-
水是万物生命之源, 认识水的太赫兹吸收谱是太赫兹技术在生物医学上应用的前提, 太赫兹频率的选择对高效、低能耗地实现太赫兹的生物效应至关重要. 水的复杂氢键网络使得其具有较宽的太赫兹吸收峰, 因此有必要研究水的氢键网络动力学与其太赫兹吸收谱之间的关系, 然而这方面的研究仍然非常缺乏. 采用分子动力学模拟方法, 本文研究了不同水模型在常温常压下的太赫兹吸收谱, 并且进一步基于温度研究了水的太赫兹吸收谱对氢键网络强弱的依赖性, 发现温度的升高会使氢键网络的太赫兹吸收谱发生红移, 这表明氢键网络的太赫兹吸收谱的中心频率与氢键相互作用的强弱具有强关联, 更进一步的研究表明水中氢键网络的氢键寿命与氢键网络振动的吸收峰的中心频率之间存在线性关系. 这一现象背后的物理能够通过将氢键网络中的氢键类比为弹簧借助弹簧振子模型加以描述. 本文的发现将有利于理解水中复杂的氢键网络动力学, 以及促进太赫兹的生物效应研究.Water is the source of all life. The understanding of the terahertz absorption spectrum of water is the prerequisite for the application of terahertz technology to biomedicine. The choice of terahertz frequency is essential for achieving the biological effects of terahertz with high efficiency and low energy consumption. The complex hydrogen bond network of water possesses a broad terahertz absorption peak. Therefore, it is necessary to study the relation between the dynamics of the hydrogen bond network of water and its terahertz absorption spectrum. However, the research in this field is still lacking. Using molecular dynamics simulation methods, the terahertz absorption spectra of different water models at room temperature and pressure are studied in this work. Furthermore, taking the temperature as a variable, the dependence of the terahertz absorption spectrum of water on the strength of the hydrogen bond network is explored. It is found that rising temperature makes the terahertz absorption spectrum of the hydrogen bond network red-shift, indicating that the center frequency of the spectrum is strongly correlated with the strength of the hydrogen bond. Further studies show that there is a linear relationship between the hydrogen bond lifetime of water and the center frequency of vibration absorption peak of the hydrogen bond network. The underlying mechanism can be disclosed by imitating the hydrogen bonds in the hydrogen bond network as springs then using the spring oscillator model. These findings are conducive to understanding in depth the complex hydrogen bond network dynamics in water and promoting the study of terahertz biological effects.
-
Keywords:
- terahertz /
- water /
- hydrogen bond
[1] Ball P 2017 Proc. Natl. Acad. Sci. U.S.A. 114 13327
 Google Scholar
Google Scholar
[2] Ball P 2008 Chem. Rev. 108 74
 Google Scholar
Google Scholar
[3] Xie Z, Li Z, Lou G, Liang Q, Chen J X, Kou J L, Wei G N 2021 Commun. Theor. Phys. 73 055602
 Google Scholar
Google Scholar
[4] Xie Z, Li Z, Li J Y, Kou J L, Yao J, Fan J T 2021 J. Chem. Phys. 154 024705
 Google Scholar
Google Scholar
[5] 王强, 曹则贤 2019 68 015101
 Google Scholar
Google Scholar
Wang Q, Cao Z X 2019 Acta Phys. Sin. 68 015101
 Google Scholar
Google Scholar
[6] 方海平 2016 65 186101
 Google Scholar
Google Scholar
Fang H P 2016 Acta Phys. Sin. 65 186101
 Google Scholar
Google Scholar
[7] 叶树集, 李传召, 张佳慧, 谈军军, 罗毅 2019 68 013101
 Google Scholar
Google Scholar
Ye S J, Li C Z, Zhang J H, Tan J J, Luo Y 2019 Acta Phys. Sin. 68 013101
 Google Scholar
Google Scholar
[8] Qi C H, Zhu Z, Wang C L, Zheng Y J 2021 J. Phys. Chem. Lett. 12 931
 Google Scholar
Google Scholar
[9] Zhang Q L, Wu Y X, Yang R Y, Zhang J L, Wang R F 2021 Chem. Phys. Lett. 762 138139
 Google Scholar
Google Scholar
[10] Zhou G B, Li L, Peng K L, Wang X P, Yang Z 2021 J. Phys. Chem. C 125 7971
 Google Scholar
Google Scholar
[11] Zhu Z, Guo H K, Jiang X K, Chen Y C, Song B, Zhu Y M, Zhuang S L 2018 J. Phys. Chem. Lett. 9 2346
 Google Scholar
Google Scholar
[12] Zhou G B, Huang L L 2021 Mol. Simul. 47 925
 Google Scholar
Google Scholar
[13] Rahman A, Stillinger F H 1971 J. Chem. Phys. 55 3336
 Google Scholar
Google Scholar
[14] Berendsen H J, Postma J P, van Gunsteren W F, Hermans J 1981 Interaction Models for Water in Relation to Protein Hydration//Pullman B 1981 Intermolecular Forces (Dordrecht: Springer) pp331–342
[15] Jorgensen W L, Chandrasekhar J, Madura J D, Impey R W, Klein M L 1983 J. Chem. Phys. 79 926
 Google Scholar
Google Scholar
[16] Harrach M F, Drossel B 2014 J. Chem. Phys. 140 174501
 Google Scholar
Google Scholar
[17] Berendsen H J C, Grigera J R, Straatsma T P 1987 J. Phys. Chem. 91 6269
 Google Scholar
Google Scholar
[18] Horn H W, Swope W C, Pitera J W, Madura J D, Dick T J, Hura G L, Head-Gordon T 2004 J. Chem. Phys. 120 9665
 Google Scholar
Google Scholar
[19] Guillot B 2002 J. Mol. Liq. 101 219
 Google Scholar
Google Scholar
[20] Akyildiz I F, Jornet J M, Han C 2014 Phys. Commun. 12 16
 Google Scholar
Google Scholar
[21] Tonouchi M 2007 Nat. Photonics 1 97
 Google Scholar
Google Scholar
[22] Liu G Z, Chang C, Qiao Z, Wu K J, Zhu Z, Cui G Q, Peng W Y, Tang Y Z, Li J, Fan C H 2019 Adv. Funct. Mater. 29 1807862
 Google Scholar
Google Scholar
[23] Zhang Z Y, Yang G, Fan F, Zhong C Z, Yuan Y, Zhang X D, Chang S J 2021 Anal. Chim. Acta 1180 338871
 Google Scholar
Google Scholar
[24] 孙怡雯, 钟俊兰, 左剑, 张存林, 但果 2015 64 168701
 Google Scholar
Google Scholar
Sun Y W, Zhong J L, Zuo J, Zhang C L, Dan G 2015 Acta Phys. Sin. 64 168701
 Google Scholar
Google Scholar
[25] Pickwell E, Wallace V 2006 J. Phys. D: Appl. Phys. 39 R301
 Google Scholar
Google Scholar
[26] Liu G Z 2018 Chin. Sci. Bull. 63 3864
 Google Scholar
Google Scholar
[27] Siegel P H 2004 IEEE Trans. Microwave Theory Tech. 52 2438
 Google Scholar
Google Scholar
[28] Zhu Z, Chang C, Shu Y S, Song B 2019 J. Phys. Chem. Lett. 11 256
 Google Scholar
Google Scholar
[29] Wu K J, Qi C H, Zhu Z, Wang C L, Song B, Chang C 2020 J. Phys. Chem. Lett. 11 7002
 Google Scholar
Google Scholar
[30] Wang K C, Yang L X, Wang S M, Guo L H, Ma J L, Tang J C, Bo W F, Wu Z, Zeng B Q, Gong Y B 2020 Phys. Chem. Chem. Phys. 22 9316
 Google Scholar
Google Scholar
[31] Li N, Peng D L, Zhang X J, Shu Y S, Zhang F, Jiang L, Song B 2021 Nano Res. 14 40
 Google Scholar
Google Scholar
[32] Li Y M, Chang C, Zhu Z, Sun L, Fan C H 2021 J. Am. Chem. Soc. 143 4311
 Google Scholar
Google Scholar
[33] Liu X, Qiao Z, Chai Y M, Zhu Z, Wu K J, Ji W L, Li D G, Xiao Y J, Mao L Q, Chang C 2021 Proc. Natl. Acad. Sci. U.S.A. 118 2015685118
 Google Scholar
Google Scholar
[34] Zhang J X, He Y, Liang S S, Liao X, Li T, Qiao Z, Chang C, Jia H B, Chen X W 2021 Nat. Commun. 12 1
 Google Scholar
Google Scholar
[35] Zhu Z, Chen C, Chang C, Song B 2020 ACS Photonics 8 781
 Google Scholar
Google Scholar
[36] Liu J, Miller W H, Paesani F, Zhang W, Case D A 2009 J. Chem. Phys. 131 164509
 Google Scholar
Google Scholar
[37] Guillot B, Guissani Y 1997 Phys. Rev. Lett. 78 2401
 Google Scholar
Google Scholar
[38] Praprotnik M, Janežič D, Mavri J 2004 J. Phys. Chem. A 108 11056
 Google Scholar
Google Scholar
[39] Srivastava A, Malik S, Debnath A 2019 Chem. Phys. 525 110396
 Google Scholar
Google Scholar
[40] Chen X W, Yuan M X, Guo H, Zhu Z 2020 Chin. Phys. B 29 030505
 Google Scholar
Google Scholar
[41] Einstein A 1905 Ann. Phys. 17 549
 Google Scholar
Google Scholar
[42] Rapaport D 1983 Mol. Phys. 50 1151
 Google Scholar
Google Scholar
[43] Guo Y W, Qin J Y, Hu J H, Cao J H, Zhu Z, Wang C L 2020 Nucl. Sci. Tech. 31 1
 Google Scholar
Google Scholar
[44] Zhu Z, Sheng N, Fang H P, Wan R Z 2016 Phys. Chem. Chem. Phys. 18 30189
 Google Scholar
Google Scholar
[45] Zhu Z, Sheng N, Wan R Z, Fang H P 2014 J. Phys. Chem. A 118 8936
 Google Scholar
Google Scholar
[46] Heyden M, Sun J, Funkner S, Mathias G, Forbert H, Havenith M, Marx D 2010 Proc. Natl. Acad. Sci. U.S.A. 107 12068
 Google Scholar
Google Scholar
[47] Glättli A, Daura X, Van Gunsteren W F 2003 J. Comput. Chem. 24 1087
 Google Scholar
Google Scholar
[48] Soper A 2000 Chem. Phys. 258 121
 Google Scholar
Google Scholar
[49] Kubo R 1966 Rep. Prog. Phys. 29 255
 Google Scholar
Google Scholar
-
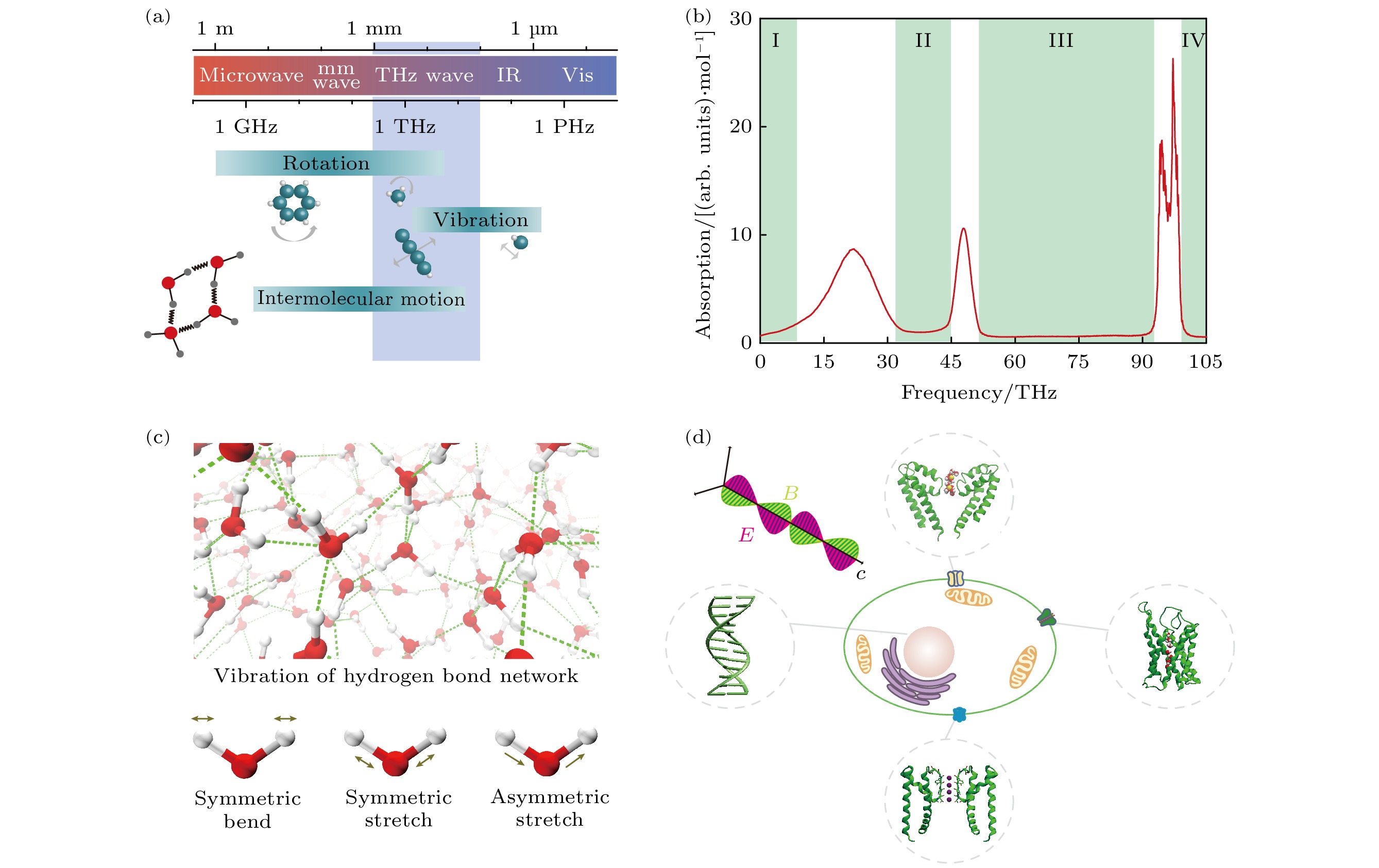
图 1 太赫兹与生物分子的密切关系以及太赫兹调控细胞动力学 (a) 生物大分子的转/振动的频率在THz频段; (b) 水的太赫兹吸收谱, 绿色区域是有望非热地调控生物分子的广义太赫兹频率的4个窗口; (c) 水的太赫兹吸收谱的振动模式[36]; (d) 太赫兹波非热调控细胞动力学, 涉及发挥细胞生物功能的水通道蛋白、DNA、钾离子通道、钙离子通道
Fig. 1. Close relationship between terahertz and biomolecules and the regulation of cell dynamics by terahertz: (a) Frequency of rotation/vibration of biological macromolecules is in the THz frequency band; (b) terahertz absorption spectrum of water, the green region is the four frequency windows in which electromagnetic wave is expected to non-thermally regulate biomolecules; (c) vibration modes of water corresponding to its terahertz absorption spectrum[36]; (d) terahertz waves non-thermally regulate the dynamics of a cell, involving aquaporins, DNA, potassium and calcium channels that perform biological functions of the cell.
图 2 不同水模型建模的体相水的太赫兹吸收谱和温度对其太赫兹吸收谱的影响 (a) 不同水模型建模的体相水的太赫兹吸收光谱以及实验测量所得体相水的太赫兹吸收谱之间的对比; (b) 对于不同水模型构成的体相水, 其氢键网络的太赫兹吸收谱; (c) 不同温度下, SPC/E水模型建模的体相水的太赫兹吸收谱; (d) 不同温度下, 对于SPC/E水模型构成的体相水, 其氢键网络的太赫兹吸收谱
Fig. 2. THz absorption spectra of bulk water modeled by different water models and effects of temperature on its spectra: (a) Comparison of THz absorption spectra of bulk water under different water models and its spectra from experimental measurement; (b) THz absorption spectra of hydrogen bond network of bulk water under different water models; (c) THz absorption spectra of bulk water modeled by SPC/E water model at different temperatures; (d) THz absorption spectra of hydrogen bond network of bulk water modeled by SPC/E water model at different temperatures.
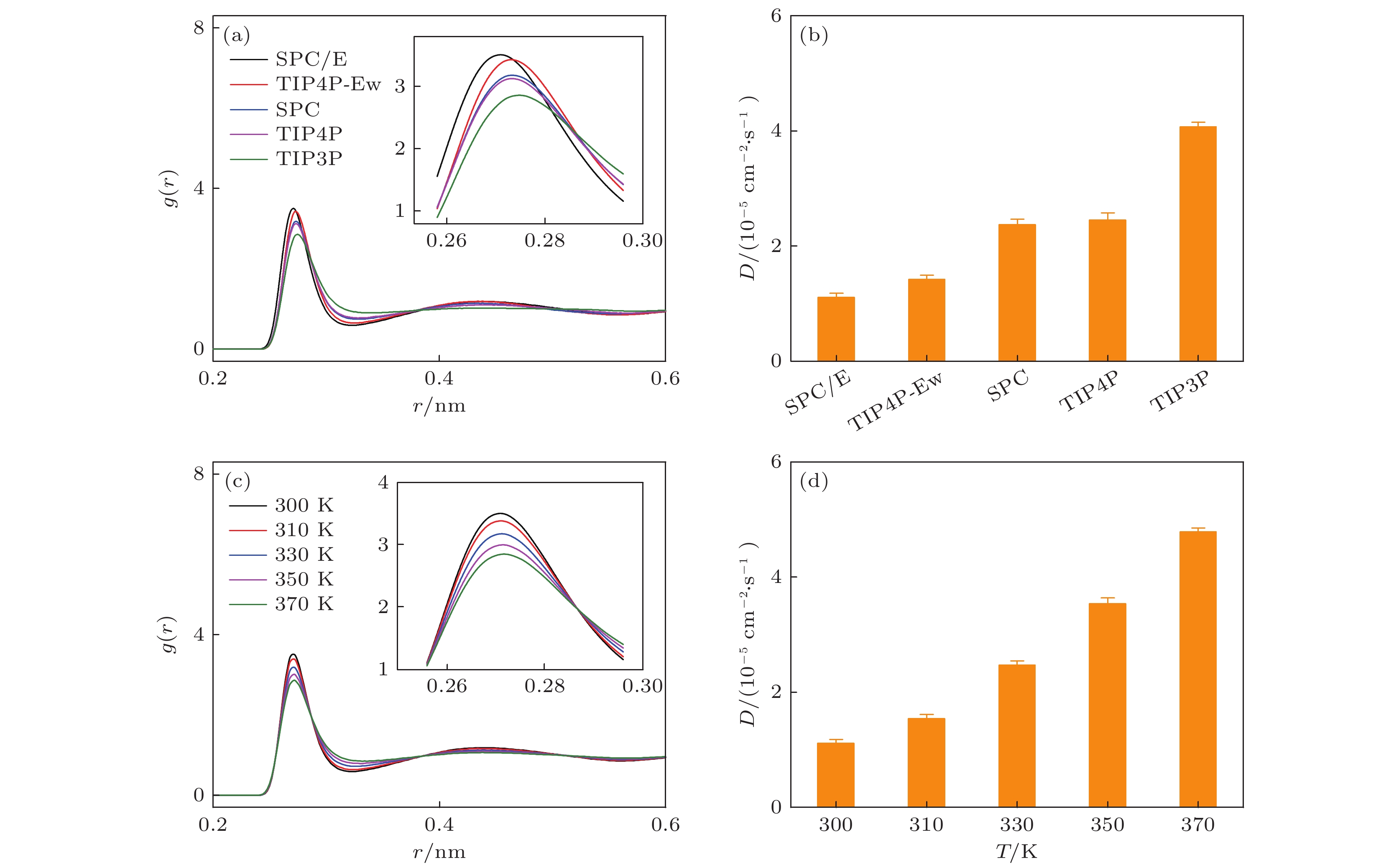
图 3 不同水模型构成的体相水的结构和动力学性质的分析 (a) 不同水模型构成的体相水的径向分布函数; (b) 不同水模型构成的体相水的扩散系数; (c) 特定的水模型(SPC/E)下, 温度对体相水的径向分布函数的影响; (d) 不同温度下SPC/E的扩散系数
Fig. 3. Analyses of structure and dynamic behavior of bulk water modeled by different water models: (a) Radial distribution function of bulk water under different water models; (b) diffusion coefficient of bulk water under different water models; (c) under specific water model (SPC/E), the effect of temperature on the radial distribution function of bulk water; (d) diffusion coefficient of bulk water at different temperatures.
图 4 氢键动力学与氢键网络振动的太赫兹吸收峰的中心频率之间的关系 (a) 由不同水模型构成的体相水的氢键的自相关函数; (b) 由不同水模型构成的体相水的氢键寿命; (c) 不同水模型下, 氢键网络振动的太赫兹吸收谱的中心频率与氢键寿命的对应关系; (d) 不同温度下, 体相水的氢键寿命; (e) 不同温度下, 水的氢键网络振动的太赫兹吸收峰的中心频率与氢键寿命的关系; (f) 氢键网络示意图
Fig. 4. Relationship between hydrogen bond dynamics and the center frequency of THz absorption spectra for the vibration of hydrogen bond network: (a) Hydrogen bond autocorrelation functions of bulk water under different water models; (b) lifetime of hydrogen bond of bulk water under different water models; (c) for bulk water under different water models, the relationship between the center frequency of THz absorption spectra for the vibration of hydrogen bond network and the lifetime of hydrogen bond; (d) lifetime of hydrogen bond for bulk water at different temperatures; (e) at different temperatures, the relationship between the center frequency of THz absorption spectra for the vibration of the hydrogen bond network and the lifetime of the hydrogen bond; (f) schematic diagram of the hydrogen bond network.
表 1 所用水模型的力场参数的比较
Table 1. Comparison of the force field parameters of different water models employed.
Water model σO—O/Å εOO/(kJ·mol–1) qH/e qO or qvir/e θ/(°) rH—O/Å SPC 3.166 0.650 0.410 –0.820 109.470 1.000 SPC/E 3.166 0.650 0.424 –0.848 109.470 1.000 TIP3P 3.151 0.636 0.417 –0.834 104.520 0.957 TIP4P 3.154 0.649 0.520 –1.040 104.520 0.957 TIP4P-Ew 3.164 0.681 0.524 –1.048 104.520 0.957 -
[1] Ball P 2017 Proc. Natl. Acad. Sci. U.S.A. 114 13327
 Google Scholar
Google Scholar
[2] Ball P 2008 Chem. Rev. 108 74
 Google Scholar
Google Scholar
[3] Xie Z, Li Z, Lou G, Liang Q, Chen J X, Kou J L, Wei G N 2021 Commun. Theor. Phys. 73 055602
 Google Scholar
Google Scholar
[4] Xie Z, Li Z, Li J Y, Kou J L, Yao J, Fan J T 2021 J. Chem. Phys. 154 024705
 Google Scholar
Google Scholar
[5] 王强, 曹则贤 2019 68 015101
 Google Scholar
Google Scholar
Wang Q, Cao Z X 2019 Acta Phys. Sin. 68 015101
 Google Scholar
Google Scholar
[6] 方海平 2016 65 186101
 Google Scholar
Google Scholar
Fang H P 2016 Acta Phys. Sin. 65 186101
 Google Scholar
Google Scholar
[7] 叶树集, 李传召, 张佳慧, 谈军军, 罗毅 2019 68 013101
 Google Scholar
Google Scholar
Ye S J, Li C Z, Zhang J H, Tan J J, Luo Y 2019 Acta Phys. Sin. 68 013101
 Google Scholar
Google Scholar
[8] Qi C H, Zhu Z, Wang C L, Zheng Y J 2021 J. Phys. Chem. Lett. 12 931
 Google Scholar
Google Scholar
[9] Zhang Q L, Wu Y X, Yang R Y, Zhang J L, Wang R F 2021 Chem. Phys. Lett. 762 138139
 Google Scholar
Google Scholar
[10] Zhou G B, Li L, Peng K L, Wang X P, Yang Z 2021 J. Phys. Chem. C 125 7971
 Google Scholar
Google Scholar
[11] Zhu Z, Guo H K, Jiang X K, Chen Y C, Song B, Zhu Y M, Zhuang S L 2018 J. Phys. Chem. Lett. 9 2346
 Google Scholar
Google Scholar
[12] Zhou G B, Huang L L 2021 Mol. Simul. 47 925
 Google Scholar
Google Scholar
[13] Rahman A, Stillinger F H 1971 J. Chem. Phys. 55 3336
 Google Scholar
Google Scholar
[14] Berendsen H J, Postma J P, van Gunsteren W F, Hermans J 1981 Interaction Models for Water in Relation to Protein Hydration//Pullman B 1981 Intermolecular Forces (Dordrecht: Springer) pp331–342
[15] Jorgensen W L, Chandrasekhar J, Madura J D, Impey R W, Klein M L 1983 J. Chem. Phys. 79 926
 Google Scholar
Google Scholar
[16] Harrach M F, Drossel B 2014 J. Chem. Phys. 140 174501
 Google Scholar
Google Scholar
[17] Berendsen H J C, Grigera J R, Straatsma T P 1987 J. Phys. Chem. 91 6269
 Google Scholar
Google Scholar
[18] Horn H W, Swope W C, Pitera J W, Madura J D, Dick T J, Hura G L, Head-Gordon T 2004 J. Chem. Phys. 120 9665
 Google Scholar
Google Scholar
[19] Guillot B 2002 J. Mol. Liq. 101 219
 Google Scholar
Google Scholar
[20] Akyildiz I F, Jornet J M, Han C 2014 Phys. Commun. 12 16
 Google Scholar
Google Scholar
[21] Tonouchi M 2007 Nat. Photonics 1 97
 Google Scholar
Google Scholar
[22] Liu G Z, Chang C, Qiao Z, Wu K J, Zhu Z, Cui G Q, Peng W Y, Tang Y Z, Li J, Fan C H 2019 Adv. Funct. Mater. 29 1807862
 Google Scholar
Google Scholar
[23] Zhang Z Y, Yang G, Fan F, Zhong C Z, Yuan Y, Zhang X D, Chang S J 2021 Anal. Chim. Acta 1180 338871
 Google Scholar
Google Scholar
[24] 孙怡雯, 钟俊兰, 左剑, 张存林, 但果 2015 64 168701
 Google Scholar
Google Scholar
Sun Y W, Zhong J L, Zuo J, Zhang C L, Dan G 2015 Acta Phys. Sin. 64 168701
 Google Scholar
Google Scholar
[25] Pickwell E, Wallace V 2006 J. Phys. D: Appl. Phys. 39 R301
 Google Scholar
Google Scholar
[26] Liu G Z 2018 Chin. Sci. Bull. 63 3864
 Google Scholar
Google Scholar
[27] Siegel P H 2004 IEEE Trans. Microwave Theory Tech. 52 2438
 Google Scholar
Google Scholar
[28] Zhu Z, Chang C, Shu Y S, Song B 2019 J. Phys. Chem. Lett. 11 256
 Google Scholar
Google Scholar
[29] Wu K J, Qi C H, Zhu Z, Wang C L, Song B, Chang C 2020 J. Phys. Chem. Lett. 11 7002
 Google Scholar
Google Scholar
[30] Wang K C, Yang L X, Wang S M, Guo L H, Ma J L, Tang J C, Bo W F, Wu Z, Zeng B Q, Gong Y B 2020 Phys. Chem. Chem. Phys. 22 9316
 Google Scholar
Google Scholar
[31] Li N, Peng D L, Zhang X J, Shu Y S, Zhang F, Jiang L, Song B 2021 Nano Res. 14 40
 Google Scholar
Google Scholar
[32] Li Y M, Chang C, Zhu Z, Sun L, Fan C H 2021 J. Am. Chem. Soc. 143 4311
 Google Scholar
Google Scholar
[33] Liu X, Qiao Z, Chai Y M, Zhu Z, Wu K J, Ji W L, Li D G, Xiao Y J, Mao L Q, Chang C 2021 Proc. Natl. Acad. Sci. U.S.A. 118 2015685118
 Google Scholar
Google Scholar
[34] Zhang J X, He Y, Liang S S, Liao X, Li T, Qiao Z, Chang C, Jia H B, Chen X W 2021 Nat. Commun. 12 1
 Google Scholar
Google Scholar
[35] Zhu Z, Chen C, Chang C, Song B 2020 ACS Photonics 8 781
 Google Scholar
Google Scholar
[36] Liu J, Miller W H, Paesani F, Zhang W, Case D A 2009 J. Chem. Phys. 131 164509
 Google Scholar
Google Scholar
[37] Guillot B, Guissani Y 1997 Phys. Rev. Lett. 78 2401
 Google Scholar
Google Scholar
[38] Praprotnik M, Janežič D, Mavri J 2004 J. Phys. Chem. A 108 11056
 Google Scholar
Google Scholar
[39] Srivastava A, Malik S, Debnath A 2019 Chem. Phys. 525 110396
 Google Scholar
Google Scholar
[40] Chen X W, Yuan M X, Guo H, Zhu Z 2020 Chin. Phys. B 29 030505
 Google Scholar
Google Scholar
[41] Einstein A 1905 Ann. Phys. 17 549
 Google Scholar
Google Scholar
[42] Rapaport D 1983 Mol. Phys. 50 1151
 Google Scholar
Google Scholar
[43] Guo Y W, Qin J Y, Hu J H, Cao J H, Zhu Z, Wang C L 2020 Nucl. Sci. Tech. 31 1
 Google Scholar
Google Scholar
[44] Zhu Z, Sheng N, Fang H P, Wan R Z 2016 Phys. Chem. Chem. Phys. 18 30189
 Google Scholar
Google Scholar
[45] Zhu Z, Sheng N, Wan R Z, Fang H P 2014 J. Phys. Chem. A 118 8936
 Google Scholar
Google Scholar
[46] Heyden M, Sun J, Funkner S, Mathias G, Forbert H, Havenith M, Marx D 2010 Proc. Natl. Acad. Sci. U.S.A. 107 12068
 Google Scholar
Google Scholar
[47] Glättli A, Daura X, Van Gunsteren W F 2003 J. Comput. Chem. 24 1087
 Google Scholar
Google Scholar
[48] Soper A 2000 Chem. Phys. 258 121
 Google Scholar
Google Scholar
[49] Kubo R 1966 Rep. Prog. Phys. 29 255
 Google Scholar
Google Scholar
计量
- 文章访问数: 12711
- PDF下载量: 483
- 被引次数: 0













 下载:
下载: