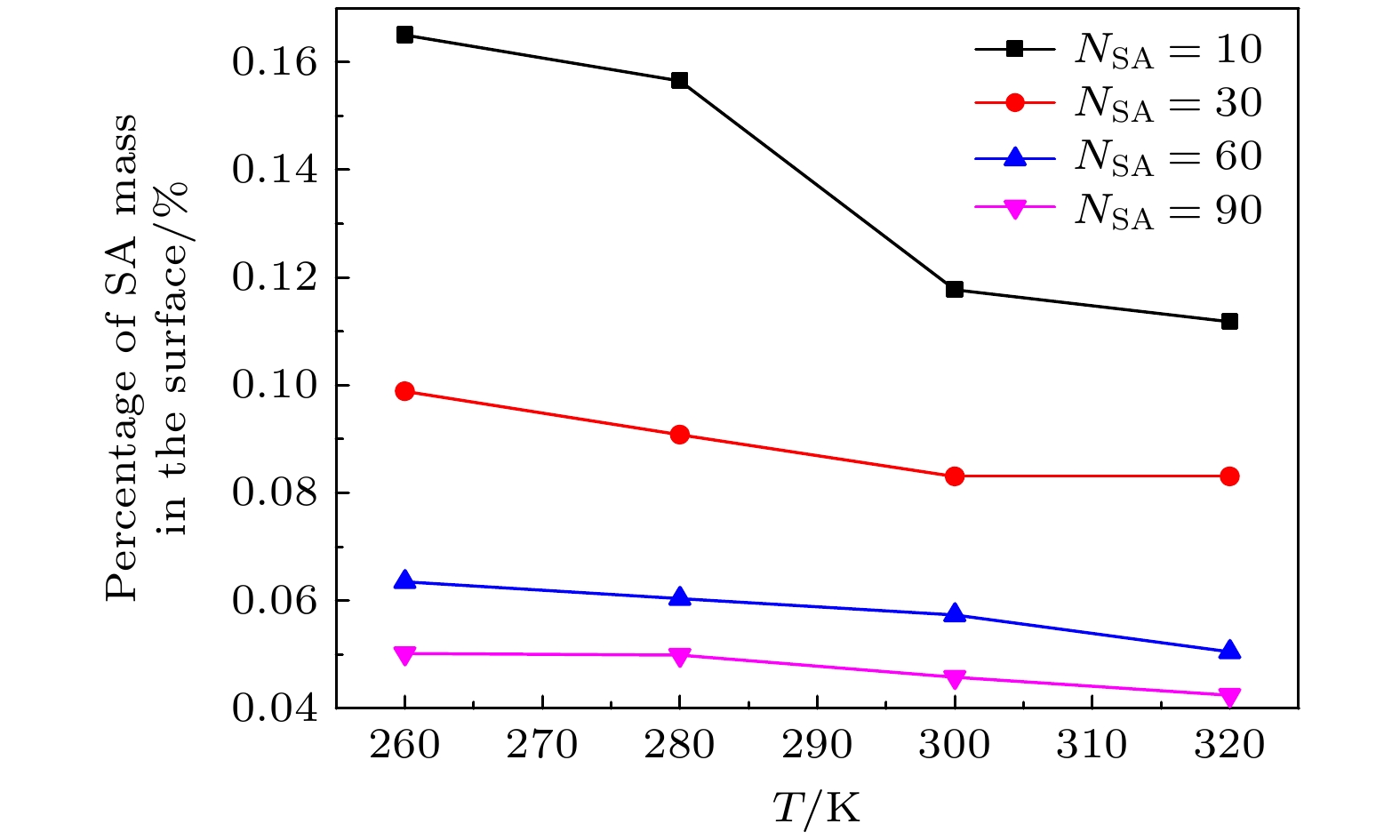
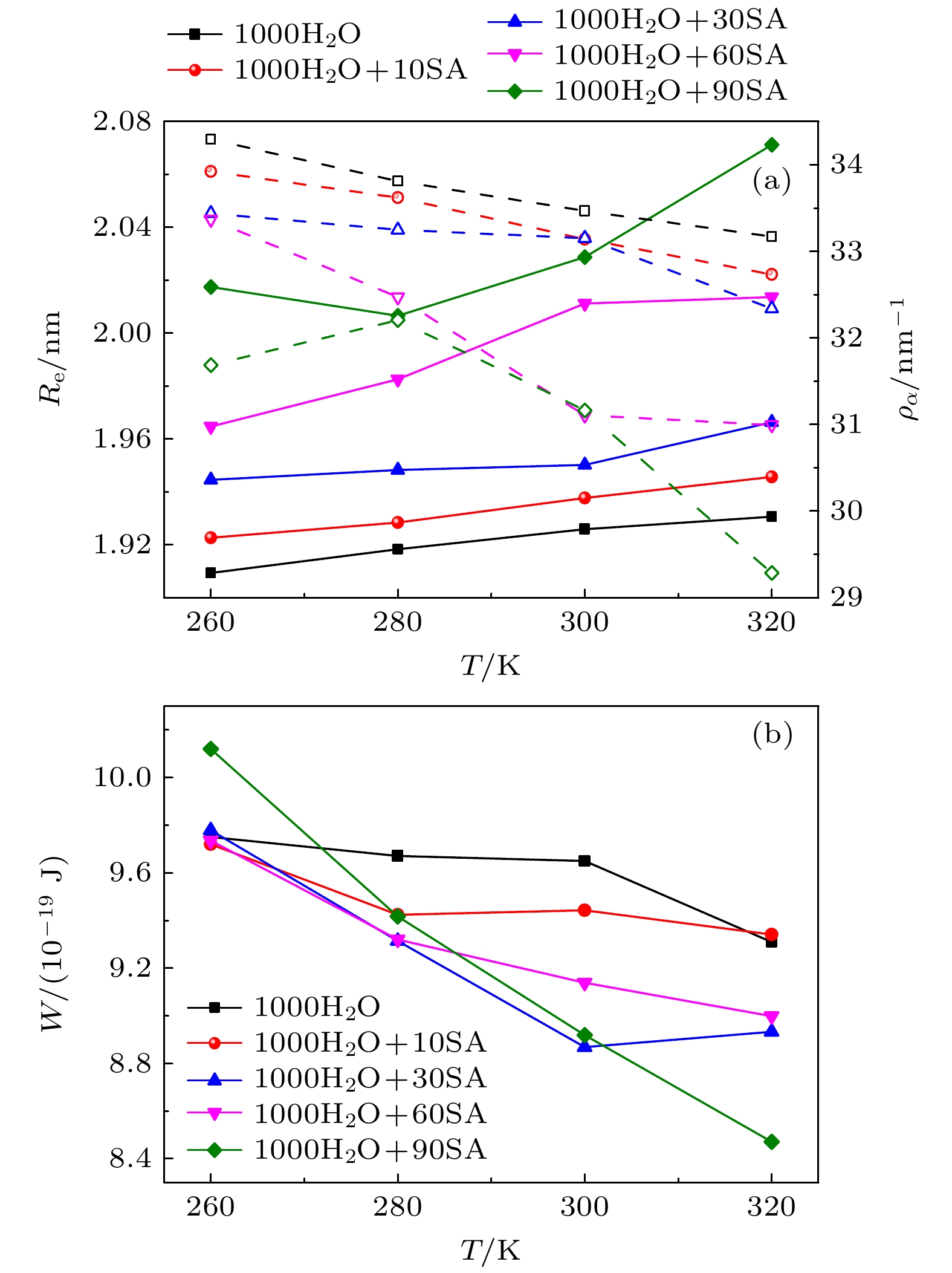
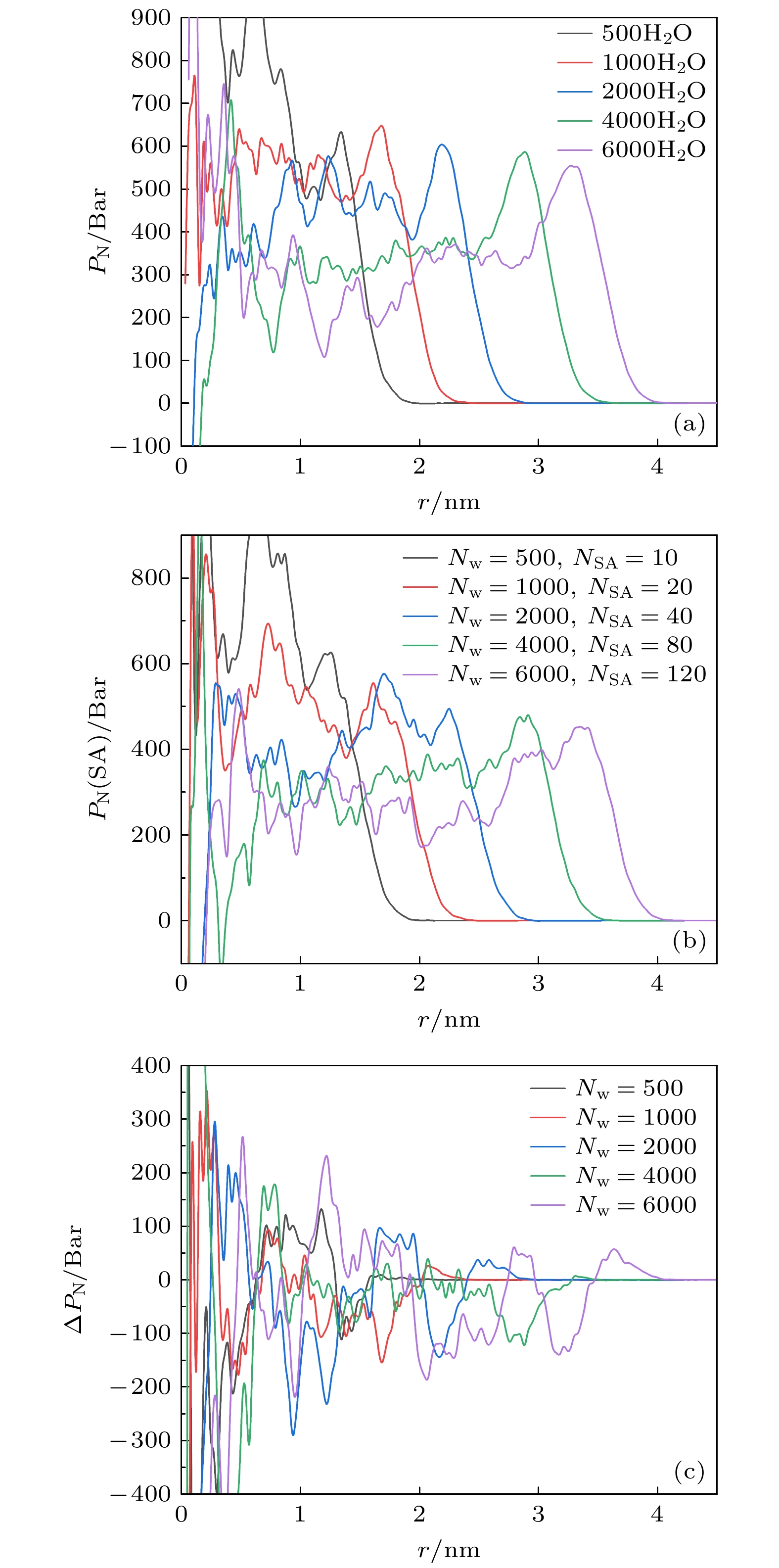
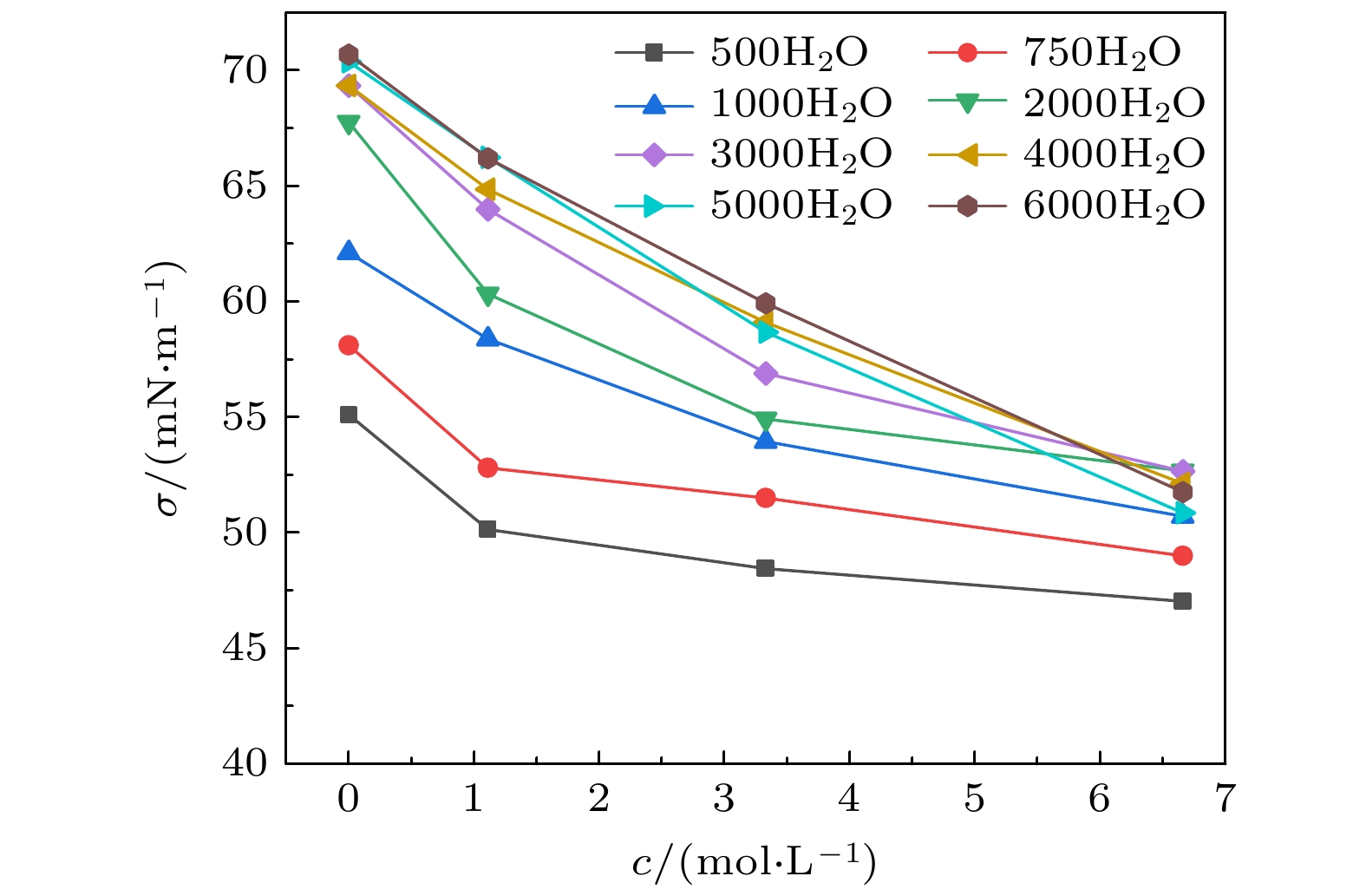
-
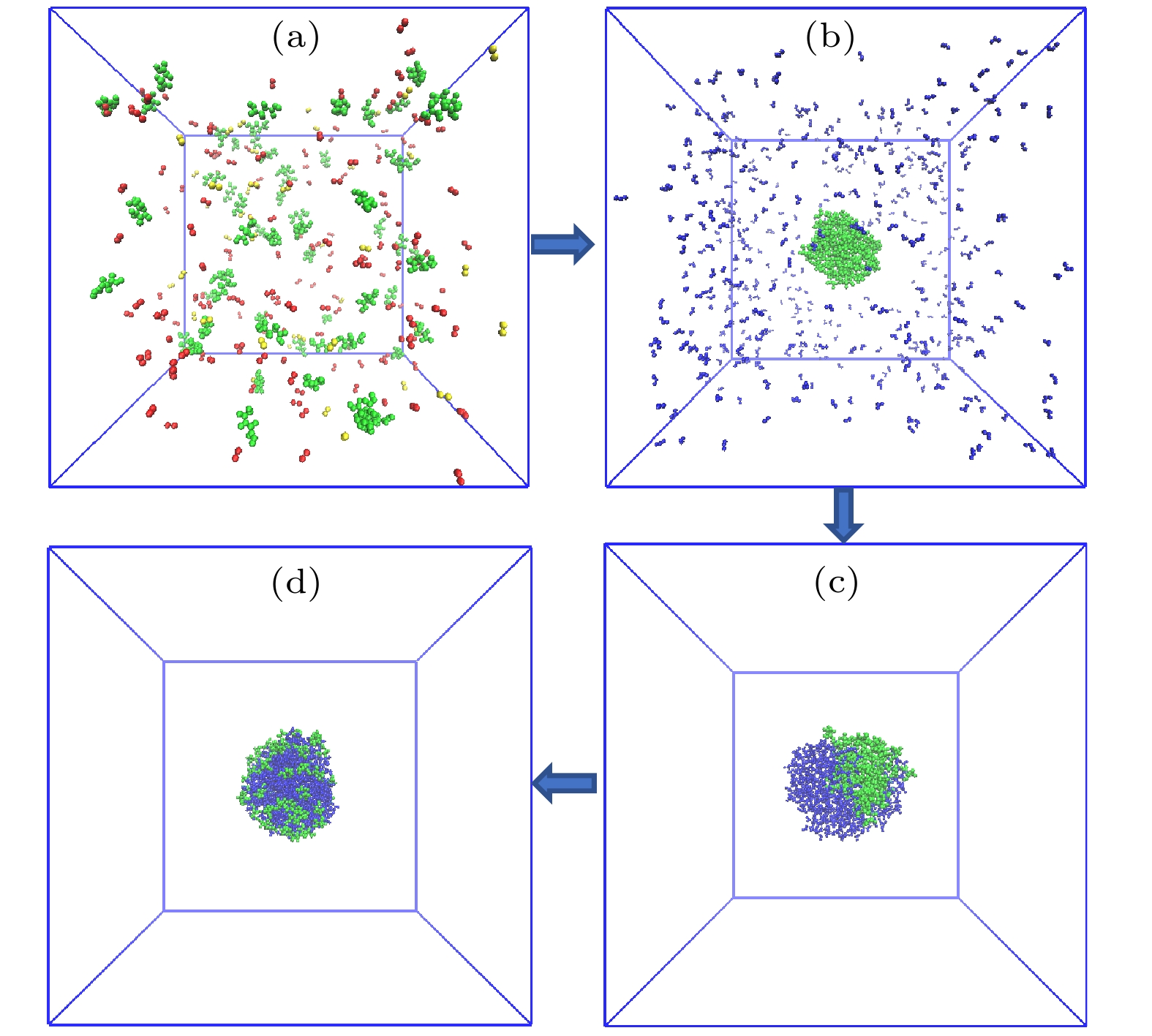
The surface tension plays a significant role in the hygroscopicity of aerosol particles on a nanoscale. However, it cannot be obtained by using the existing measurement techniques. In this study, we simulate the hygroscopic growth of one single succinic acid (SA) particle by using the molecular dynamic (MD) method. Based on the MD simulation results, the surface tension of the stable SA-water droplet is calculated by using a numerical model. Furthermore, the influencing mechanisms of temperature, diameter and concentration of SA on the surface tension of the nanoscale droplet are investigated. The results show that with the temperature increasing from 260 K to 320 K, the surface tension of the droplet decreases, which is mainly caused by the weakening of the intermolecular forces inside the droplet. Besides, the sensitivity of the surface tension to the temperature increases with the increasing SA concentration, which can be explained by the effect of the temperature and the SA concentration on the radial distribution of SA molecules. With the increase of the particle diameter, the surface tension of droplet first increases and then tends to be constant. The normal components of the Irving-Kirkwood pressure tensors are calculated to explain the effect of diameter and SA on the surface tension. In addition, when the SA concentration is increased, the particle diameter range which has an obvious effect on the surface tension is reduced. Moreover, the surface tension of the nanodroplet is negatively correlated with the SA concentration, and the correlation fits into the logarithmic function form, especially for droplet with a diameter smaller than 6.12 nm. The Szyszkowski equation is employed to fit the relationship between SA concentration and the surface tension of droplet. These findings can provide parameter support for improving the theoretical model of particle hygroscopicity and related kinetic processes. This study emphasizes further research on the surface tension of nano-droplets with more complex components.
-
Keywords:
-
aerosol droplets / - surface tension /
- molecular dynamics /
- numerical simulation
[1] Mahowald N 2011 Science 334 794
 Google Scholar
Google Scholar
[2] Scott C E, Arnold S R, Monks S A, Asmi A, Paasonen P, Spracklen D V 2018 Nat. Geosci. 11 44
 Google Scholar
Google Scholar
[3] Fan F X, Zhang S H, Wang W Y, Yan J P, Su M X 2019 Process Saf. Environ. Prot. 125 197
 Google Scholar
Google Scholar
[4] Fan F X, Zhang S H, Peng Z B, Chen J, Su M X, Moghtaderi B, Doroodchi E 2019 Can. J. Chem. Eng. 97 930
 Google Scholar
Google Scholar
[5] Cheng Y, Su H, Koop T, Mikhailov E, Poschl U 2015 Nat. Commun. 6 5923
 Google Scholar
Google Scholar
[6] Yamada T, Sakai K 2012 Phys. Fluids 24 022103
 Google Scholar
Google Scholar
[7] Morris H S, Grassian V H, Tivanski A V 2015 Chem. Sci. 6 3242
 Google Scholar
Google Scholar
[8] Dutcher C S, Wexler A S, Clegg S L 2010 J. Phys. Chem. A 114 12216
 Google Scholar
Google Scholar
[9] Wexler A S, Clegg S L 2002 J. Geophys. Res. Atmos. 107 ACH 14
[10] Chapela G A, Saville G, Thompson S M, Rowlinson J S 1977 J. Chem. Soc. Faraday Trans. 2 73 1133
 Google Scholar
Google Scholar
[11] Blokhuis E M, Bedeaux D, Holcomb C D, Zollweg J A 1995 Mol. Phys. 85 665
 Google Scholar
Google Scholar
[12] Chen F, Smith P E 2007 J. Chem. Phys. 126 221101
 Google Scholar
Google Scholar
[13] Wang X X, Chen C C, Binder K, Kuhn U, Pöschl U, Su H, Cheng Y F 2018 Atmos. Chem. Phys. 18 17077
 Google Scholar
Google Scholar
[14] Sun L, Li X, Hede T, Tu Y Q, Leck C, Ågren H 2012 J. Phys. Chem. B 116 3198
 Google Scholar
Google Scholar
[15] Liu L, Guo S, Zhao Z, Li H 2022 J. Phys. Chem. A 126 2407
 Google Scholar
Google Scholar
[16] Petters S S, Petters M D 2016 J. Geophys. Res. Atmos. 121 1878
 Google Scholar
Google Scholar
[17] Zhang C, Zhang Z C, Bu L X, Yang Y, Xiong W, Wang Y S 2023 Particuology 77 128
 Google Scholar
Google Scholar
[18] Nosé S C 1984 Mol. Phys. 52 255
 Google Scholar
Google Scholar
[19] Hoover W G 1985 Phys. Rev. A 31 1695
 Google Scholar
Google Scholar
[20] Plimpton S 1995 J. Comput. Phys. 117 1
 Google Scholar
Google Scholar
[21] Jorgensen W L, Maxwell D S, Tirado-Rives J 1996 J. Am. Chem. Soc. 118 11225
 Google Scholar
Google Scholar
[22] Li X, Hede T, Tu Y, Leck C, Ågren H 2011 Atmos. Chem. Phys. 11 519
 Google Scholar
Google Scholar
[23] Wang B B, Wang X D, Duan Y Y, Chen M 2014 Int. J. Heat Mass Transfer 73 533
 Google Scholar
Google Scholar
[24] Eastwood J W, Hockney R W, Lawrence D N 1980 Comput. Phys. Commun. 19 215
 Google Scholar
Google Scholar
[25] Thompson S M, Gubbins K E, Walton J P R B, Chantry R A R, Rowlinson J S 1984 J. Chem. Phys. 81 530
 Google Scholar
Google Scholar
[26] Köhler H 1936 Trans. Faraday Soc. 32 1152
 Google Scholar
Google Scholar
[27] Vargaftik N B, Volkov B N, Voljak L D 1983 J. Phys. Chem. Ref. Data 12 817
 Google Scholar
Google Scholar
[28] Berendsen H J C, Grigera J R, Straatsma T P 1987 J. Phys. Chem. 91 6269
 Google Scholar
Google Scholar
[29] Booth A M, Topping D O, McFiggans G, Percival C J 2009 Phys. Chem. Chem. Phys. 11 8021
 Google Scholar
Google Scholar
[30] Liu L Y, Li H 2023 Atmos. Environ. 294 119500
 Google Scholar
Google Scholar
[31] Szyszkowski B V 1908 Z. Phys. Chem. 64 385
 Google Scholar
Google Scholar
-
-
表 1 不同液滴中Szyszkowski公式中拟合参数Γmax和γ
Table 1. Fitted coefficients Γmax and γ in Szyszkowski equation for different droplets.
Nw 500 750 1000 2000 3000 4000 5000 6000 Re/nm 1.528 1.751 1.926 2.427 2.780 3.061 3.296 3.504 σw/(mN·m–1) 55.100 58.099 62.112 67.760 69.334 69.339 70.371 70.688 Γmax/(10–6 mol·m-2) 0.711 0.872 2.598 1.910 3.743 7.368 10.963 11.018 γ/(mol·L-1) 0.074 0.123 1.363 1.274 1.297 4.326 6.366 6.755 -
[1] Mahowald N 2011 Science 334 794
 Google Scholar
Google Scholar
[2] Scott C E, Arnold S R, Monks S A, Asmi A, Paasonen P, Spracklen D V 2018 Nat. Geosci. 11 44
 Google Scholar
Google Scholar
[3] Fan F X, Zhang S H, Wang W Y, Yan J P, Su M X 2019 Process Saf. Environ. Prot. 125 197
 Google Scholar
Google Scholar
[4] Fan F X, Zhang S H, Peng Z B, Chen J, Su M X, Moghtaderi B, Doroodchi E 2019 Can. J. Chem. Eng. 97 930
 Google Scholar
Google Scholar
[5] Cheng Y, Su H, Koop T, Mikhailov E, Poschl U 2015 Nat. Commun. 6 5923
 Google Scholar
Google Scholar
[6] Yamada T, Sakai K 2012 Phys. Fluids 24 022103
 Google Scholar
Google Scholar
[7] Morris H S, Grassian V H, Tivanski A V 2015 Chem. Sci. 6 3242
 Google Scholar
Google Scholar
[8] Dutcher C S, Wexler A S, Clegg S L 2010 J. Phys. Chem. A 114 12216
 Google Scholar
Google Scholar
[9] Wexler A S, Clegg S L 2002 J. Geophys. Res. Atmos. 107 ACH 14
[10] Chapela G A, Saville G, Thompson S M, Rowlinson J S 1977 J. Chem. Soc. Faraday Trans. 2 73 1133
 Google Scholar
Google Scholar
[11] Blokhuis E M, Bedeaux D, Holcomb C D, Zollweg J A 1995 Mol. Phys. 85 665
 Google Scholar
Google Scholar
[12] Chen F, Smith P E 2007 J. Chem. Phys. 126 221101
 Google Scholar
Google Scholar
[13] Wang X X, Chen C C, Binder K, Kuhn U, Pöschl U, Su H, Cheng Y F 2018 Atmos. Chem. Phys. 18 17077
 Google Scholar
Google Scholar
[14] Sun L, Li X, Hede T, Tu Y Q, Leck C, Ågren H 2012 J. Phys. Chem. B 116 3198
 Google Scholar
Google Scholar
[15] Liu L, Guo S, Zhao Z, Li H 2022 J. Phys. Chem. A 126 2407
 Google Scholar
Google Scholar
[16] Petters S S, Petters M D 2016 J. Geophys. Res. Atmos. 121 1878
 Google Scholar
Google Scholar
[17] Zhang C, Zhang Z C, Bu L X, Yang Y, Xiong W, Wang Y S 2023 Particuology 77 128
 Google Scholar
Google Scholar
[18] Nosé S C 1984 Mol. Phys. 52 255
 Google Scholar
Google Scholar
[19] Hoover W G 1985 Phys. Rev. A 31 1695
 Google Scholar
Google Scholar
[20] Plimpton S 1995 J. Comput. Phys. 117 1
 Google Scholar
Google Scholar
[21] Jorgensen W L, Maxwell D S, Tirado-Rives J 1996 J. Am. Chem. Soc. 118 11225
 Google Scholar
Google Scholar
[22] Li X, Hede T, Tu Y, Leck C, Ågren H 2011 Atmos. Chem. Phys. 11 519
 Google Scholar
Google Scholar
[23] Wang B B, Wang X D, Duan Y Y, Chen M 2014 Int. J. Heat Mass Transfer 73 533
 Google Scholar
Google Scholar
[24] Eastwood J W, Hockney R W, Lawrence D N 1980 Comput. Phys. Commun. 19 215
 Google Scholar
Google Scholar
[25] Thompson S M, Gubbins K E, Walton J P R B, Chantry R A R, Rowlinson J S 1984 J. Chem. Phys. 81 530
 Google Scholar
Google Scholar
[26] Köhler H 1936 Trans. Faraday Soc. 32 1152
 Google Scholar
Google Scholar
[27] Vargaftik N B, Volkov B N, Voljak L D 1983 J. Phys. Chem. Ref. Data 12 817
 Google Scholar
Google Scholar
[28] Berendsen H J C, Grigera J R, Straatsma T P 1987 J. Phys. Chem. 91 6269
 Google Scholar
Google Scholar
[29] Booth A M, Topping D O, McFiggans G, Percival C J 2009 Phys. Chem. Chem. Phys. 11 8021
 Google Scholar
Google Scholar
[30] Liu L Y, Li H 2023 Atmos. Environ. 294 119500
 Google Scholar
Google Scholar
[31] Szyszkowski B V 1908 Z. Phys. Chem. 64 385
 Google Scholar
Google Scholar
Catalog
Metrics
- Abstract views: 6129
- PDF Downloads: 141
- Cited By: 0














 DownLoad:
DownLoad: