-
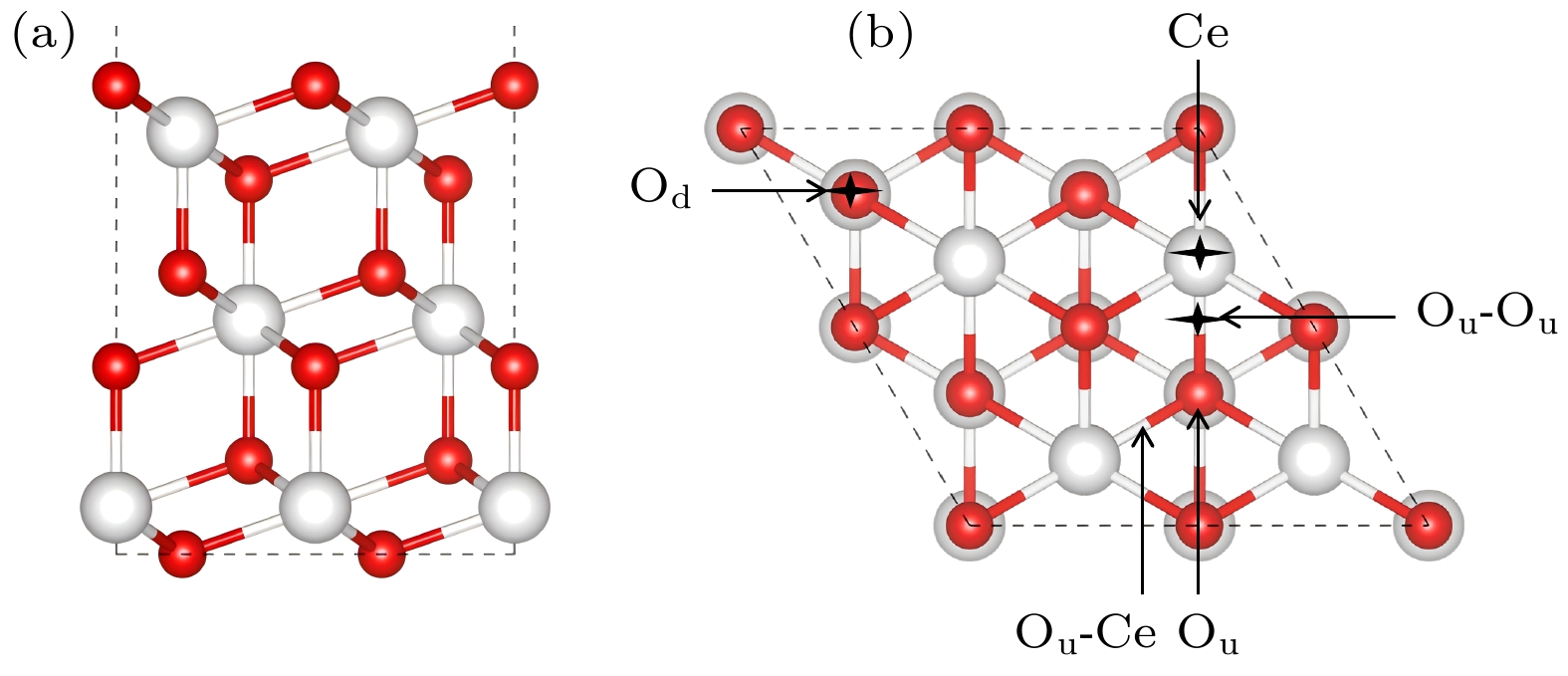
Au/CeO2(111), as an important catalyst system, has demonstrated excellent catalytic performances in a variety of fields such as the catalytic oxidation and the water-gas shift reactions. In order to reveal in depth the Au/CeO2(111) catalytic mechanism, especially to understand the interaction of the active components on an atomic scale, in this work, the adsorption properties on the Au/CeO2(111) surface are investigated by calculating the adsorption energy, differential charge density, Bader charge, and the density of states by using density functional theory (DFT+U). First, five adsorption sites of Au/CeO2(111) are identified in the planar region of CeO2(111), and the most stable adsorption configuration is found to be located at the bridging position between surface oxygen atoms (the oxygen-oxygen bridging site), which suggests that Au interacts more closely with the oxygen-oxygen bridging sites. Further, the differential charge density and Bader charge reveal the charge transfer mechanism in the adsorption process. Specifically, the Au atoms are oxidized into Au+, while the Ce4+ ions in the second nearest neighbor of Au are reduced to Ce3+, and the adsorption process is accompanied by a charge transfer phenomenon. Au also exhibits a unique adsorption behavior in the CeO2(111) step-edge region, where a highly under-allocated environment is formed due to the decrease in the coordination number of atoms in the step edge, which enhances the adsorption of Au in a highly under-allocated environment. The adsorption of Au at the step edge is enhanced by the lower coordinated environment due to the reduced coordination number of the atoms at the step edge. By comparing four different types of step structures (Type I, Type II, Type II*, and Type III), it is found that the higher adsorption energy of Au at Type II* site and that at Type III site are both mainly due to the lower coordinated state of Ce atoms at these sites. Charge transfer is also particularly pronounced at the Type III sites. It is also accompanied by electron transferring from Au to Ce4+ ions, making Type III the preferred adsorption site for Au atoms. By constructing a more comprehensive Au/CeO2 model, this study breaks through the previous limitation of focusing only on planar adsorption and reveals the adsorption mechanism of Au/CeO2 at the edge of the step, which provides a new perspective for understanding in depth the catalytic mechanism of Au/CeO2(111).
-
Keywords:
- Au/CeO2(111) /
- charge transfer /
- adsorption energy /
- first principles calculations
-
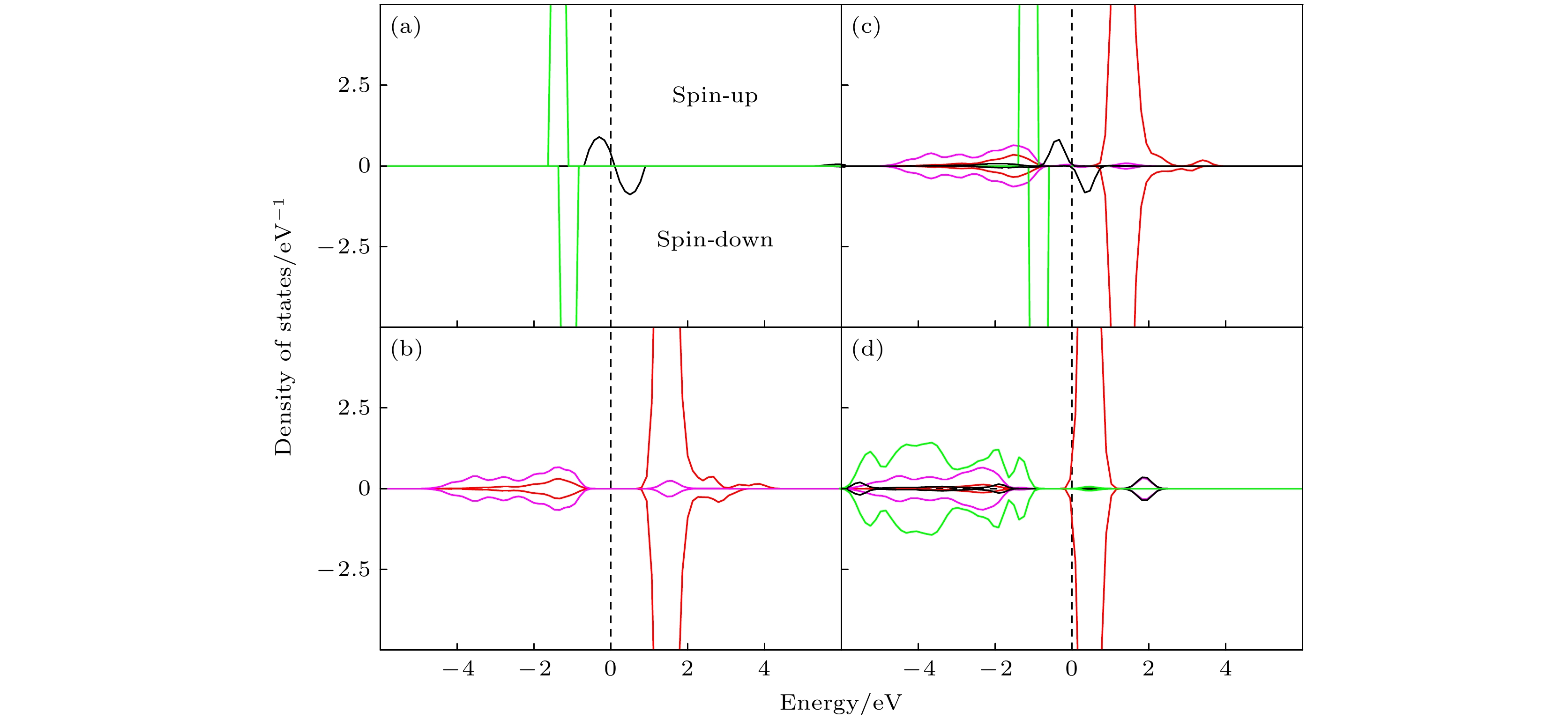
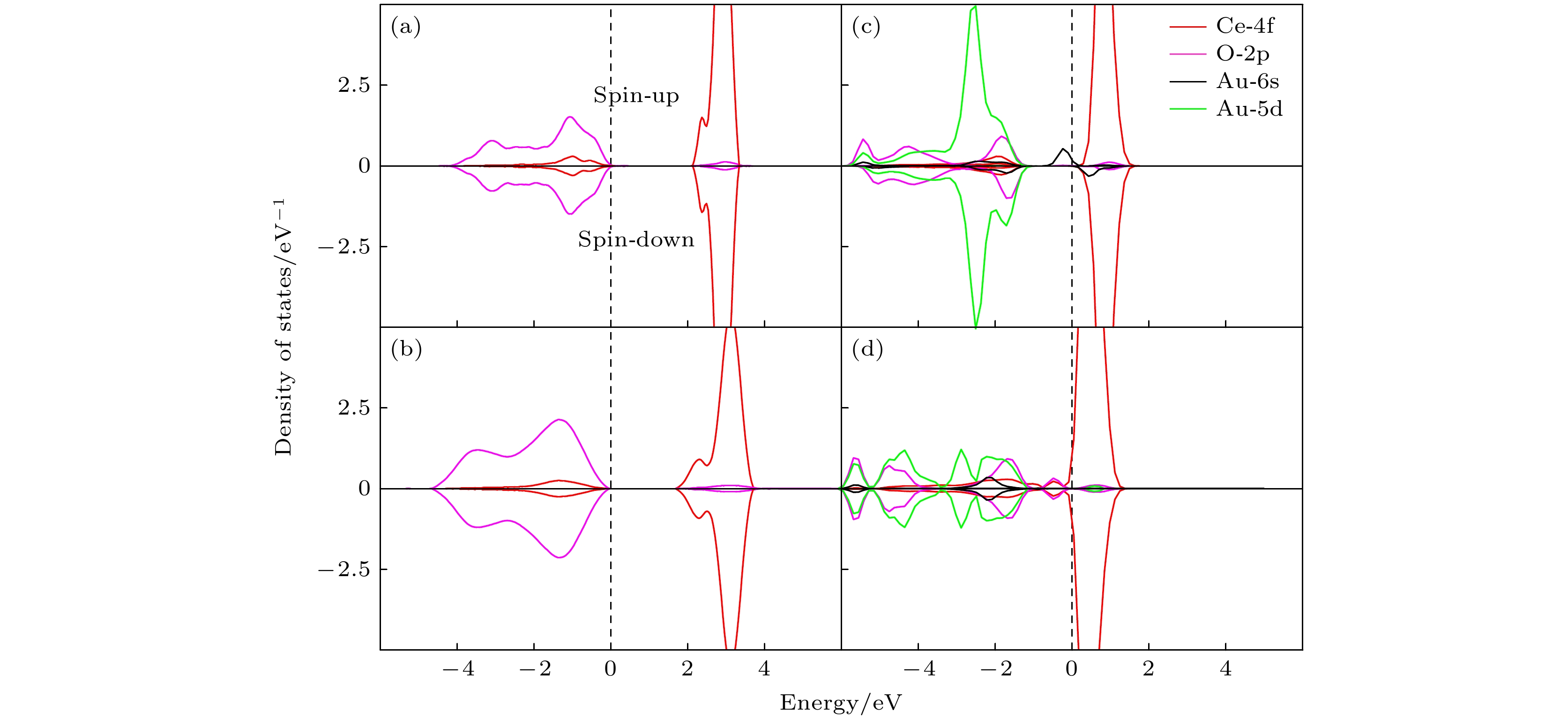
图 4 (a)纯金(Au)的态密度; (b)纯净的CeO2(111) 表面的态密度; (c) Au/CeO2(111) 表面铈顶位的态密度; (d) Au/CeO2(111) 表面氧-氧桥位的态密度, spin-up 和 spin-down 分别代表自旋向上和自旋向下的电子态
Figure 4. Presents the DOS for various systems: (a) pure gold (Au); (b) pristine CeO2(111) surface; (c) Au/CeO2(111) surface at the cerium top site; (d) Au/CeO2(111) surface at the oxygen-oxygen bridge site, within each plot, spin-up and spin-down represent the electronic states with spin aligned upwards and downwards.
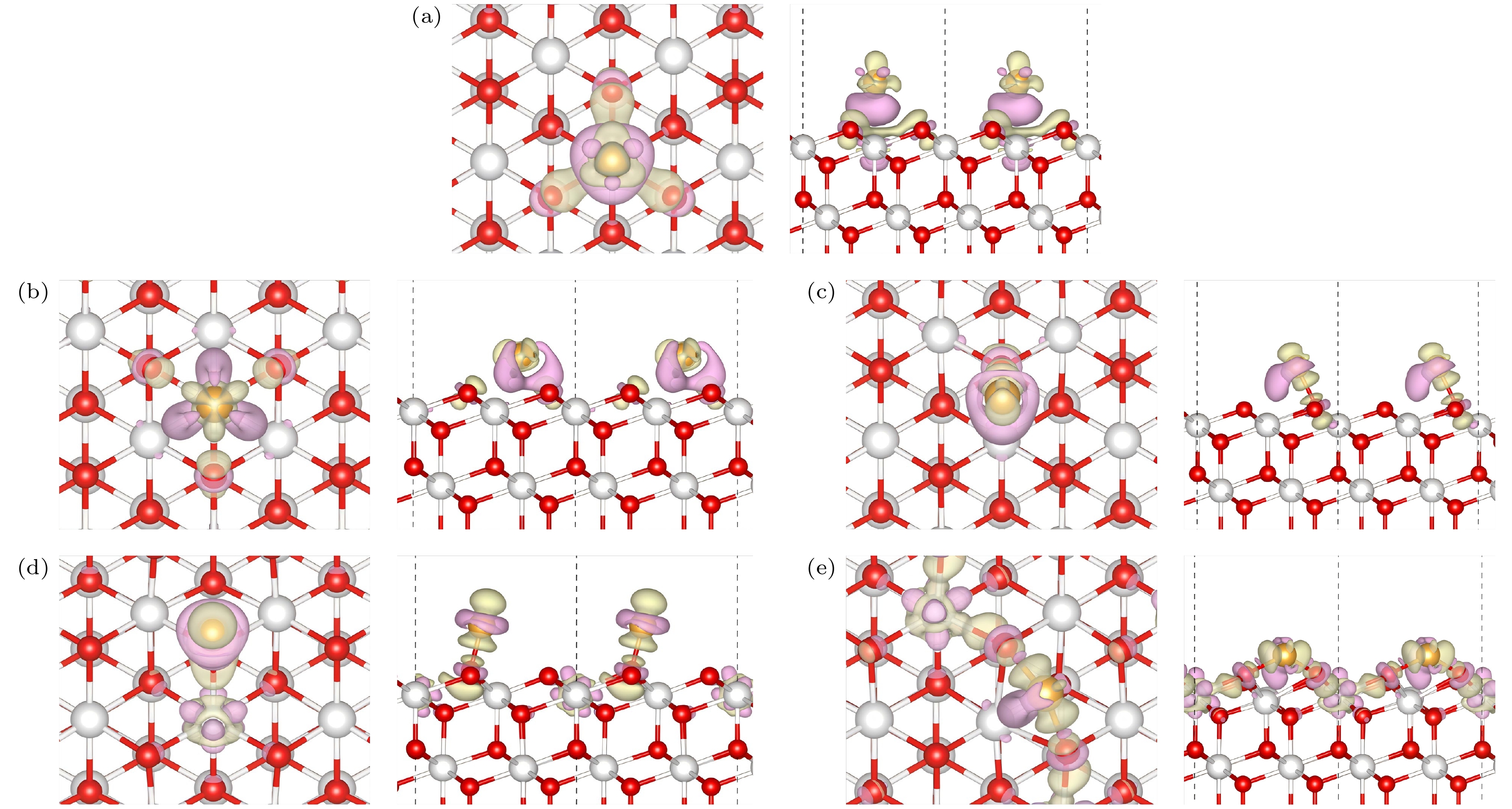
图 3 Au/CeO2(111)表面上的差分电荷密度 (a)铈顶位(Ce); (b)次氧位(Od); (c)顶氧-铈位(Ou-Ce); (d)氧顶位(Ou); (e)氧-氧桥位(Ou-Ou), 图中紫色部分表示该位置电荷减少, 绿色部分表示电荷的增加
Figure 3. Differential charge density on the Au/CeO2(111) surface: (a) Cerium top site (Ce); (b) sub-oxygen site (Od); (c) top oxygen-cerium site (Ou-Ce); (d) oxygen top site (Ou); (e) oxygen-oxygen bridge site (Ou-Ou), the purple regions in the figure indicate a decrease in charge at that location, while the green regions indicate an increase in charge.
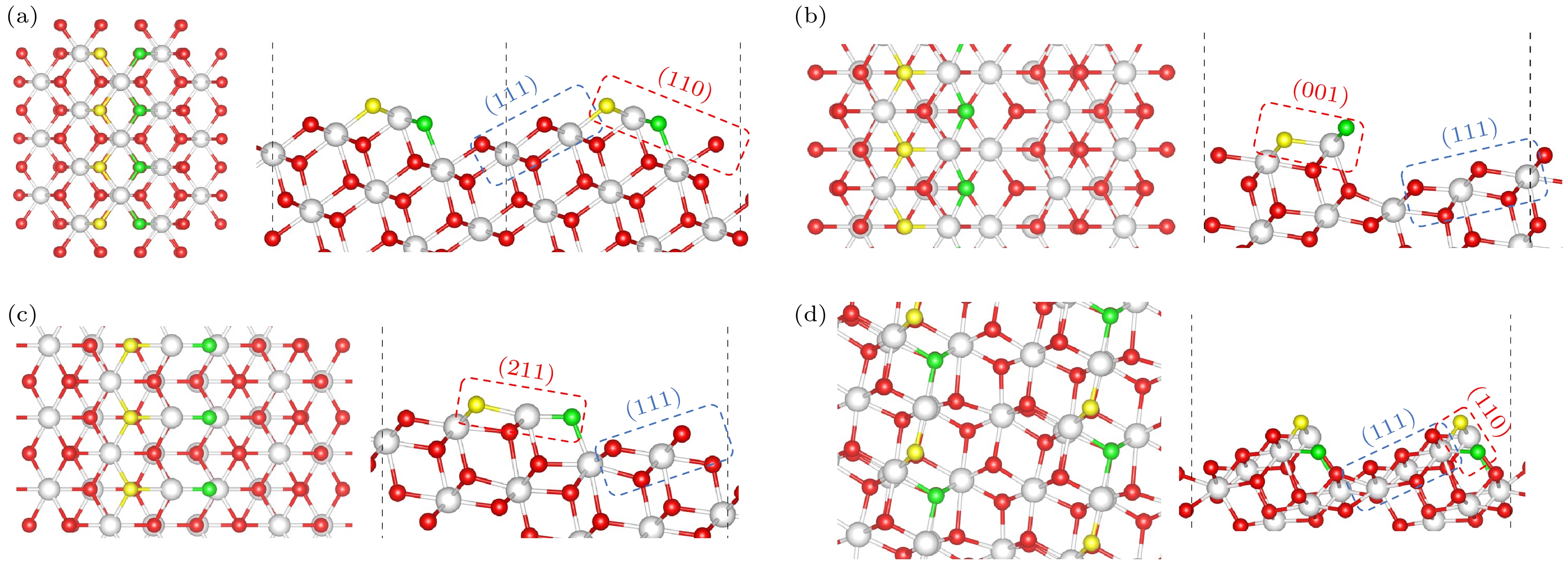
图 5 (a) I型台阶; (b) II型台阶; (c) II*型台阶; (d) III型台阶构建的化学计量CeO2表面; 台阶上边缘的氧(OT)和靠近台阶下边缘的氧(OE)分别用黄色和绿色代表(左:俯视图;右:侧视图)
Figure 5. Calculated structures of stoichiometric CeO2 vicinal surfaces built for: (a) Type I steps; (b) Type II steps; (c) Type II* steps; (d) Type III steps; the oxygen at the border of the upper (111) terrace (OT) and the oxygen at the edge close to the lower (111) terrace (OE) are highlighted in yellow and green, respectively (left: top view; right: side view).
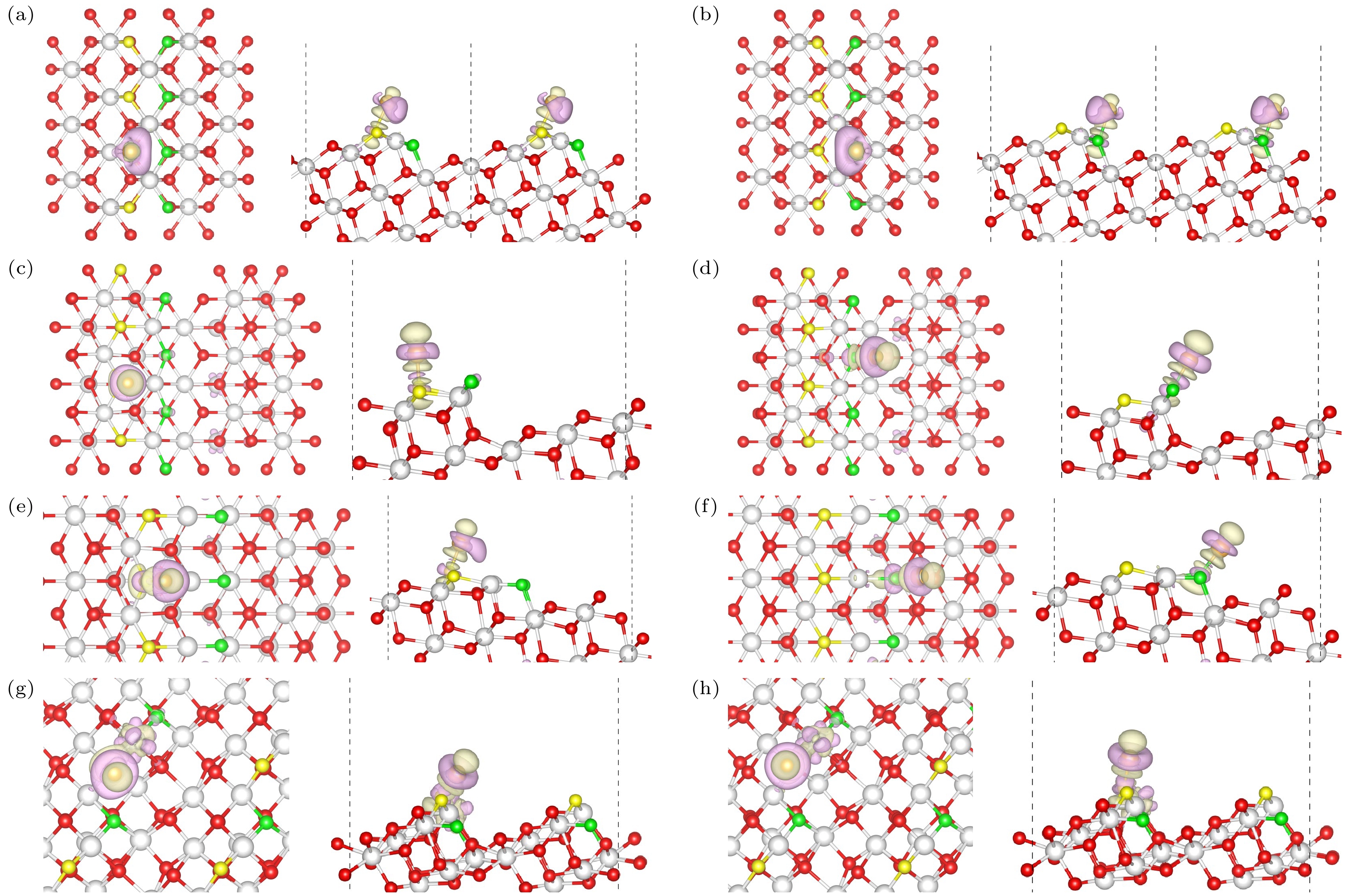
图 6 Au/CeO2(111)台阶上的差分电荷密度(左:俯视图;右:侧视图) (a), (b)Type I; (c), (d) Type II ; (e), (f) Type II*; (g), (h) Type III; 图中紫色部分表示该位置电荷减少, 绿色部分表示电荷的增加
Figure 6. Differential charge density on stepped Au/CeO2(111) surfaces (Left: Top view; Right: Side view): (a), (b) Type I step; (c), (d) Type II step; (e), (f) Type II* step; (g), (h) Type III step; the purple regions indicate charge depletion, while the green regions indicate charge accumulation at those locations.
图 7 CeO2(111)表面上的 (a)Type II*, (b)Type III; (c) Au吸附Type II* OE位点; (d) Au吸附Type III OT位点DOS; spin-up 和 spin-down 分别代表自旋向上和自旋向下的电子态
Figure 7. DOS for: (a) Type II* on CeO2(111) surface; (b) Type III on CeO2(111) surface; (c) Au adsorbed at OE site of Type II*; (d) Au adsorbed at OT site of Type III; spin-up and spin-down represent the electronic states with spin directed upwards and downwards, respectively.
表 1 Au/CeO2(111)表面吸附构型的能量和几何性质, Eads是吸附能, d[Au-O]和d[Au-Ce]是附着原子到表面原子的距离, d[Ce-O]是铈原子到氧原子的距离
Table 1. Energy and geometric properties of adsorption configurations on the Au/CeO2(111) surface, Eads is the adsorption energy, d[Au–O] and d[Au–Ce] are distances from the adsorbed atoms to surface atoms, and d[Ce-O] is the distance between the cerium atom and the oxygen atom.
Site Eads/eV $ d\text{[Au-O]/}\stackrel{. }{\text{A}} $ $ d\text{[Au-Ce]/}\stackrel{. }{\text{A}} $ $ d\text{[Ce-O]/}\stackrel{. }{\text{A}} $ Ce 0.39 eV 2×3.15
1×3.101×2.99
2×4.86
1×4.913×2.36
3×2.39Od 0.61 eV 1×2.73
1×2.75
1×2.791×3.26
1×3.28
1×3.29
1×5.099×2.38 Ou-Ce 0.77 eV 2.15 1×3.17
1×3.98
1×4.01
1×4.971×2.34
1×2.35
3×2.36
1×2.37Ou 0.95 eV 1.97 1×3.68
1×3.70
1×4.07
1×5.631×2.38
2×2.42
2×2.45
1×2.53Ou-Ou 1.19 eV 2×2.14 1×2.84
2×3.31
1×4.611×2.41
2×2.42
1×2.46
2×2.55表 2 Au/CeO2(111)台阶吸附构型的能量和几何性质, Eads是吸附能, d[Au-O] 和d[Au-Ce]是附着原子到表面原子的距离, d[Ce-O]是铈原子到氧原子的距离, 及不同配位数O和Ce原子
Table 2. Energy and geometric properties of adsorption configurations on stepped Au/CeO2(111) surfaces. Eads is the adsorption energy, d[Au-O] and d[Au-Ce] are the distances from the adsorbed atom to the surface atoms, d[Ce-O] is the distance between the cerium atom and the oxygen atom, along with the coordination numbers of different O and Ce atoms.
Step type Eads/eV $ d\text{[Au-O]/}\stackrel{. }{\text{A}} $ $ d\text{[Au-Ce]/}\stackrel{. }{\text{A}} $ $ d\text{[Ce-O]/}\stackrel{. }{\text{A}} $ Ocoordination Cecoordination OT of TypeⅠ 1.00 1×2.14 1×3.30
1×3.361×2.31
1×2.51
1×2.533 6 OE of TypeⅠ 0.97 1×2.16 1×3.31
1×3.291×2.38
1×2.39
1×2.473 6 OT of TypeⅡ 1.25 1×1.95 1×3.59
2×3.951×2.51
2×2.563 6 OE of TypeⅡ 1.32 1×1.94 1×3.82
1×3.86
1×5.952×2.42 2 6 OT of TypeⅡ* 1.36 1×1.97 1×3.40
1×3.94
1×3.982×2.46 3 5 OE of TypeⅡ* 1.74 1×1.93 1×3.79
1×5.21
1×5.381×2.21
1×2.692 5 OT of Type Ⅲ 2.15 1×1.94 1×3.73
1×3.94
1×4.331×2.42
1×2.472 5 OE of Type Ⅲ 2.14 1×1.93 1×3.79
1×4.00
1×4.791×2.41
1×2.493 5 -
[1] Haruta M, Kobayashi T, Sano H, Yamada N 1987 Chem. Lett. 16 405
 Google Scholar
Google Scholar
[2] Li Y J, Wen H, Zhang Q, Adachi Y, Arima E, Kinoshita Y, Nomura H, Ma Z M, Kou L, Tsukuda Y, Naitoh Y, Sugawara Y, Xu R, Cheng Z 2018 Ultramicroscopy 191 51
 Google Scholar
Google Scholar
[3] Zielinski M, Juszczyk W, Kaszkur Z 2022 RSC Adv. 12 5312
 Google Scholar
Google Scholar
[4] Berrichi A, Bailiche Z, Bachir R 2022 Res. Chem. Intermed. 48 4119
 Google Scholar
Google Scholar
[5] Megías-Sayago C, Lolli A, Ivanova S, Albonetti S, Cavani F, Odriozola J A 2019 Catal. Today 333 169
 Google Scholar
Google Scholar
[6] Li Q L, Xie W, Chen G Q, Li Y F, Huang Y J, Chen X D 2015 Nano Res. 8 3075
 Google Scholar
Google Scholar
[7] Liu H P, Cao Z L, Yang S Y, Ren Q Y, Dong Z J, Liu W, Li Z A, Chen X, Luo L L 2024 Nano Res. 17 4986
 Google Scholar
Google Scholar
[8] Kim C, Thompson L 2006 J. Catal. 244 248
 Google Scholar
Google Scholar
[9] Chen Y, Hu P, Lee M H, Wang H 2008 Surf. Sci. 602 1736
 Google Scholar
Google Scholar
[10] Hernández N C, Grau-Crespo R, De Leeuw N H, Sanz J Fdez 2009 Phys. Chem. Chem. Phys. 11 5246
 Google Scholar
Google Scholar
[11] Zhang C, Michaelides A, King D A, Jenkins S J 2010 J. Am. Chem. Soc. 132 2175
 Google Scholar
Google Scholar
[12] Engel J, Francis S, Roldan A 2019 Phys. Chem. Chem. Phys. 21 19011
 Google Scholar
Google Scholar
[13] Shu P L, Tian X, Guo Q, Ren X, Zhao B, Wen H F, Tang J, Li Y J, Yasuhiro S, Ma Z M, Liu J 2024 Phys. Scr. 99 105990
 Google Scholar
Google Scholar
[14] Teng B T, Wu F M, Huang W X, Wen X D, Zhao L H, Luo M F 2012 ChemPhysChem 13 1261
 Google Scholar
Google Scholar
[15] Lu Y, Quardokus R, Lent C S, Justaud F, Lapinte C, Kandel S A 2010 J. Am. Chem. Soc. 132 13519
 Google Scholar
Google Scholar
[16] Lustemberg P G, Pan Y, Shaw B J, Grinter D, Pang C, Thornton G, Pérez R, Ganduglia-Pirovano M V, Nilius N 2016 Phys. ReV. Lett. 116 236101
 Google Scholar
Google Scholar
[17] Fu Q, Saltsburg H, Flytzani-Stephanopoulos M 2003 Science 301 935
 Google Scholar
Google Scholar
[18] Bezkrovnyi O, Bruix A, Blaumeiser D, Piliai L, Schötz S, Bauer T, Khalakhan I, Skála T, Matvija P, Kraszkiewicz P, Pawlyta M, Vorokhta M, Matolínová I, Libuda J, Neyman K M, Kȩpiński L 2022 Chem. Mater. 34 7916
 Google Scholar
Google Scholar
[19] Tibiletti D, Amieiro-Fonseca A, Burch R, Chen Y, Fisher J M, Goguet A, Hardacre C, Hu P, Thompsett D 2005 J. Phys. Chem. B 109 22553
 Google Scholar
Google Scholar
[20] 冯婕, 郭强, 舒鹏丽, 温阳, 温焕飞, 马宗敏, 李艳君, 刘俊, 伊戈尔·弗拉基米罗维奇·雅明斯基 2023 72 110701
 Google Scholar
Google Scholar
Feng J, Guo Q, Shu P L, Wen Y, Wen H F, Ma Z M, Li Y J, Liu J 2023 Acta Phys. Sin. 72 110701
 Google Scholar
Google Scholar
[21] Perdew J P, Burke K, Ernzerhof M 1996 Phys. ReV. Lett. 77 3865
 Google Scholar
Google Scholar
[22] Cococcioni M, De Gironcoli S 2005 Phys. Rev. B 71 035105
[23] Zheng Z Y, Wang D, Zhang Y, Yang F, Gong X Q 2020 Chin. J. Cata. 41 1360
 Google Scholar
Google Scholar
[24] Wilson E L, Grau-Crespo R, Pang C L, Cabailh G, Chen Q, Purton J A, Catlow C R A, Brown W A, De Leeuw N H, Thornton G 2008 J. Phys. Chem. C 112 10918
 Google Scholar
Google Scholar
[25] Ma Z M, Shi Y B, Mu J L, Qu Z, Zhang X M, Li Q, Liu J 2017 Appl. Surf. Sci. 394 472
 Google Scholar
Google Scholar
[26] Owen C J, Jenkins S J 2021 J. Chem. Phys. 154 164703
 Google Scholar
Google Scholar
[27] Chen L J, Tang Y, Cui L, Ouyang C, Shi S 2013 J. of Power Sources 234 69
 Google Scholar
Google Scholar
[28] Kim H Y, Henkelman G 2013 J. Phys. Chem. Lett. 4 216
 Google Scholar
Google Scholar
[29] Kozlov S M, Neyman K M 2014 Phys. Chem. Chem. Phys. 16 7823
 Google Scholar
Google Scholar
[30] Olbrich R, Pieper H H, Oelke R, Wilkens H, Wollschläger J, Zoellner M H, Schroeder T, Reichling M 2014 Appl. Phys. Lett. 104 081910
 Google Scholar
Google Scholar
[31] Barth C, Laffon C, Olbrich R, Ranguis A, Parent Ph, Reichling M 2016 Sci Rep. 6 21165
 Google Scholar
Google Scholar
[32] Shu P L, Guo Q, Tian X, Wei J, Qu Z, Ren X, Wen H F, Tang J, Li Y J, Sugawara Y, Ma Z M, Liu J 2024 Surf. Interfaces 51 104738
 Google Scholar
Google Scholar
[33] Kozlov S M, Viñes F, Nilius N, Shaikhutdinov S, Neyman K M 2012 J. Phys. Chem. Lett. 3 1956
 Google Scholar
Google Scholar
[34] Chu D R, Wang Z Q, Gong X Q 2022 Surf. Sci 722 122096
 Google Scholar
Google Scholar
[35] 温焕飞, 菅原康弘, 李艳君 2020 69 210701
 Google Scholar
Google Scholar
Wen H F, Sugawara Y, Li Y J 2020 Acta Phys. Sin. 69 210701
 Google Scholar
Google Scholar
[36] Zhou H, Wang D, Gong X Q 2020 Phys. Chem. Chem. Phys. 22 7738
 Google Scholar
Google Scholar
[37] Zhou C Y, Wang D, Gong X Q 2019 Phys. Chem. Chem. Phys. 21 19987
 Google Scholar
Google Scholar
[38] Piliai L, Matvija P, Dinhová T N, Khalakhan I, Skála T, Doležal Z, Bezkrovnyi O, Kepinski L, Vorokhta M, Matolínová I 2022 ACS Appl. Mater. Interfaces 14 56280
 Google Scholar
Google Scholar
[39] Janssen E M W, Wiegers G A 1978 J. Less-Common Met. 57 P47
 Google Scholar
Google Scholar
Metrics
- Abstract views: 500
- PDF Downloads: 10
- Cited By: 0














 DownLoad:
DownLoad: