-
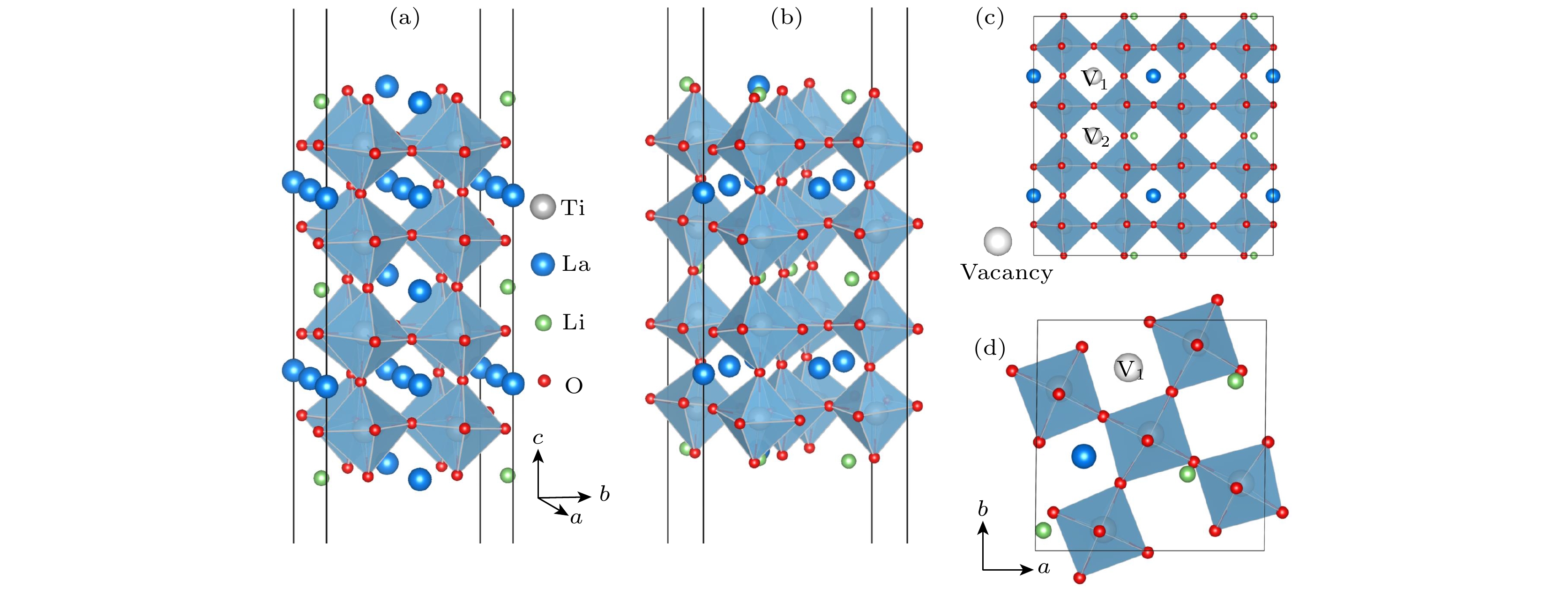
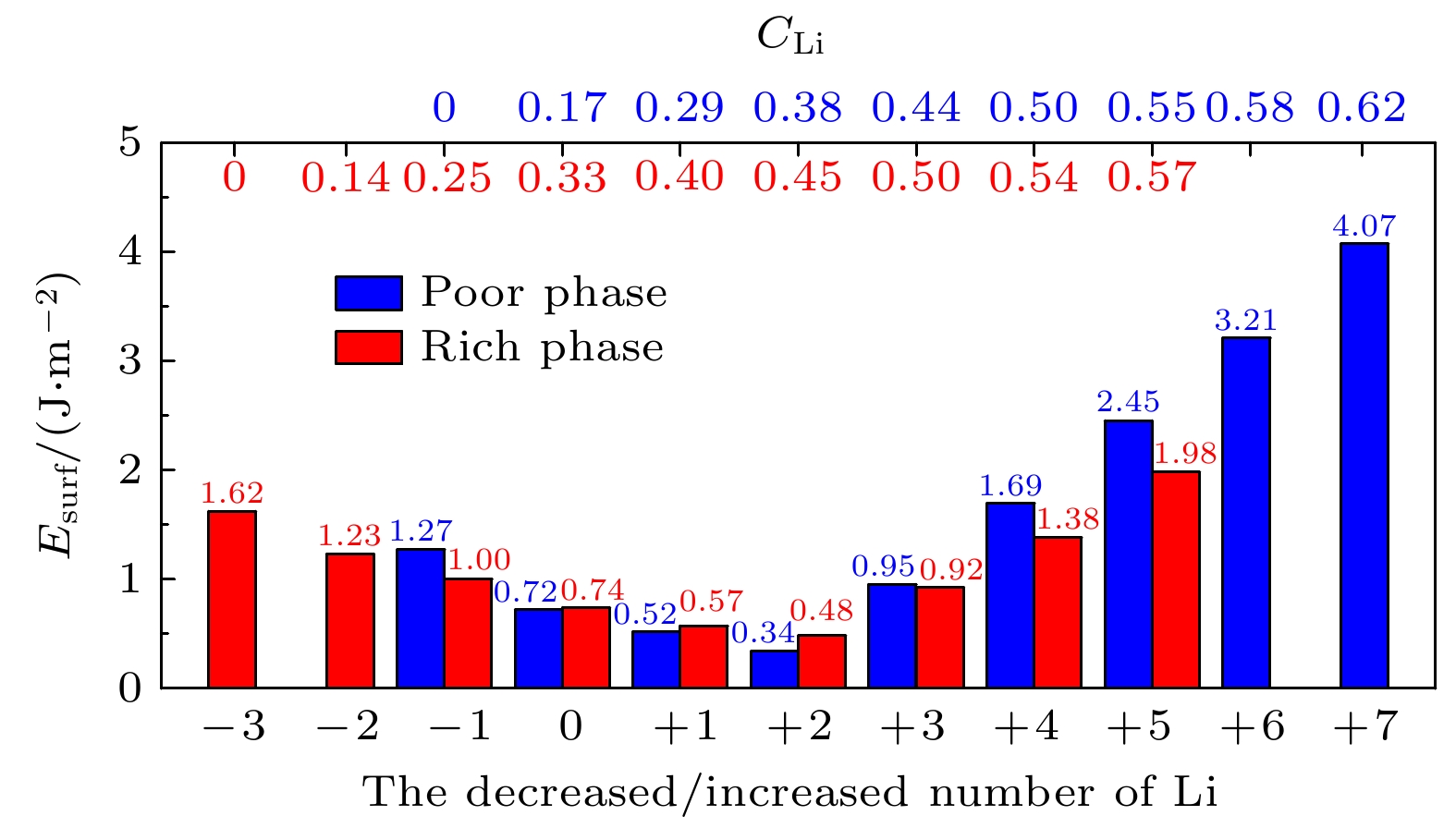
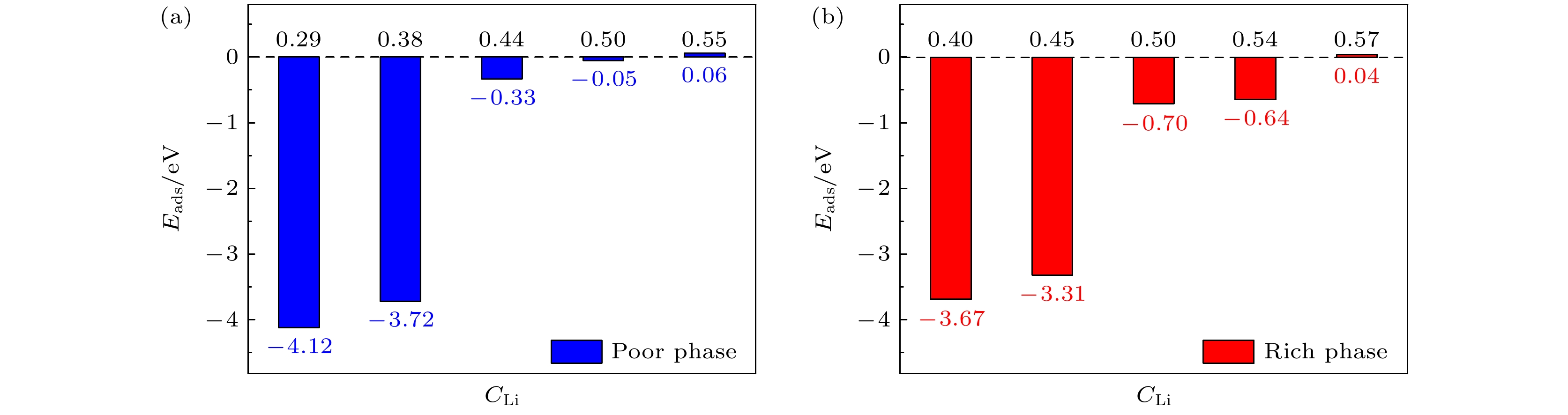
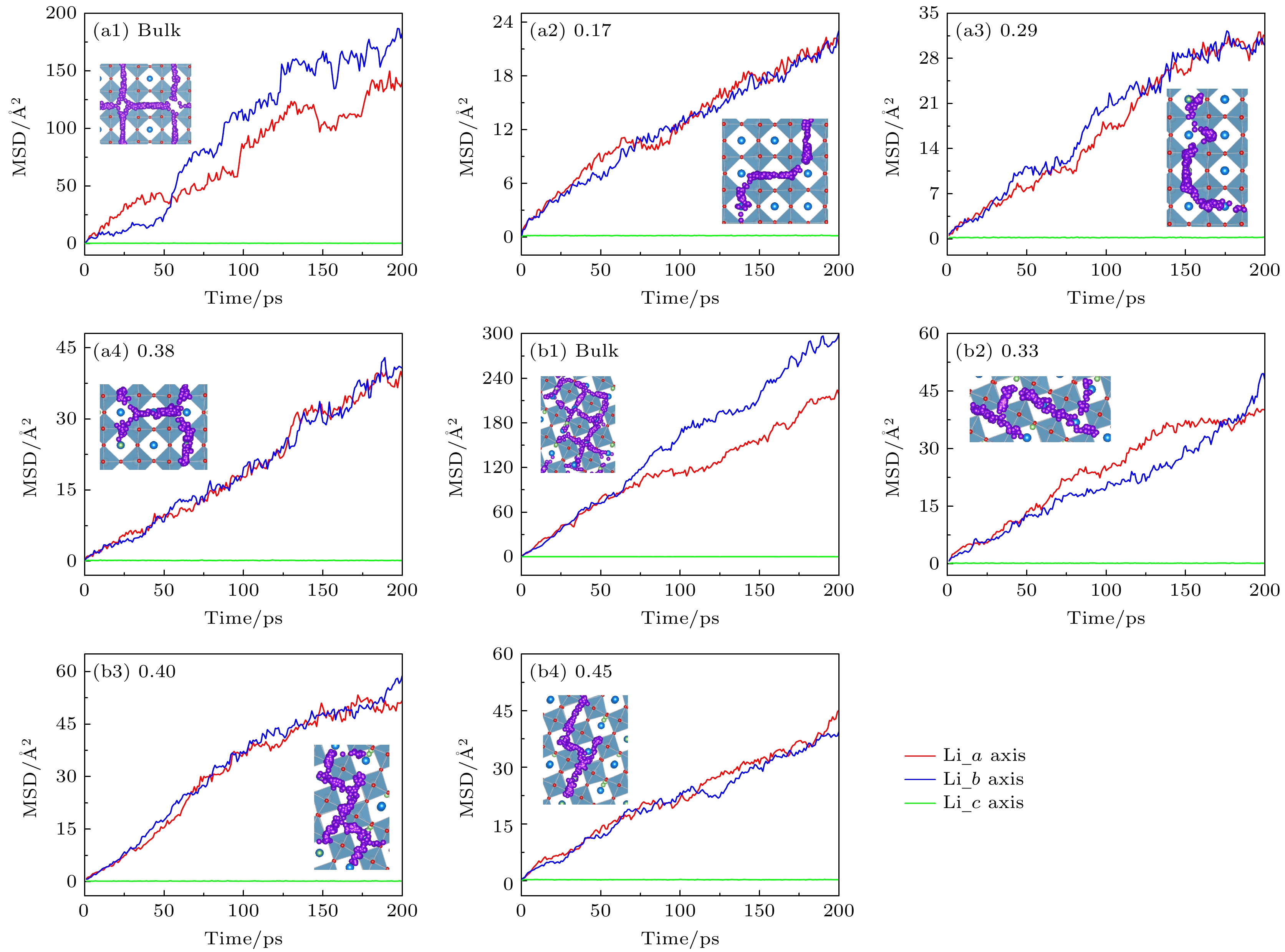
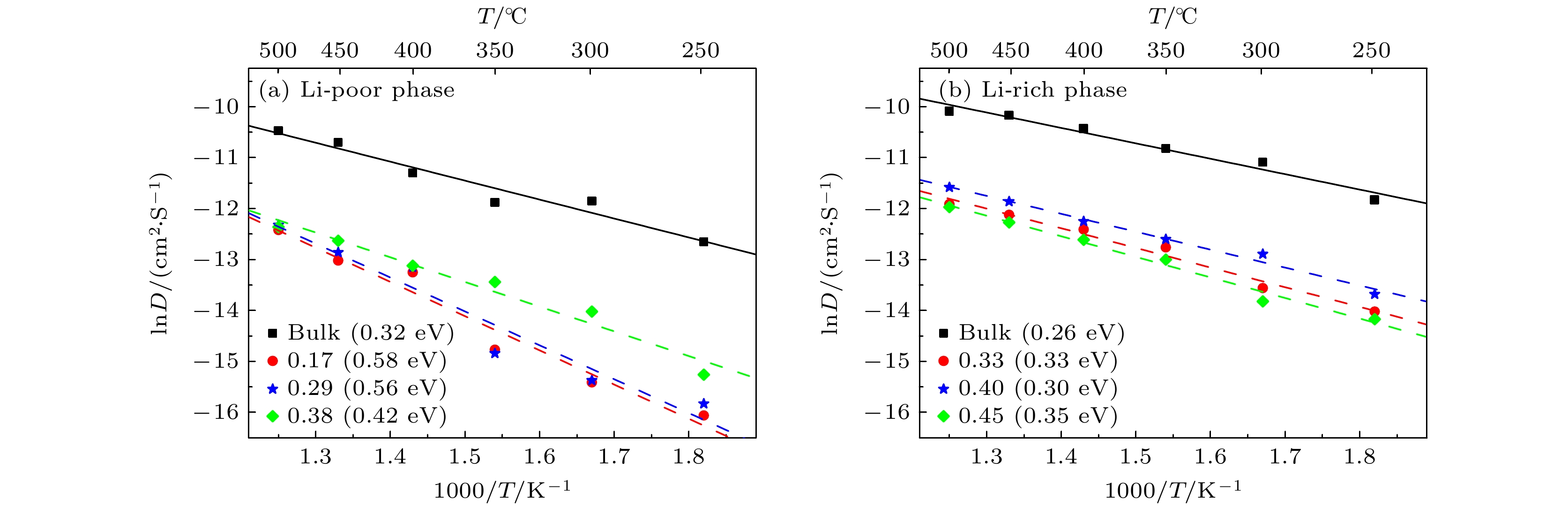
Li3xLa(2/3)–x†(1/3)–2xTiO3 (LLTO)是一类颇具前景的锂离子电池固态电解质. 本文采用第一性原理结合分子动力学方法对贫锂相和富锂相两种类型的LLTO表面进行研究, 分析表面Li含量对其稳定性、电子结构及Li离子输运性质的影响. 结果表明, 具有La/O/Li-原子终端的(001)面为最稳定晶面. 对于LLTO (001)面, 当贫锂相/富锂相终端Li含量为0.17/0.33, 0.29/0.40, 0.38/0.45时, 其表面结构更为稳定. 电子结构分析表明, 随着Li含量的增大, 不论是贫锂相还是富锂相, 其(001)表面均发现金属至半导体的转变. Li离子输运性质的研究结果表明, 贫锂相和富锂相LLTO (001)表面均具有沿ab平面的二维扩散通道, 且当终端Li含量分别达到0.38和0.40时具有最大的Li离子扩散系数及最低的Li离子扩散能垒, 最低扩散能垒分别为0.42 eV和0.30 eV. 因而, 改变终端Li含量有利于提高LLTO(001)表面稳定性、打开表面带隙、改善Li离子迁移性能, 这有助于抑制LLTO表面锂枝晶的生长.
-
关键词:
- 全固态锂离子电池 /
- 锂含量 /
- Li3xLa(2/3)–x†(1/3)–2xTiO3表面
Li3xLa(2/3)–x†(1/3)–2xTiO3(LLTO) is a promising solid-state electrolyte for Li-ion batteries. We study the effect of Li content on the stability, electronic and Li-ion diffusion properties of LLTO surface based on first-principles and molecular dynamics simulations. We consider both Li-poor and Li-rich LLTO surfaces. The results show that La/O/Li-terminated LLTO (001) is the most stable crystal surface. Further, LLTO (001) surface gives better stability when Li content is 0.17, 0.29, and 0.38 for Li-poor phase, while 0.33, 0.40, and 0.45 for Li-rich phase . Electronic structure calculations infer that in both Li-poor and Li-rich LLTO(001) surfaces there occurs the transition from conductor to semiconductor with the increase of Li content. Besides, we find that Li-ion always keeps a two-dimensional diffusion path for different Li content. As Li content increases from 0.17 to 0.38 for Li-poor LLTO (001) surface, Li-ion diffusion coefficient increases gradually and Li-ion diffusion barrier decreases from 0.58 eV to 0.42 eV. Differently, when Li content increases from 0.33 to 0.45 for Li-rich LLTO(001) surface, it does not follow a monotonic trend for diffusion coefficient nor for diffusion barrier of Li-ion. In this case, Li-ion diffusion coefficient is the largest and Li-ion diffusion barrier is the lowest (0.30 eV) when Li content is 0.40. Thus, our study suggests that by varying Li content, the stability, band gap, and Li-ion diffusion performance of LLTO (001) can be changed favorably. These advantages can inhibit the formation of lithium dendrites on the LLTO (001) surface.[1] Famprikis T, Canepa P, Dawson J A, Islam M S, Masquelier C 2019 Nat. Mater. 18 1278
 Google Scholar
Google Scholar
[2] Manthiram A, Yu X W, Wang S F 2017 Nat. Rev. Mater. 2 1
 Google Scholar
Google Scholar
[3] Zhao Q, Stalin S, Zhao C-Z, Archer L A 2020 Nat. Rev. Mater. 5 229
 Google Scholar
Google Scholar
[4] Wu M S, Xu B, Lei X L, Huang K, Ouyang C Y 2018 J. Mater. Chem. A 6 1150
 Google Scholar
Google Scholar
[5] Yan S, Yim C H, Pankov V, Bauer M, Baranova E, Weck A, Merati A, Abu-Lebdeh Y 2021 Batteries 7 75
 Google Scholar
Google Scholar
[6] Sun Y D, Guan P Y, Liu Y J, Xu H L, Li S, Chu D W 2018 Crit. Rev. Solid State 44 265
 Google Scholar
Google Scholar
[7] Hua C, Fang X, Wang Z, Chen L 2013 Electrochem. Commun. 32 5
 Google Scholar
Google Scholar
[8] Stramare S, Thangadurai V, Weppner W 2003 Chem. Mater. 15 3974
 Google Scholar
Google Scholar
[9] Chen C H, Amine K 2001 Solid State Ion. 144 51
 Google Scholar
Google Scholar
[10] Inaguma o, Liquan C, Itoh M, Nakamura T 1993 Solid State Commun. 86 689
 Google Scholar
Google Scholar
[11] Han F D, Westover A S, Yue J, Fan X L, Wang F, Chi M F, Leonard D N, Dudney N, Wang H, Wang C S 2019 Nat. Energy 4 187
 Google Scholar
Google Scholar
[12] Wu B B, Wang S Y, Lochala J S, Desrochers D, Liu B, Zhang W Q, Yang J H, Xiao J 2018 Energy Environ. Sci. 11 1803
 Google Scholar
Google Scholar
[13] Cervantes J M, Pilo J, Rosas-Huerta J L, Antonio J E, Muñoz H, Oviedo-Roa R, Carvajal E 2021 J. Electrochem. Soc. 168 080516
 Google Scholar
Google Scholar
[14] Zhao Q S, Xue H T, Tang F L, Wei C D 2021 Solid State Ion. 373 115797
 Google Scholar
Google Scholar
[15] Cheng L, Chen W, Kunz M, Persson K, Tamura N, Chen G Y, Doeff M 2015 ACS Appl. Mater. Interface 7 2073
 Google Scholar
Google Scholar
[16] Belousov V V 2007 Russ. J. Phys. Chem. A 81 441
 Google Scholar
Google Scholar
[17] Wu M S, Xu B, Luo W W, Sun B Z, Shi J, Ouyang C Y 2020 Appl. Surf. Sci. 510 145394
 Google Scholar
Google Scholar
[18] Jung S C, Han Y K 2011 Phys. Chem. Chem. Phys. 13 21282
 Google Scholar
Google Scholar
[19] Nakayama M, Usui T, Uchimoto Y, Wakihara M, Yamamoto M 2005 J. Phys. Chem. B 109 4135
 Google Scholar
Google Scholar
[20] Inaguma Y, Itoh M 1996 Solid State Ion. 86-88 257
[21] Maruyama Y, Ogawa H, Kamimura M, Kobayashi M 2006 J. Phys. Soc. Jpn. 75 064602
 Google Scholar
Google Scholar
[22] Ren Y Y, Shen Y, Lin Y H, Nan C W 2019 ACS Appl. Mater. Interface 11 5928
 Google Scholar
Google Scholar
[23] Catti M 2008 J. Phys. Chem. C 112 11068
 Google Scholar
Google Scholar
[24] Qian D N, Xu B, Cho H M, Hatsukade T, Carroll K J, Meng Y S 2012 Chem. Mater. 24 2744
 Google Scholar
Google Scholar
[25] Kresse G, Furthmuller J 1996 Phys. Rev. B 54 11169
 Google Scholar
Google Scholar
[26] Kresse G, Hafner J 1994 Phys. Rev. B 49 14251
 Google Scholar
Google Scholar
[27] Perdew J P, Burke K, Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[28] Perdew J P, Ernzerhof M, Burke K 1996 J. Chem. Phys. 105 9982
 Google Scholar
Google Scholar
[29] Blochl P E 1994 Phys. Rev. B 50 17953
 Google Scholar
Google Scholar
[30] Kresse G, Joubert D 1999 Phys. Rev. B 59 1758
 Google Scholar
Google Scholar
[31] Monkhorst H J, Pack J D 1976 Phys. Rev. B 13 5188
 Google Scholar
Google Scholar
[32] Plimpton S 1995 J. Comput. Phys. 117 1
 Google Scholar
Google Scholar
[33] Chen C H, Du J C, Chen L Q 2015 J. Am. Ceram. Soc. 98 534
 Google Scholar
Google Scholar
[34] Symington A R, Molinari M, Dawson J A, Statham J M, Purton J, Canepa P, Parker S C 2021 J. Mater. Chem. A 9 6487
 Google Scholar
Google Scholar
[35] Ono S, Seki Y, Kashida S, Kobayashi M 2006 Solid State Ion. 177 1145
 Google Scholar
Google Scholar
[36] Kim D H, Kim D H, Jeong Y C, Seo H I, Kim Y C 2012 Ceram. Int. 38 S S467
[37] Bohnke O 2008 Solid State Ion. 179 9
 Google Scholar
Google Scholar
-
表 1 不同泛函计算所得贫锂相LLTO体相的晶格参数(a, b, c)及带隙 (Eg)
Table 1. The calculated lattice parameters (a, b, c) and band gap (Eg) of Li-poor LLTO bulk with different functional.
表 2 富锂相LLTO不同表面终端的表面能(Esurf)和化学式(SFs), 括号中的值对应贫锂相
Table 2. Surface energy (Esurf) and structural formulas (SFs) of Li-rich LLTO surfaces with different terminations. The data of Li-poor LLTO (001) is shown in parentheses.
Facets Termination SFs Esurf/(J·m–2) (001) La/O- Li3La11Ti10O35 (Li2La14Ti16O52) 2.89 (1.95) Ti/O- Li3La6Ti15O40 (LiLa9Ti16O44) 1.40 (1.33) La/O/Li- Li10La12Ti20O65 (Li3La11Ti16O52) 0.69 (0.78) Li/O- Li11La11Ti20O65 0.78 (010) La/O- Li7La13Ti20O64 0.93 Ti/O- Li7La11Ti24O68 0.87 La/O/Li- Li9La13Ti20O64 0.82 (100) La/O- Li7La13Ti20O64 1.05 Ti/O- Li7La11Ti24O68 0.90 La/O/Li- Li9La13Ti20O64 0.83 (110) O- Li7La11Ti20O68 0.98 Ti/La/O- Li7La13Ti24O64 3.40 Ti/O/La/Li- Li9La14Ti24O72 1.21 (111) La/O- Li9La13Ti24O72 2.21 Ti/O- Li7La11Ti20O60 0.85 Ti/O/La/Li- Li7La11Ti20O60 0.93 表 3 不同温度下贫锂相和富锂相LLTO(001)表面结构中全部Li+的最小(Dmin)和最大(Dmax)扩散系数
Table 3. The minimum (Dmin) and maximum (Dmax) Li+ diffusion coefficient of Li-poor and Li-rich LLTO(001) surfaces at different temperatures.
T/K Li-poor phase/(cm2·S–1) Li-rich phase/(cm2·S–1) Dmin Dmax Dmin Dmax 550 1.06×10–7 2.37×10–7 7.02×10–7 1.14×10–6 600 2.02×10–7 8.12×10–7 9.96×10–7 2.53×10–6 650 3.84×10–7 1.46×10–6 2.26×10–6 3.38×10–6 700 1.77×10–6 2.01×10–6 3.34×10–6 4.80×10–6 750 2.22×10–6 3.27×10–6 4.69×10–6 7.08×10–6 800 4.03×10–6 4.28×10–6 6.33×10–6 9.36×10–6 -
[1] Famprikis T, Canepa P, Dawson J A, Islam M S, Masquelier C 2019 Nat. Mater. 18 1278
 Google Scholar
Google Scholar
[2] Manthiram A, Yu X W, Wang S F 2017 Nat. Rev. Mater. 2 1
 Google Scholar
Google Scholar
[3] Zhao Q, Stalin S, Zhao C-Z, Archer L A 2020 Nat. Rev. Mater. 5 229
 Google Scholar
Google Scholar
[4] Wu M S, Xu B, Lei X L, Huang K, Ouyang C Y 2018 J. Mater. Chem. A 6 1150
 Google Scholar
Google Scholar
[5] Yan S, Yim C H, Pankov V, Bauer M, Baranova E, Weck A, Merati A, Abu-Lebdeh Y 2021 Batteries 7 75
 Google Scholar
Google Scholar
[6] Sun Y D, Guan P Y, Liu Y J, Xu H L, Li S, Chu D W 2018 Crit. Rev. Solid State 44 265
 Google Scholar
Google Scholar
[7] Hua C, Fang X, Wang Z, Chen L 2013 Electrochem. Commun. 32 5
 Google Scholar
Google Scholar
[8] Stramare S, Thangadurai V, Weppner W 2003 Chem. Mater. 15 3974
 Google Scholar
Google Scholar
[9] Chen C H, Amine K 2001 Solid State Ion. 144 51
 Google Scholar
Google Scholar
[10] Inaguma o, Liquan C, Itoh M, Nakamura T 1993 Solid State Commun. 86 689
 Google Scholar
Google Scholar
[11] Han F D, Westover A S, Yue J, Fan X L, Wang F, Chi M F, Leonard D N, Dudney N, Wang H, Wang C S 2019 Nat. Energy 4 187
 Google Scholar
Google Scholar
[12] Wu B B, Wang S Y, Lochala J S, Desrochers D, Liu B, Zhang W Q, Yang J H, Xiao J 2018 Energy Environ. Sci. 11 1803
 Google Scholar
Google Scholar
[13] Cervantes J M, Pilo J, Rosas-Huerta J L, Antonio J E, Muñoz H, Oviedo-Roa R, Carvajal E 2021 J. Electrochem. Soc. 168 080516
 Google Scholar
Google Scholar
[14] Zhao Q S, Xue H T, Tang F L, Wei C D 2021 Solid State Ion. 373 115797
 Google Scholar
Google Scholar
[15] Cheng L, Chen W, Kunz M, Persson K, Tamura N, Chen G Y, Doeff M 2015 ACS Appl. Mater. Interface 7 2073
 Google Scholar
Google Scholar
[16] Belousov V V 2007 Russ. J. Phys. Chem. A 81 441
 Google Scholar
Google Scholar
[17] Wu M S, Xu B, Luo W W, Sun B Z, Shi J, Ouyang C Y 2020 Appl. Surf. Sci. 510 145394
 Google Scholar
Google Scholar
[18] Jung S C, Han Y K 2011 Phys. Chem. Chem. Phys. 13 21282
 Google Scholar
Google Scholar
[19] Nakayama M, Usui T, Uchimoto Y, Wakihara M, Yamamoto M 2005 J. Phys. Chem. B 109 4135
 Google Scholar
Google Scholar
[20] Inaguma Y, Itoh M 1996 Solid State Ion. 86-88 257
[21] Maruyama Y, Ogawa H, Kamimura M, Kobayashi M 2006 J. Phys. Soc. Jpn. 75 064602
 Google Scholar
Google Scholar
[22] Ren Y Y, Shen Y, Lin Y H, Nan C W 2019 ACS Appl. Mater. Interface 11 5928
 Google Scholar
Google Scholar
[23] Catti M 2008 J. Phys. Chem. C 112 11068
 Google Scholar
Google Scholar
[24] Qian D N, Xu B, Cho H M, Hatsukade T, Carroll K J, Meng Y S 2012 Chem. Mater. 24 2744
 Google Scholar
Google Scholar
[25] Kresse G, Furthmuller J 1996 Phys. Rev. B 54 11169
 Google Scholar
Google Scholar
[26] Kresse G, Hafner J 1994 Phys. Rev. B 49 14251
 Google Scholar
Google Scholar
[27] Perdew J P, Burke K, Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[28] Perdew J P, Ernzerhof M, Burke K 1996 J. Chem. Phys. 105 9982
 Google Scholar
Google Scholar
[29] Blochl P E 1994 Phys. Rev. B 50 17953
 Google Scholar
Google Scholar
[30] Kresse G, Joubert D 1999 Phys. Rev. B 59 1758
 Google Scholar
Google Scholar
[31] Monkhorst H J, Pack J D 1976 Phys. Rev. B 13 5188
 Google Scholar
Google Scholar
[32] Plimpton S 1995 J. Comput. Phys. 117 1
 Google Scholar
Google Scholar
[33] Chen C H, Du J C, Chen L Q 2015 J. Am. Ceram. Soc. 98 534
 Google Scholar
Google Scholar
[34] Symington A R, Molinari M, Dawson J A, Statham J M, Purton J, Canepa P, Parker S C 2021 J. Mater. Chem. A 9 6487
 Google Scholar
Google Scholar
[35] Ono S, Seki Y, Kashida S, Kobayashi M 2006 Solid State Ion. 177 1145
 Google Scholar
Google Scholar
[36] Kim D H, Kim D H, Jeong Y C, Seo H I, Kim Y C 2012 Ceram. Int. 38 S S467
[37] Bohnke O 2008 Solid State Ion. 179 9
 Google Scholar
Google Scholar
计量
- 文章访问数: 9416
- PDF下载量: 162
- 被引次数: 0














 下载:
下载: