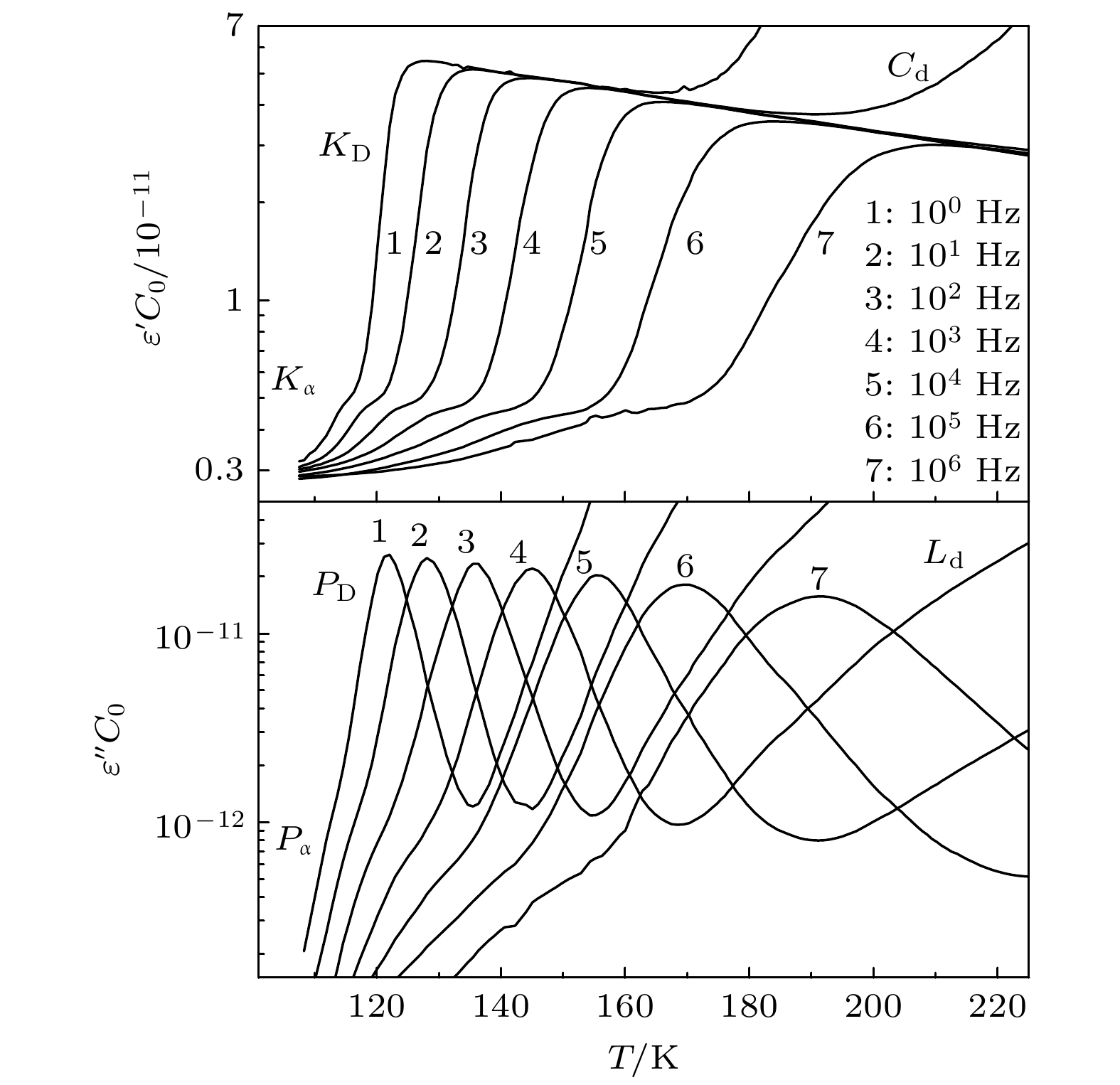
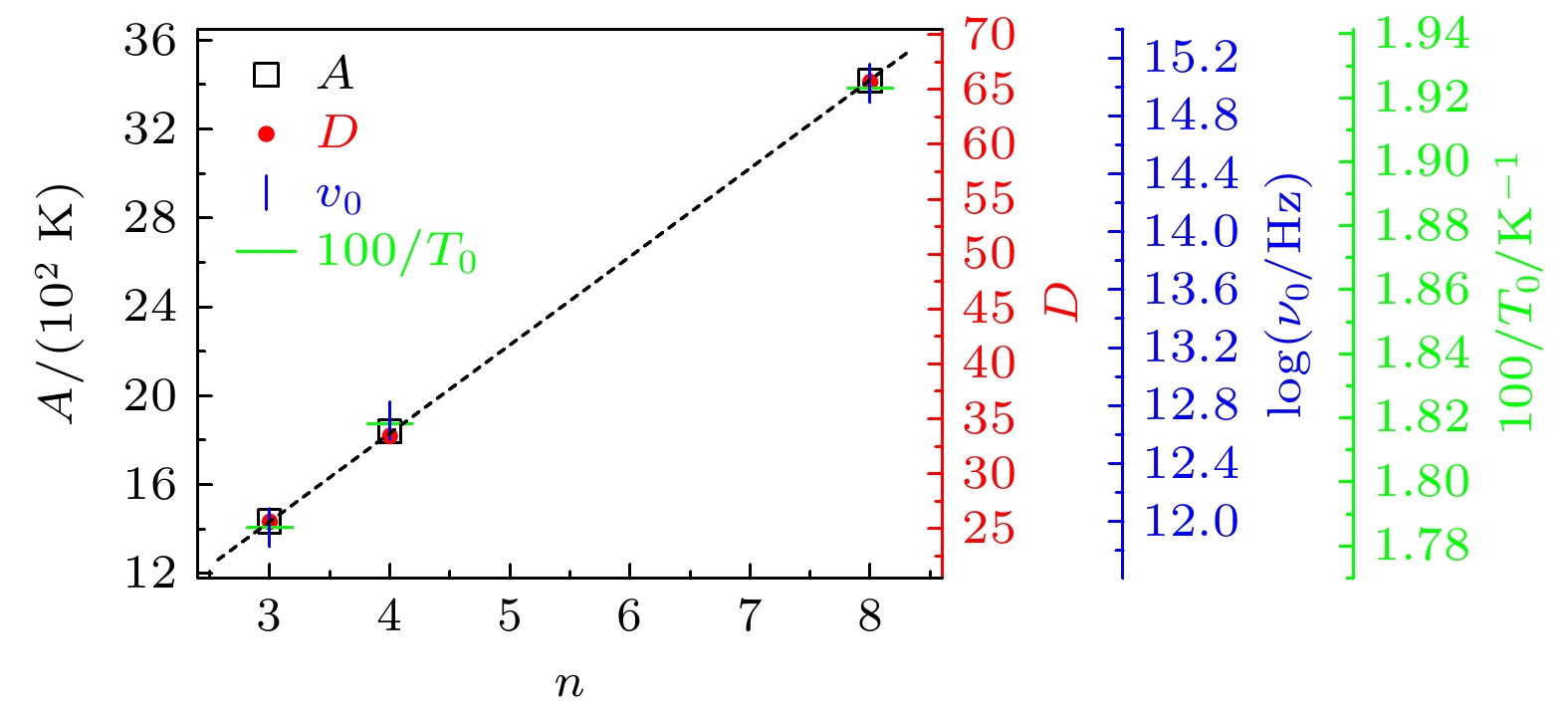
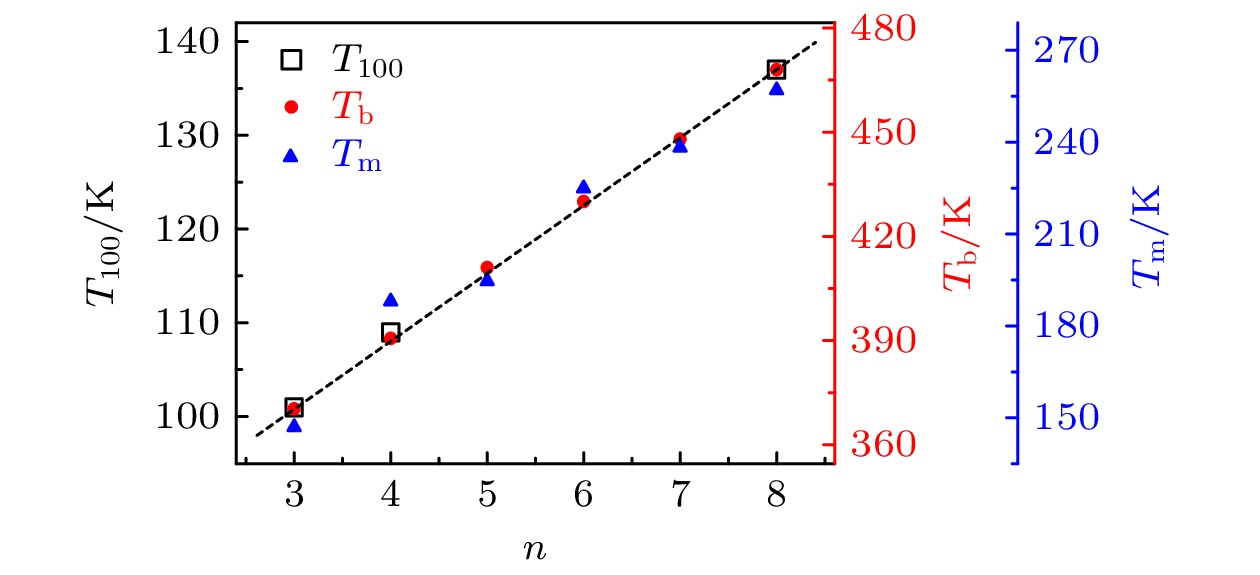
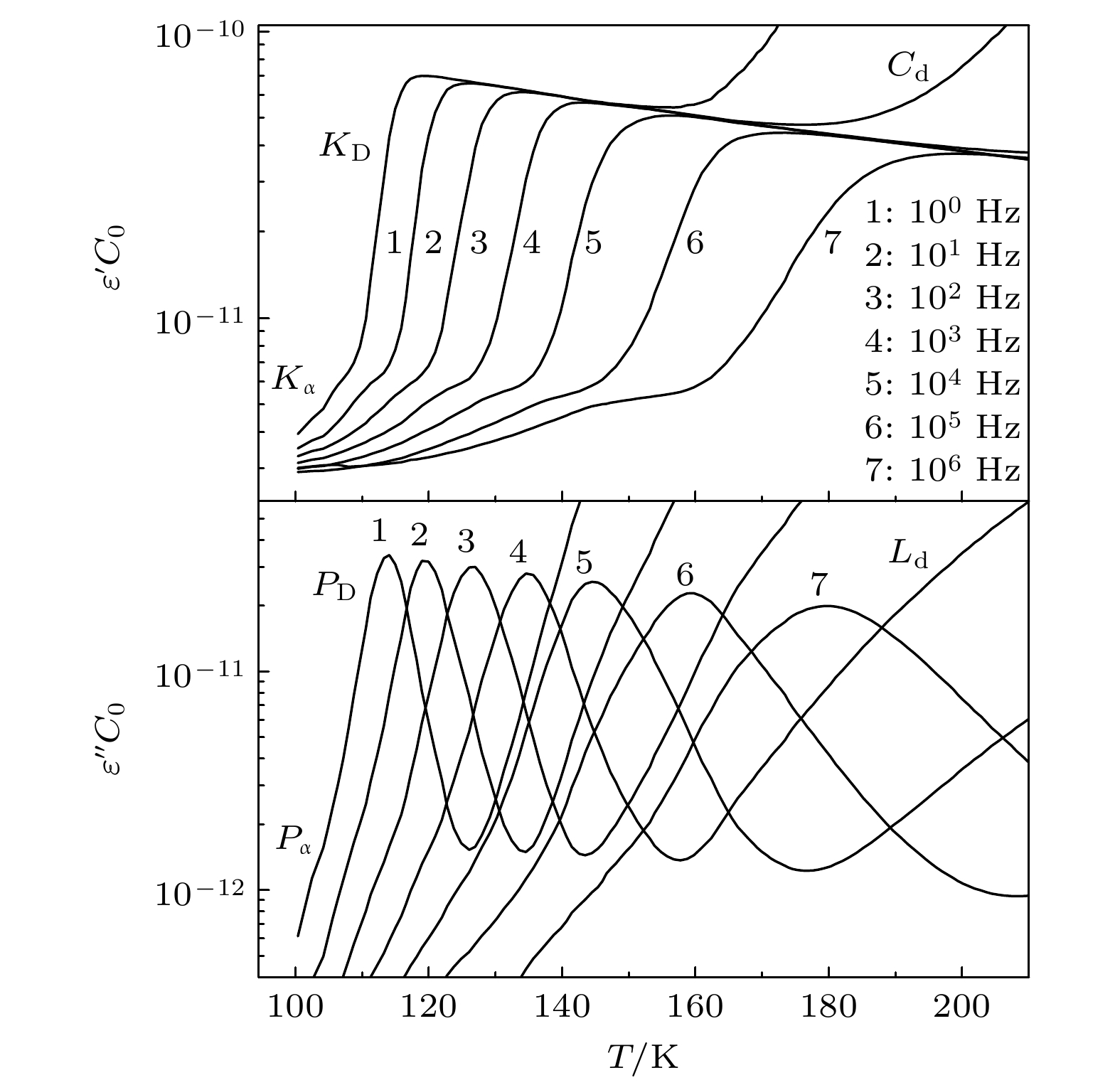
-
Monohydroxy alcohol has a Debye relaxation process that other liquids usually do not have, and with the further research, some new phenomena and new problems related to the process have been gradually discovered, deepening the understanding of material structure and dynamics. In order to further investigate the dynamics of Debye relaxation processes and the influence of molecular constitutions on them, the Debye processes of three primary alcohols without branched chains or side groups are studied by dielectric spectroscopy method, and some important information about the processes is revealed. A number of dynamic parameters of Debye relaxation in n-propanol, n-butanol and n-octanol almost all increase linearly with the rising of the number of carbon atoms in the molecules, which include the characteristic temperature, the reciprocal of Vogel-Fulcher-Tammann (VFT) temperature, the strength parameter of Debye process, the activation energy and the logarithm of the intrinsic vibration frequency of relaxation units under high temperature limit. However, the values of VFT temperatures change little and have consistency, illustrating that the relaxation units of Debye processes in these three monohydroxy alcohols should be the same, further verifying the view that the Debye relaxation originates from the hydroxyl groups in hydrogen bonded molecular chains. Comparing Boiling temperatures and melting temperatures of those samples with the evolution of the above activation energy, it is shown that there is a positive correlation between the interaction among hydrogen bonds and the whole one among molecules. In addition, combining the information about the strength parameter with that from the relevant theories, a possible perspective is gained for further investigation of liquid fragility. The comparison of those three samples with ethanol displays that the degree of separation of Debye relaxation and α relaxation is influenced by the molecular chain length, which can provide a breakthrough point to explore Debye relaxation. These results in this work will promote the further understanding and research of Debye relaxation in monohydroxy alcohols, and also provide experimental information for relevant theories.
-
Keywords:
- Debye relaxation /
- monohydroxy alcohol /
- dielectric spectroscopy /
- glass transition
[1] Böhmer R, Gainaru C, Richert R 2014 Phys. Rep. 545 125
 Google Scholar
Google Scholar
[2] Huth H, Wang L M, Schick C, Richert R 2007 J. Chem. Phys. 126 104503
 Google Scholar
Google Scholar
[3] Jakobsen B, Maggi C, Christensen T, Dyre J C 2008 J. Chem. Phys. 129 184502
 Google Scholar
Google Scholar
[4] Gainaru C, Kastner S, Mayr F, Lunkenheimer P, Schildmann S, Weber H J, Hiller W, Loidl A, Böhmer R 2011 Phys. Rev. Lett. 107 118304
 Google Scholar
Google Scholar
[5] Bauer S, Burlafinger K, Gainaru C, Lunkenheimer P, Hiller W, Loidl A, Böhmer R 2013 J. Chem. Phys. 138 94505
 Google Scholar
Google Scholar
[6] Wang L N, Zhao X Y, Huang Y N 2019 Int. J. Mod. Phys. B 33 1950313
 Google Scholar
Google Scholar
[7] Wang L N, Zhao X Y, Huang Y N 2019 Chin. Phys. Lett. 36 097701
 Google Scholar
Google Scholar
[8] Zhao X Y, Wang L N, He Y F, Zhou H W, Huang Y N 2020 Chem. Phys. 528 110473
 Google Scholar
Google Scholar
[9] Lu G H, Wang L N, Zhao X Y, He Y F, Huang Y N 2021 Int. J. Mod. Phys. B 35 2150014
 Google Scholar
Google Scholar
[10] Wang L M, Tian Y J, Liu R P, Richert R 2008 J. Chem. Phys. 128 084503
 Google Scholar
Google Scholar
[11] 李艳伟, 孙昭艳, 安立佳 2016 大学化学 31 1
 Google Scholar
Google Scholar
Li Y W, Sun Z Y, An L J 2016 Univ. Chem. 31 1
 Google Scholar
Google Scholar
[12] Dyre J C 2006 Rev. Mod. Phys. 78 953
 Google Scholar
Google Scholar
[13] Angell C A, Ngai K L, McKenna G B, McMillan P F, Martin S W 2000 J. Appl. Phys. 88 3113
 Google Scholar
Google Scholar
[14] 汪卫华 2013 物理学进展 33 177
Wang W H 2013 Prog. Phys. 33 177
[15] Gainaru C, Meier R, Schildmann S, Lederle C, Hiller W, Rössler E A, Böhmer R 2010 Phys. Rev. Lett. 105 258303
 Google Scholar
Google Scholar
[16] Gao Y Q, Tu W K, Chen Z M, Tian Y J, Liu R P, Wang L M 2013 J. Chem. Phys. 139 164504
 Google Scholar
Google Scholar
[17] Zhao X Y, Wang L N, Yin H M, Zhou H W, Huang Y N 2019 Chin. Phys. B 28 086601
 Google Scholar
Google Scholar
[18] Xu D, Feng S D, Wang J Q, Wang L M, Richert R 2020 J. Phys. Chem. Lett. 11 5792
 Google Scholar
Google Scholar
[19] Havriliak S, Negami S 1967 Polymer 8 161
 Google Scholar
Google Scholar
[20] Richert R 2010 J. Chem. Phys. 133 74502
 Google Scholar
Google Scholar
[21] Wang J, Zhao K S, Wu L X 2014 J. Chem. Phys. 141 54502
 Google Scholar
Google Scholar
[22] Ishai P B, Talary M S, Caduff A, Levy E, Feldman Y 2013 Meas. Sci. Technol. 24 102001
 Google Scholar
Google Scholar
[23] Brand R, Lunkenheimer P, Loidl A 2002 J. Chem. Phys. 116 10386
 Google Scholar
Google Scholar
[24] Chua Y Z, Young-Gonzales A R, Richert R, Ediger M D, Schick C 2017 J. Chem. Phys. 147 014502
 Google Scholar
Google Scholar
[25] Scherer G W 1992 J. Am. Ceram. Soc. 75 1060
 Google Scholar
Google Scholar
[26] Vogel H 1921 Phys. Zeit. 22 645
[27] Fulcher G S 1925 J. Am. Ceram. Soc. 8 339
 Google Scholar
Google Scholar
[28] Tammann G, Hesse W 1926 Z. Anorg. Allg. Chem. 156 245
 Google Scholar
Google Scholar
[29] Böhmer R, Ngai K L, Angell C A, Plazek D J 1993 J. Chem. Phys. 99 4201
 Google Scholar
Google Scholar
[30] Huang Y N, Wang C J, Riande E 2005 J. Chem. Phys. 122 144502
 Google Scholar
Google Scholar
[31] 赵兴宇, 王丽娜, 樊小辉, 张丽丽, 卫来, 张晋鲁, 黄以能 2011 60 036403
 Google Scholar
Google Scholar
Zhao X Y, Wang L N, Fan X H, Zhang L L, Wei L, Zhang J-L, Huang Y N 2011 Acta Phys. Sin. 60 036403
 Google Scholar
Google Scholar
[32] Angell C A 1995 Science 267 1924
 Google Scholar
Google Scholar
[33] Lunkenheimer P, Schneider U, Brand R, Loidl A 2000 Contemp. Phys. 41 15
 Google Scholar
Google Scholar
[34] Tschamler H, Richter E, Wettig F 1949 Monatsh. Chem. 80 749
 Google Scholar
Google Scholar
[35] Wang L M, Richert R 2007 J. Phys. Chem. B 111 3201
 Google Scholar
Google Scholar
-
表 1 正丙醇、正丁醇和正辛醇的
$ n $ ,$ A $ ,$ {\nu }_{0} $ ,$ {T}_{0} $ ,$ {T}_{100} $ 以及D值Table 1. Values of
$ n $ ,$ A $ ,$ {\nu }_{0} $ ,$ {T}_{0} $ ,$ {T}_{100} $ and D in n-propanol, n-butanol and n-octanol.样品 $ n $ $ A $/K $ {\nu }_{0} $/Hz $ {T}_{0} $/K $ {T}_{100} $/K $ D $ 正丙醇 3 1434 9.08$ \times $1011 56 101 25.6 正丁醇 4 1839 4.97$ \times $1012 55 109 33.4 正辛醇 8 3416 1.06$ \times $1015 52 137 65.7 -
[1] Böhmer R, Gainaru C, Richert R 2014 Phys. Rep. 545 125
 Google Scholar
Google Scholar
[2] Huth H, Wang L M, Schick C, Richert R 2007 J. Chem. Phys. 126 104503
 Google Scholar
Google Scholar
[3] Jakobsen B, Maggi C, Christensen T, Dyre J C 2008 J. Chem. Phys. 129 184502
 Google Scholar
Google Scholar
[4] Gainaru C, Kastner S, Mayr F, Lunkenheimer P, Schildmann S, Weber H J, Hiller W, Loidl A, Böhmer R 2011 Phys. Rev. Lett. 107 118304
 Google Scholar
Google Scholar
[5] Bauer S, Burlafinger K, Gainaru C, Lunkenheimer P, Hiller W, Loidl A, Böhmer R 2013 J. Chem. Phys. 138 94505
 Google Scholar
Google Scholar
[6] Wang L N, Zhao X Y, Huang Y N 2019 Int. J. Mod. Phys. B 33 1950313
 Google Scholar
Google Scholar
[7] Wang L N, Zhao X Y, Huang Y N 2019 Chin. Phys. Lett. 36 097701
 Google Scholar
Google Scholar
[8] Zhao X Y, Wang L N, He Y F, Zhou H W, Huang Y N 2020 Chem. Phys. 528 110473
 Google Scholar
Google Scholar
[9] Lu G H, Wang L N, Zhao X Y, He Y F, Huang Y N 2021 Int. J. Mod. Phys. B 35 2150014
 Google Scholar
Google Scholar
[10] Wang L M, Tian Y J, Liu R P, Richert R 2008 J. Chem. Phys. 128 084503
 Google Scholar
Google Scholar
[11] 李艳伟, 孙昭艳, 安立佳 2016 大学化学 31 1
 Google Scholar
Google Scholar
Li Y W, Sun Z Y, An L J 2016 Univ. Chem. 31 1
 Google Scholar
Google Scholar
[12] Dyre J C 2006 Rev. Mod. Phys. 78 953
 Google Scholar
Google Scholar
[13] Angell C A, Ngai K L, McKenna G B, McMillan P F, Martin S W 2000 J. Appl. Phys. 88 3113
 Google Scholar
Google Scholar
[14] 汪卫华 2013 物理学进展 33 177
Wang W H 2013 Prog. Phys. 33 177
[15] Gainaru C, Meier R, Schildmann S, Lederle C, Hiller W, Rössler E A, Böhmer R 2010 Phys. Rev. Lett. 105 258303
 Google Scholar
Google Scholar
[16] Gao Y Q, Tu W K, Chen Z M, Tian Y J, Liu R P, Wang L M 2013 J. Chem. Phys. 139 164504
 Google Scholar
Google Scholar
[17] Zhao X Y, Wang L N, Yin H M, Zhou H W, Huang Y N 2019 Chin. Phys. B 28 086601
 Google Scholar
Google Scholar
[18] Xu D, Feng S D, Wang J Q, Wang L M, Richert R 2020 J. Phys. Chem. Lett. 11 5792
 Google Scholar
Google Scholar
[19] Havriliak S, Negami S 1967 Polymer 8 161
 Google Scholar
Google Scholar
[20] Richert R 2010 J. Chem. Phys. 133 74502
 Google Scholar
Google Scholar
[21] Wang J, Zhao K S, Wu L X 2014 J. Chem. Phys. 141 54502
 Google Scholar
Google Scholar
[22] Ishai P B, Talary M S, Caduff A, Levy E, Feldman Y 2013 Meas. Sci. Technol. 24 102001
 Google Scholar
Google Scholar
[23] Brand R, Lunkenheimer P, Loidl A 2002 J. Chem. Phys. 116 10386
 Google Scholar
Google Scholar
[24] Chua Y Z, Young-Gonzales A R, Richert R, Ediger M D, Schick C 2017 J. Chem. Phys. 147 014502
 Google Scholar
Google Scholar
[25] Scherer G W 1992 J. Am. Ceram. Soc. 75 1060
 Google Scholar
Google Scholar
[26] Vogel H 1921 Phys. Zeit. 22 645
[27] Fulcher G S 1925 J. Am. Ceram. Soc. 8 339
 Google Scholar
Google Scholar
[28] Tammann G, Hesse W 1926 Z. Anorg. Allg. Chem. 156 245
 Google Scholar
Google Scholar
[29] Böhmer R, Ngai K L, Angell C A, Plazek D J 1993 J. Chem. Phys. 99 4201
 Google Scholar
Google Scholar
[30] Huang Y N, Wang C J, Riande E 2005 J. Chem. Phys. 122 144502
 Google Scholar
Google Scholar
[31] 赵兴宇, 王丽娜, 樊小辉, 张丽丽, 卫来, 张晋鲁, 黄以能 2011 60 036403
 Google Scholar
Google Scholar
Zhao X Y, Wang L N, Fan X H, Zhang L L, Wei L, Zhang J-L, Huang Y N 2011 Acta Phys. Sin. 60 036403
 Google Scholar
Google Scholar
[32] Angell C A 1995 Science 267 1924
 Google Scholar
Google Scholar
[33] Lunkenheimer P, Schneider U, Brand R, Loidl A 2000 Contemp. Phys. 41 15
 Google Scholar
Google Scholar
[34] Tschamler H, Richter E, Wettig F 1949 Monatsh. Chem. 80 749
 Google Scholar
Google Scholar
[35] Wang L M, Richert R 2007 J. Phys. Chem. B 111 3201
 Google Scholar
Google Scholar
Catalog
Metrics
- Abstract views: 4734
- PDF Downloads: 72
- Cited By: 0














 DownLoad:
DownLoad: