentration, the strength of Ti—O bond remains almost unchanged. The electronic structure calculations exhibit that with the increase of Al doping concentration, the bandgap of LATP does not change much, and LATP shows semiconductor characteristic. The differential charge results indicate that more electrons are localized on O-atoms surrounding the Al-dopant, causing the AlO6 groups to form polarization centers. The study on the migration properties of Li+ indicates that Li+ exhibits different migration characteristics in three different migration modes (vacancy migration, interstitial migration, and cooperative migration). With the increase of Al doping concentration, the migration barrier of Li+ increases via vacancies involving only lattice site migration, and the migration barrier for LATP-0.16 is lowest (0.369 eV). While in interstitial migration involving only interstitial sites, the migration barrier of Li+ decreases accordingly. When the Al doping concentration is x = 0.50, the migration barrier is lowest (0.342 eV). In terms of cooperative migration, this migration mode involves both vacancy and interstitial sites, so the migration barrier first decreases and then increases with the increase of Al doping concentration. Thus, our study suggests that by varying the concentration of Al doping, the interstitial Li+ content, migration channel structure, and the migration performance of Li+ can be changed favorably. Our results provide a theoretical basis for improving the ion conductivity of Li in LATP by varying the Al doping concentration in experiment.-
Keywords:
- solid-state electrolyte /
- Al doped /
- Li1+xAlxTi2–x(PO4)3 /
- Li+ migration
[1] Nitta N, Wu F X, Lee J T, Yushin G 2015 Mater. Today 18 252
 Google Scholar
Google Scholar
[2] Abraham K M 2015 J. Phys. Chem. Lett. 6 830
 Google Scholar
Google Scholar
[3] Tarascon J M 2010 Phil. Trans. R. Soc. A 368 3227
 Google Scholar
Google Scholar
[4] Quartarone E, Mustarelli P 2011 Chem. Soc. Rev. 40 2525
 Google Scholar
Google Scholar
[5] Zhang B K, Tan R, Yang L Y, Zheng J X, Zhang K C, Mo S J, Lin Z, Pan F 2018 Energy Storage Mater. 10 139
 Google Scholar
Google Scholar
[6] Chen R, Qu W, Guo X, Li L, Wu F 2016 Mater. Horiz. 3 487
 Google Scholar
Google Scholar
[7] Wang Y, Zhong W H 2015 Chem. Electro. Chem. 2 3
 Google Scholar
Google Scholar
[8] Bruce P G, Freunberger S A, Hardwick L J, Tarascon J M 2011 Nat. Mater. 11 19
 Google Scholar
Google Scholar
[9] Kato Y, Hori S, Saito T, Suzuki K, Hirayama M, Mitsui A, Yonemura M, Iba H, Kanno R 2016 Nat. Energy 1 16030
 Google Scholar
Google Scholar
[10] Bachman J C, Muy S, Grimaud A, Chang H H, Pour N, Lux S F, Paschos O, Maglia F, Lupart S, Lamp P, Giordano L, Shao-Horn Y 2016 Chem. Rev. 116 140
 Google Scholar
Google Scholar
[11] Croce F, Appetecchi G B, Persi L, Scrosati B 1998 Nature 394 456
 Google Scholar
Google Scholar
[12] Xiao Z L, Long T Y, Song L B, Zheng Y H, Wang C 2022 Ionics 28 15
 Google Scholar
Google Scholar
[13] Xi G, Xiao M, Wang S J, Han D M, Li Y N, Meng Y Z 2020 Adv. Funct. Mater. 31 2007598
 Google Scholar
Google Scholar
[14] Zhang Z Z, Wenzel S, Zhu Y Z, Sann J, Shen L, Yang J, Yao X Y, Hu Y S, Wolverton C, Li H, Chen L Q, Janek J 2020 ACS Appl. Energy Mater. 3 7427
 Google Scholar
Google Scholar
[15] Chen X F, Guan Z Q, Chu F L, Xue Z C, Wu F X, Yu Y 2021 Info. Mat. 4 e12248
 Google Scholar
Google Scholar
[16] 吴洁, 江小标, 杨旸, 吴勇民, 朱蕾, 汤卫平 2020 储能科学与技术 9 1472
 Google Scholar
Google Scholar
Wu J, Jiang X B, Yang Y, Wu Y M, Zhu L, Tang W P 2020 Energy Stor. Sci. Tech. 9 1472
 Google Scholar
Google Scholar
[17] Zhang L X, Liu Y M, Han J, Yang C, Zhou X, Yuan Y, You Y 2023 ACS Appl. Mater. Interfaces 15 44867
 Google Scholar
Google Scholar
[18] Subramanian M A, Subramanian R, Clearfield A 1986 Solid State Ionics 18-19 562
 Google Scholar
Google Scholar
[19] Kresse G, Furthmüller J 1996 Phys. Rev. B 54 11169
 Google Scholar
Google Scholar
[20] Adachi G Y, Imanaka N, Aono H 1996 Adv. Mater. 8 127
 Google Scholar
Google Scholar
[21] Aono H, Sugimoto E, Sadaoka Y, Imanaka N, Adachi G Y 1990 J. Electrochem. Soc. 137 1023
 Google Scholar
Google Scholar
[22] Schroeder M, Glatthaar S, Binder J R 2011 Solid State Ionics. 201 49
 Google Scholar
Google Scholar
[23] Mariappan C R, Gellert M, Yada C, Rosciano F, Roling B 2012 Electrochem. Commun. 14 25
 Google Scholar
Google Scholar
[24] Yin F S, Zhang Z J, Fang Y L, Sun C W 2023 J. Energy Stor. 73 108950
 Google Scholar
Google Scholar
[25] Arbi K, Lazarraga M G, Ben Hassen Chehimi D, Ayadi-Trabelsi M, Rojo J, Sanz J 2004 Chem. Mater. 16 255
 Google Scholar
Google Scholar
[26] Monchak M, Hupfer T, Senyshyn A, Boysen H, Chernyshov D, Hansen T, Schell K G, Bucharsky E C, Hoffmann M J, Ehrenberg H 2016 Inorg. Chem. 55 2941
 Google Scholar
Google Scholar
[27] Arbi K, Hoelzel M, Kuhn A, Garcia-Alvarado F, Sanz J 2013 Inorg. Chem. 52 9290
 Google Scholar
Google Scholar
[28] Rao A V, Veeraiah V, Rao A P, Babu B K, Latha B S, Rao K R 2014 B Mater. Sci. 37 883
 Google Scholar
Google Scholar
[29] Luo Y Y, Liu X Y, Wen C J, Ning T X, Jiang X X, Lu A X 2023 Appl. Phys. A 129 518
 Google Scholar
Google Scholar
[30] Liang Y J, Peng C, Kamiike Y, Kuroda K, Okido M 2019 J. Alloys Compd. 775 1147
 Google Scholar
Google Scholar
[31] Luo Y Y, Jiang X X, Yu Y J, Liu L D, Lin X T, Wang Z K, Han L, Luo Z W, Lu A X 2023 Solid State Ionics 390 116111
 Google Scholar
Google Scholar
[32] Tian H K, Jalem R, Gao B, Yamamoto Y, Muto S, Sakakura M, Iriyama Y, Tateyama Y 2020 ACS Appl. Mater. Interfaces 12 54752
 Google Scholar
Google Scholar
[33] Aono H, Sugimota E, Sadaoka Y, Imanaka N, Adachi Gi Y 1993 J. Electrochem. Soc. 140 1827
 Google Scholar
Google Scholar
[34] Wu P F, Zhou W W, Su X, Li J Y, Su M, Zhou X C, Sheldon B W, Lu W Q 2022 Adv. Energy Mater. 13 2203440
 Google Scholar
Google Scholar
[35] 任元, 邹喆乂, 赵倩, 王达, 喻嘉, 施思齐 2020 69 226601
 Google Scholar
Google Scholar
Ren Y, Zou Z Y, Zhao Q, Wang D, Yu J, Shi S Q 2020 Acta Phys. Sin. 69 226601
 Google Scholar
Google Scholar
[36] Yang K, Chen L K, Ma J B, He Y B, Kang F Y 2021 Info. Mat. 3 1195
 Google Scholar
Google Scholar
[37] Zhang B K, Lin Z, Dong H F, Wang L W, Pan F 2020 J. Mater. Chem. A 8 342
 Google Scholar
Google Scholar
[38] Kotobuki M, Koishi M 2019 J. Asian Ceram 7 69
 Google Scholar
Google Scholar
[39] Vinod Chandran C, Pristat S, Witt E, Tietz F, Heitjans P 2016 J. Phys. Chem. C 120 8436
 Google Scholar
Google Scholar
[40] Shi S Q, Gao J, Liu Y, Zhao Y, Wu Q, Ju W W, Ouyang C Y, Xiao R J 2016 Chin. Phys. B 25 018212
 Google Scholar
Google Scholar
[41] Dashjav E, Tietz F 2014 Z. Anorg. Allg. Chem. 640 3070
 Google Scholar
Google Scholar
[42] 王田田 2022 博士学位论文 (上海: 中国科学院上海硅酸盐研究所)
Wang T T 2022 Ph. D. Dissertation (Shanghai: Shanghai Institude of Ceramics, Chinese Academy of Sciences
[43] Francisco B E, Stoldt C R, M’Peko J C 2014 Chem. Mater. 26 4741
 Google Scholar
Google Scholar
[44] He S N, Xu Y L, Zhang B F, Sun X F, Chen Y J, Jin Y L 2018 Chem. Eng. J. 345 483
 Google Scholar
Google Scholar
[45] Lang B, Ziebarth B, Elsässer C 2015 Chem. Mater. 27 5040
 Google Scholar
Google Scholar
[46] Alami M, Brochu R, Soubeyroux J L, Gravereau P, Flem G L, Hagenmuller P 1991 J. Solid State Chem. 90 185
 Google Scholar
Google Scholar
[47] Qui D T, Hamdoune S, Soubeyroux J L, Prince E 1988 J. Solid State Chem. 72 309
 Google Scholar
Google Scholar
[48] Redhammer G J, Rettenwander D, Pristat S, Dashjav E, Kumar C M N, Topa D, Tietz F 2016 Solid State Sci. 60 99
 Google Scholar
Google Scholar
[49] Pérez-Estébanez M, Isasi-Marín J, Többens D M, Rivera-Calzada A, León C 2014 Solid State Ionics 266 1
 Google Scholar
Google Scholar
[50] Kresse G, Joubert D 1999 Phys. Rev. B 59 1758
 Google Scholar
Google Scholar
[51] Perdew J P, Burke K, Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[52] Perdew J P, Ernzerhof M, Burke K 1996 J. Chem. Phys. 105 9982
 Google Scholar
Google Scholar
[53] James D P, Hendrik J M 1977 Phys. Rev. B 16 1748
 Google Scholar
Google Scholar
[54] Henkelman G, Uberuaga B P, Jónsson H 2000 J. Chem. Phys. 113 9901
 Google Scholar
Google Scholar
[55] Daniel P, Daniel M, Daniel F U 2021 Solid State Ionics 359 115521
 Google Scholar
Google Scholar
[56] 姚登浪, 黄泽琛, 郭祥, 丁召, 王一 2024 原子与分子 41 153
 Google Scholar
Google Scholar
Yao D L, Huang Z C, Guo X, Ding Z, Wang Y 2024 J. At. Mol. Phys. 41 153
 Google Scholar
Google Scholar
[57] Tian H K, Liu Z, Ji Y Z, Chen L Q, Qi Y 2019 Chem. Mater. 31 7351
 Google Scholar
Google Scholar
[58] Lu X, Wang S H, Xiao R J, Shi S Q, Li H, Chen L Q 2017 Nano Energy 41 626
 Google Scholar
Google Scholar
[59] Woodcock D A, Lightfoot P 1999 J. Mater. Chem. 9 2907
 Google Scholar
Google Scholar
[60] He X F, Zhu Y Z, Mo Y F 2017 Nat. Commun. 8 15893
 Google Scholar
Google Scholar
[61] Kosova N V, Devyatkina E T, Stepanov A P, Buzlukov A L 2008 Ionics 14 303
 Google Scholar
Google Scholar
[62] Case D, McSloy A J, Sharpe R, Yeandel S R, Bartlett T, Cookson J, Dashjav E, Tietz F, Naveen Kumar C M, Goddard P 2020 Solid State Ionics 346 115192
 Google Scholar
Google Scholar
-
图 1 (a) LTP晶体结构, TiO6八面体和PO4四面体共顶点连接形成三维骨架, 蓝色八面体为[Ti/Al]O6, 灰色四面体为PO4; (b) LTP晶体结构中Li的间隙位点, 绿色球为Li6b位点, 紫红色球为Li18e位点, 橙色球为Li36f位点
Fig. 1. (a) LTP crystal structure, TO6 octahedron and PO4 tetrahedron are connected together to form a three dimensional skeleton, the blue octahedron is [Ti/Al]O6, and the gray tetrahedron is PO4; (b) the interstitial sites of Li in the LTP crystal structure: the green sphere is the Li6b site, the purple-red sphere is the Li18e site, and the orange sphere is the Li36f site.
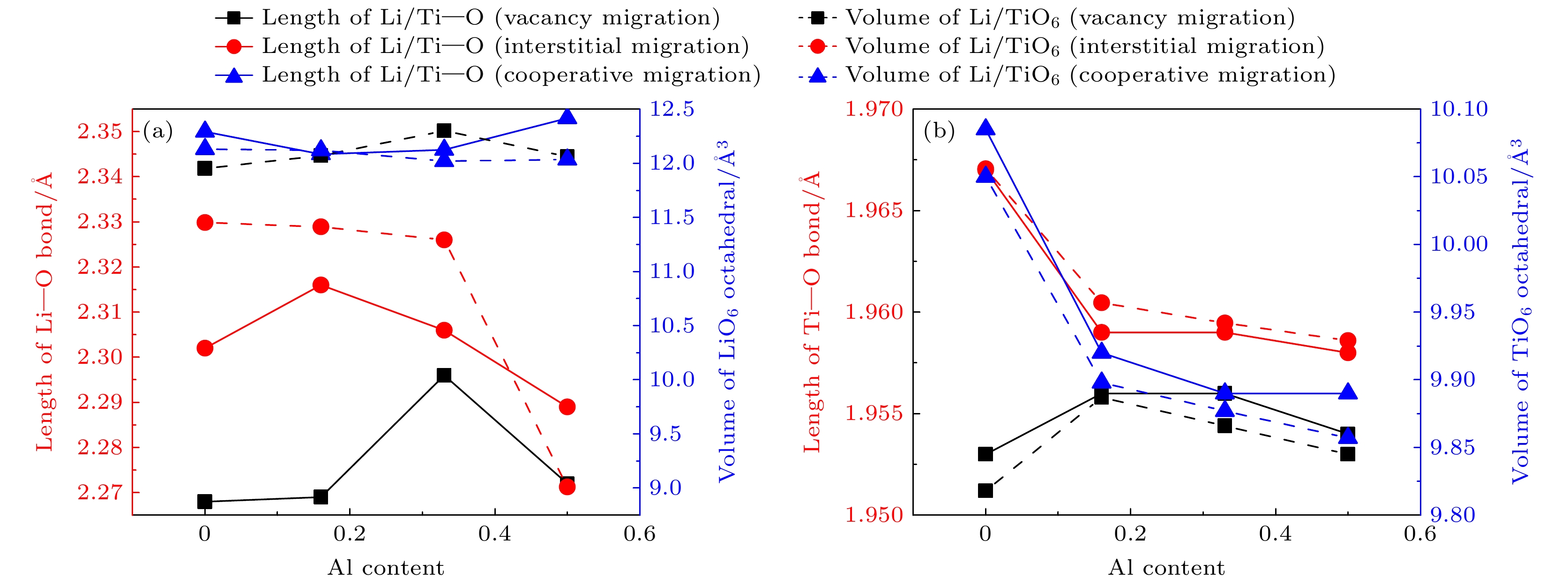
图 3 LTP和LATP中Li+在迁移过程中势垒最高点处的迁移离子的Li—O键长和邻近Ti离子的Ti—O键长及其八面体体积(a) Li—O键键长及LiO6八面体体积; (b) Ti—O键键长及TiO6八面体体积
Fig. 3. Li—O bond length and octahedral volume of the migrating ion and the nearest ion at the highest barrier during migration in LTP and LATP: (a) Length of Li—O and the volume of LiO6 octahedral; (b) length of Ti—O and the volume of TiO6 octahedral.
图 4 LATP (x = 0.16, 0.33, 0.50)相对于LTP和Al的差分电荷密度 (a) LATP-0.16; (b) LATP-0.33; (c) LATP-0.50. 黄色区域表示电荷积累, 等值面值为1×10–3 e/Å3
Fig. 4. Charge density differences of LATP (x = 0.16, 0.33, 0.50) with respect to LTP and Al: (a) LATP-0.16; (b) LATP-0.33; (c) LATP-0.50. The yellow region represents charge accumulation, the isosurface value is 1×10–3 e/Å3.
表 1 LTP及不同Al掺杂浓度下LATP结构的晶格参数, 晶胞体积与掺杂能
Table 1. Lattice constants, volume of hexagonal unit cell, Al defect formation energies of LTP and LATP structures with different Al-doping concentration.
体系 a/Å b/Å c/Å V/Å3 掺杂能/eV LTP 8.615 8.615 21.097 1355.97 — Theory[5] 8.63 8.63 21.13 — — Experiment[41] 8.511 8.511 20.843 1307.53 — LATP-0.16 8.619 8.620 21.021 1352.33 –4.14 LATP-0.33 8.614 8.614 20.924 1343.79 –3.97 Experiment[55] 8.50 8.50 20.82 — — LATP-0.50 8.607 8.603 20.876 1337.75 –4.01 Experiment[48] 8.4897 8.4897 20.7635 1296.03 — 表 2 优化后的LTP和LATP结构中的平均键长和八面体体积
Table 2. Average bond lengths and the octahedral volume in relaxed LTP and LATP structures.
结构 Li—O/Å $ {V}_{{\mathrm{L}}{\mathrm{i}}{\mathrm{O}}_6} $/Å3 Ti—O/Å $ {V}_{{\mathrm{T}}{\mathrm{i}}{\mathrm{O}}_6} $/Å3 LTP 2.276 13.656 1.960 9.936 LATP-0.16 2.284 13.681 1.960 9.935 LATP-0.33 2.272 13.521 1.958 9.912 LATP-0.50 2.279 13.492 1.959 9.840 -
[1] Nitta N, Wu F X, Lee J T, Yushin G 2015 Mater. Today 18 252
 Google Scholar
Google Scholar
[2] Abraham K M 2015 J. Phys. Chem. Lett. 6 830
 Google Scholar
Google Scholar
[3] Tarascon J M 2010 Phil. Trans. R. Soc. A 368 3227
 Google Scholar
Google Scholar
[4] Quartarone E, Mustarelli P 2011 Chem. Soc. Rev. 40 2525
 Google Scholar
Google Scholar
[5] Zhang B K, Tan R, Yang L Y, Zheng J X, Zhang K C, Mo S J, Lin Z, Pan F 2018 Energy Storage Mater. 10 139
 Google Scholar
Google Scholar
[6] Chen R, Qu W, Guo X, Li L, Wu F 2016 Mater. Horiz. 3 487
 Google Scholar
Google Scholar
[7] Wang Y, Zhong W H 2015 Chem. Electro. Chem. 2 3
 Google Scholar
Google Scholar
[8] Bruce P G, Freunberger S A, Hardwick L J, Tarascon J M 2011 Nat. Mater. 11 19
 Google Scholar
Google Scholar
[9] Kato Y, Hori S, Saito T, Suzuki K, Hirayama M, Mitsui A, Yonemura M, Iba H, Kanno R 2016 Nat. Energy 1 16030
 Google Scholar
Google Scholar
[10] Bachman J C, Muy S, Grimaud A, Chang H H, Pour N, Lux S F, Paschos O, Maglia F, Lupart S, Lamp P, Giordano L, Shao-Horn Y 2016 Chem. Rev. 116 140
 Google Scholar
Google Scholar
[11] Croce F, Appetecchi G B, Persi L, Scrosati B 1998 Nature 394 456
 Google Scholar
Google Scholar
[12] Xiao Z L, Long T Y, Song L B, Zheng Y H, Wang C 2022 Ionics 28 15
 Google Scholar
Google Scholar
[13] Xi G, Xiao M, Wang S J, Han D M, Li Y N, Meng Y Z 2020 Adv. Funct. Mater. 31 2007598
 Google Scholar
Google Scholar
[14] Zhang Z Z, Wenzel S, Zhu Y Z, Sann J, Shen L, Yang J, Yao X Y, Hu Y S, Wolverton C, Li H, Chen L Q, Janek J 2020 ACS Appl. Energy Mater. 3 7427
 Google Scholar
Google Scholar
[15] Chen X F, Guan Z Q, Chu F L, Xue Z C, Wu F X, Yu Y 2021 Info. Mat. 4 e12248
 Google Scholar
Google Scholar
[16] 吴洁, 江小标, 杨旸, 吴勇民, 朱蕾, 汤卫平 2020 储能科学与技术 9 1472
 Google Scholar
Google Scholar
Wu J, Jiang X B, Yang Y, Wu Y M, Zhu L, Tang W P 2020 Energy Stor. Sci. Tech. 9 1472
 Google Scholar
Google Scholar
[17] Zhang L X, Liu Y M, Han J, Yang C, Zhou X, Yuan Y, You Y 2023 ACS Appl. Mater. Interfaces 15 44867
 Google Scholar
Google Scholar
[18] Subramanian M A, Subramanian R, Clearfield A 1986 Solid State Ionics 18-19 562
 Google Scholar
Google Scholar
[19] Kresse G, Furthmüller J 1996 Phys. Rev. B 54 11169
 Google Scholar
Google Scholar
[20] Adachi G Y, Imanaka N, Aono H 1996 Adv. Mater. 8 127
 Google Scholar
Google Scholar
[21] Aono H, Sugimoto E, Sadaoka Y, Imanaka N, Adachi G Y 1990 J. Electrochem. Soc. 137 1023
 Google Scholar
Google Scholar
[22] Schroeder M, Glatthaar S, Binder J R 2011 Solid State Ionics. 201 49
 Google Scholar
Google Scholar
[23] Mariappan C R, Gellert M, Yada C, Rosciano F, Roling B 2012 Electrochem. Commun. 14 25
 Google Scholar
Google Scholar
[24] Yin F S, Zhang Z J, Fang Y L, Sun C W 2023 J. Energy Stor. 73 108950
 Google Scholar
Google Scholar
[25] Arbi K, Lazarraga M G, Ben Hassen Chehimi D, Ayadi-Trabelsi M, Rojo J, Sanz J 2004 Chem. Mater. 16 255
 Google Scholar
Google Scholar
[26] Monchak M, Hupfer T, Senyshyn A, Boysen H, Chernyshov D, Hansen T, Schell K G, Bucharsky E C, Hoffmann M J, Ehrenberg H 2016 Inorg. Chem. 55 2941
 Google Scholar
Google Scholar
[27] Arbi K, Hoelzel M, Kuhn A, Garcia-Alvarado F, Sanz J 2013 Inorg. Chem. 52 9290
 Google Scholar
Google Scholar
[28] Rao A V, Veeraiah V, Rao A P, Babu B K, Latha B S, Rao K R 2014 B Mater. Sci. 37 883
 Google Scholar
Google Scholar
[29] Luo Y Y, Liu X Y, Wen C J, Ning T X, Jiang X X, Lu A X 2023 Appl. Phys. A 129 518
 Google Scholar
Google Scholar
[30] Liang Y J, Peng C, Kamiike Y, Kuroda K, Okido M 2019 J. Alloys Compd. 775 1147
 Google Scholar
Google Scholar
[31] Luo Y Y, Jiang X X, Yu Y J, Liu L D, Lin X T, Wang Z K, Han L, Luo Z W, Lu A X 2023 Solid State Ionics 390 116111
 Google Scholar
Google Scholar
[32] Tian H K, Jalem R, Gao B, Yamamoto Y, Muto S, Sakakura M, Iriyama Y, Tateyama Y 2020 ACS Appl. Mater. Interfaces 12 54752
 Google Scholar
Google Scholar
[33] Aono H, Sugimota E, Sadaoka Y, Imanaka N, Adachi Gi Y 1993 J. Electrochem. Soc. 140 1827
 Google Scholar
Google Scholar
[34] Wu P F, Zhou W W, Su X, Li J Y, Su M, Zhou X C, Sheldon B W, Lu W Q 2022 Adv. Energy Mater. 13 2203440
 Google Scholar
Google Scholar
[35] 任元, 邹喆乂, 赵倩, 王达, 喻嘉, 施思齐 2020 69 226601
 Google Scholar
Google Scholar
Ren Y, Zou Z Y, Zhao Q, Wang D, Yu J, Shi S Q 2020 Acta Phys. Sin. 69 226601
 Google Scholar
Google Scholar
[36] Yang K, Chen L K, Ma J B, He Y B, Kang F Y 2021 Info. Mat. 3 1195
 Google Scholar
Google Scholar
[37] Zhang B K, Lin Z, Dong H F, Wang L W, Pan F 2020 J. Mater. Chem. A 8 342
 Google Scholar
Google Scholar
[38] Kotobuki M, Koishi M 2019 J. Asian Ceram 7 69
 Google Scholar
Google Scholar
[39] Vinod Chandran C, Pristat S, Witt E, Tietz F, Heitjans P 2016 J. Phys. Chem. C 120 8436
 Google Scholar
Google Scholar
[40] Shi S Q, Gao J, Liu Y, Zhao Y, Wu Q, Ju W W, Ouyang C Y, Xiao R J 2016 Chin. Phys. B 25 018212
 Google Scholar
Google Scholar
[41] Dashjav E, Tietz F 2014 Z. Anorg. Allg. Chem. 640 3070
 Google Scholar
Google Scholar
[42] 王田田 2022 博士学位论文 (上海: 中国科学院上海硅酸盐研究所)
Wang T T 2022 Ph. D. Dissertation (Shanghai: Shanghai Institude of Ceramics, Chinese Academy of Sciences
[43] Francisco B E, Stoldt C R, M’Peko J C 2014 Chem. Mater. 26 4741
 Google Scholar
Google Scholar
[44] He S N, Xu Y L, Zhang B F, Sun X F, Chen Y J, Jin Y L 2018 Chem. Eng. J. 345 483
 Google Scholar
Google Scholar
[45] Lang B, Ziebarth B, Elsässer C 2015 Chem. Mater. 27 5040
 Google Scholar
Google Scholar
[46] Alami M, Brochu R, Soubeyroux J L, Gravereau P, Flem G L, Hagenmuller P 1991 J. Solid State Chem. 90 185
 Google Scholar
Google Scholar
[47] Qui D T, Hamdoune S, Soubeyroux J L, Prince E 1988 J. Solid State Chem. 72 309
 Google Scholar
Google Scholar
[48] Redhammer G J, Rettenwander D, Pristat S, Dashjav E, Kumar C M N, Topa D, Tietz F 2016 Solid State Sci. 60 99
 Google Scholar
Google Scholar
[49] Pérez-Estébanez M, Isasi-Marín J, Többens D M, Rivera-Calzada A, León C 2014 Solid State Ionics 266 1
 Google Scholar
Google Scholar
[50] Kresse G, Joubert D 1999 Phys. Rev. B 59 1758
 Google Scholar
Google Scholar
[51] Perdew J P, Burke K, Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[52] Perdew J P, Ernzerhof M, Burke K 1996 J. Chem. Phys. 105 9982
 Google Scholar
Google Scholar
[53] James D P, Hendrik J M 1977 Phys. Rev. B 16 1748
 Google Scholar
Google Scholar
[54] Henkelman G, Uberuaga B P, Jónsson H 2000 J. Chem. Phys. 113 9901
 Google Scholar
Google Scholar
[55] Daniel P, Daniel M, Daniel F U 2021 Solid State Ionics 359 115521
 Google Scholar
Google Scholar
[56] 姚登浪, 黄泽琛, 郭祥, 丁召, 王一 2024 原子与分子 41 153
 Google Scholar
Google Scholar
Yao D L, Huang Z C, Guo X, Ding Z, Wang Y 2024 J. At. Mol. Phys. 41 153
 Google Scholar
Google Scholar
[57] Tian H K, Liu Z, Ji Y Z, Chen L Q, Qi Y 2019 Chem. Mater. 31 7351
 Google Scholar
Google Scholar
[58] Lu X, Wang S H, Xiao R J, Shi S Q, Li H, Chen L Q 2017 Nano Energy 41 626
 Google Scholar
Google Scholar
[59] Woodcock D A, Lightfoot P 1999 J. Mater. Chem. 9 2907
 Google Scholar
Google Scholar
[60] He X F, Zhu Y Z, Mo Y F 2017 Nat. Commun. 8 15893
 Google Scholar
Google Scholar
[61] Kosova N V, Devyatkina E T, Stepanov A P, Buzlukov A L 2008 Ionics 14 303
 Google Scholar
Google Scholar
[62] Case D, McSloy A J, Sharpe R, Yeandel S R, Bartlett T, Cookson J, Dashjav E, Tietz F, Naveen Kumar C M, Goddard P 2020 Solid State Ionics 346 115192
 Google Scholar
Google Scholar
计量
- 文章访问数: 4197
- PDF下载量: 87
- 被引次数: 0














 下载:
下载: