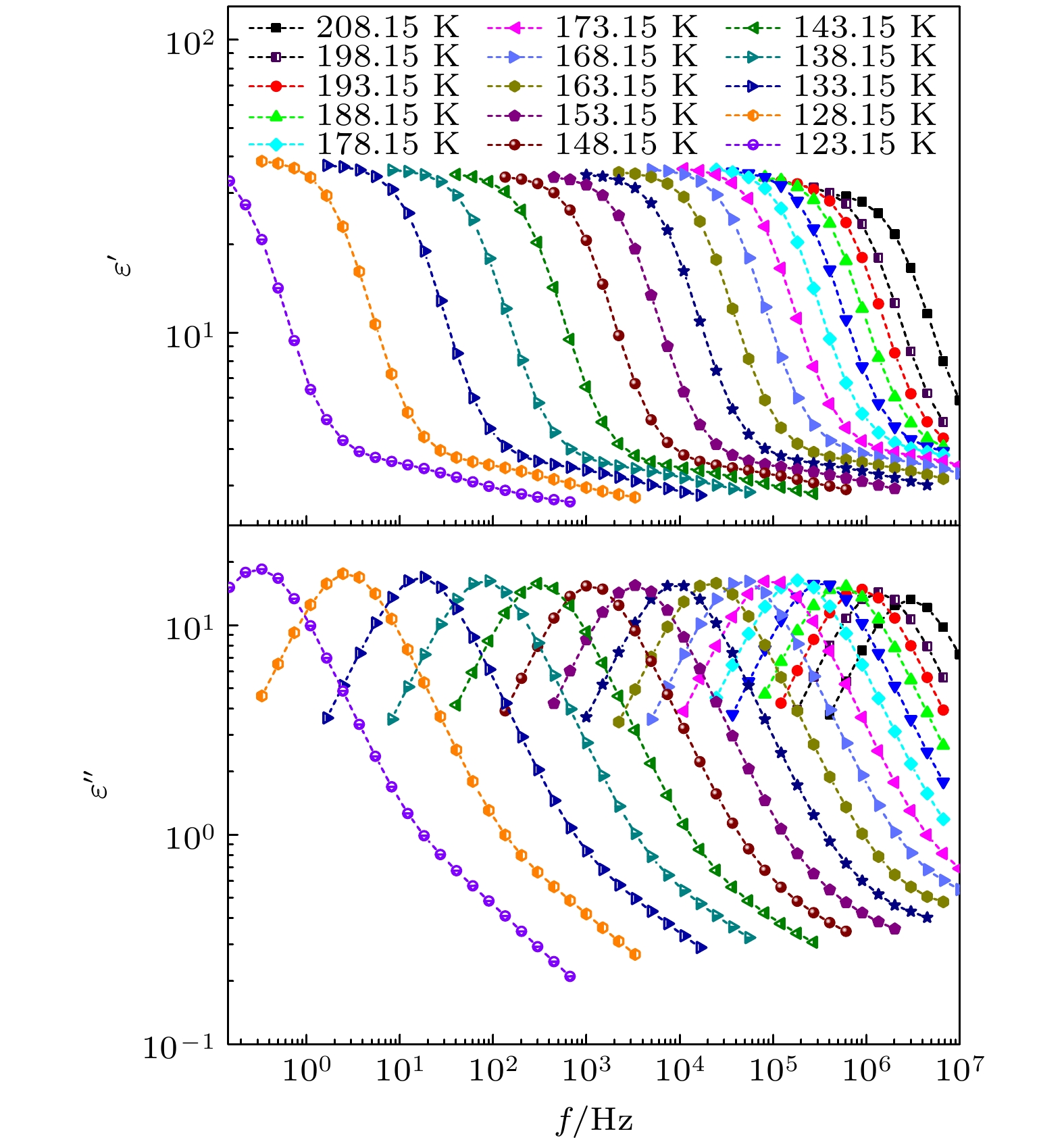
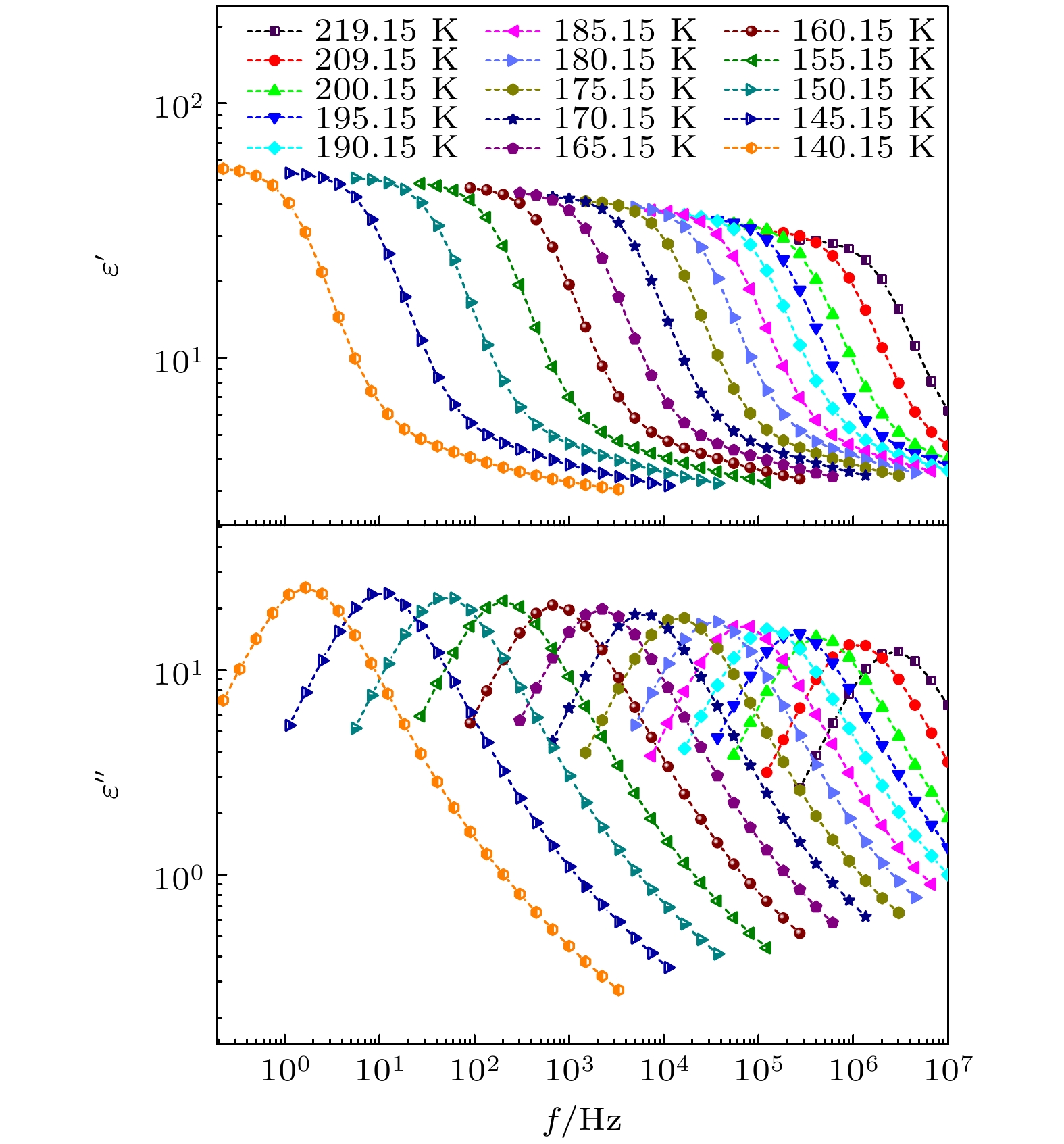
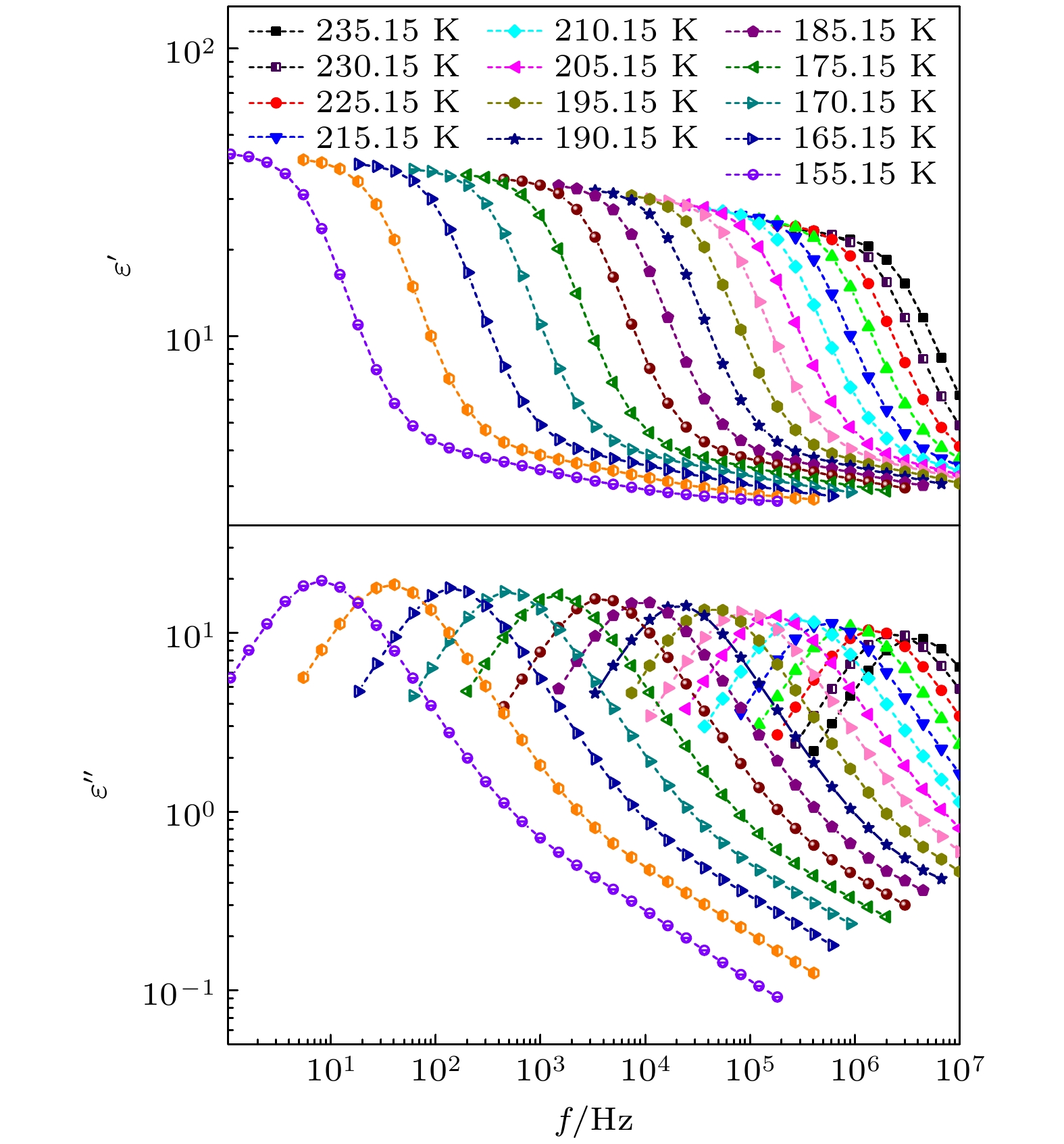
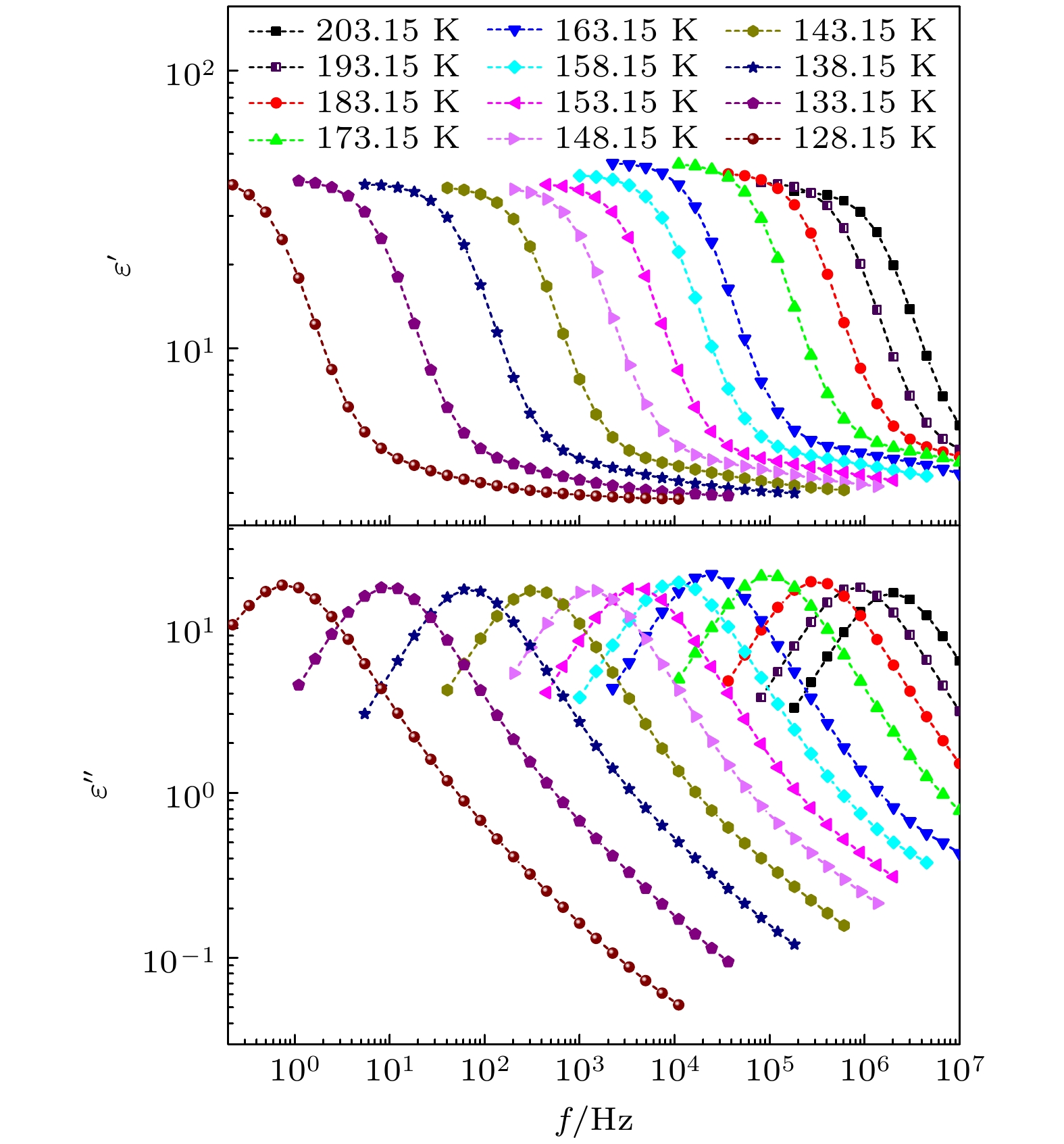
-
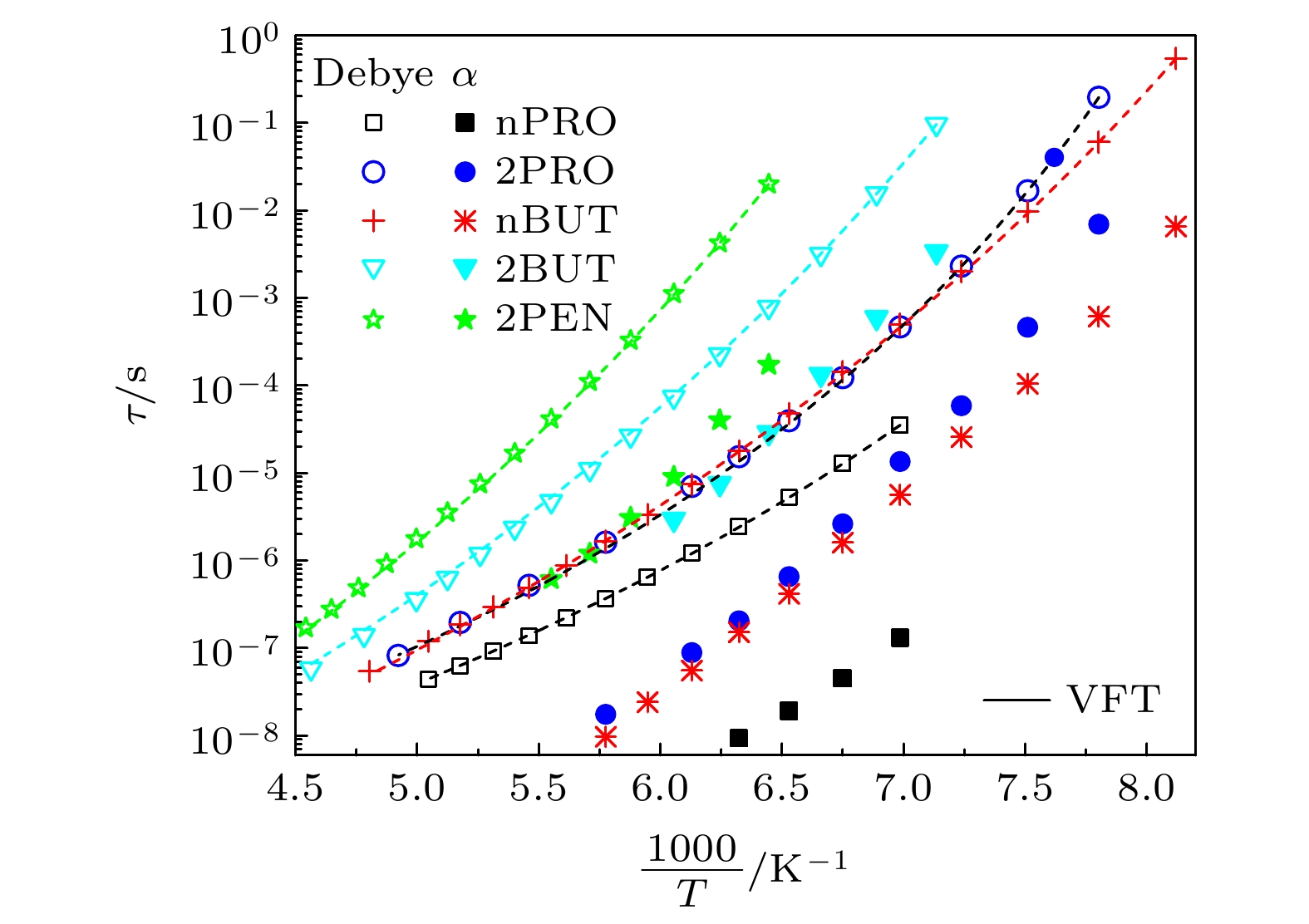
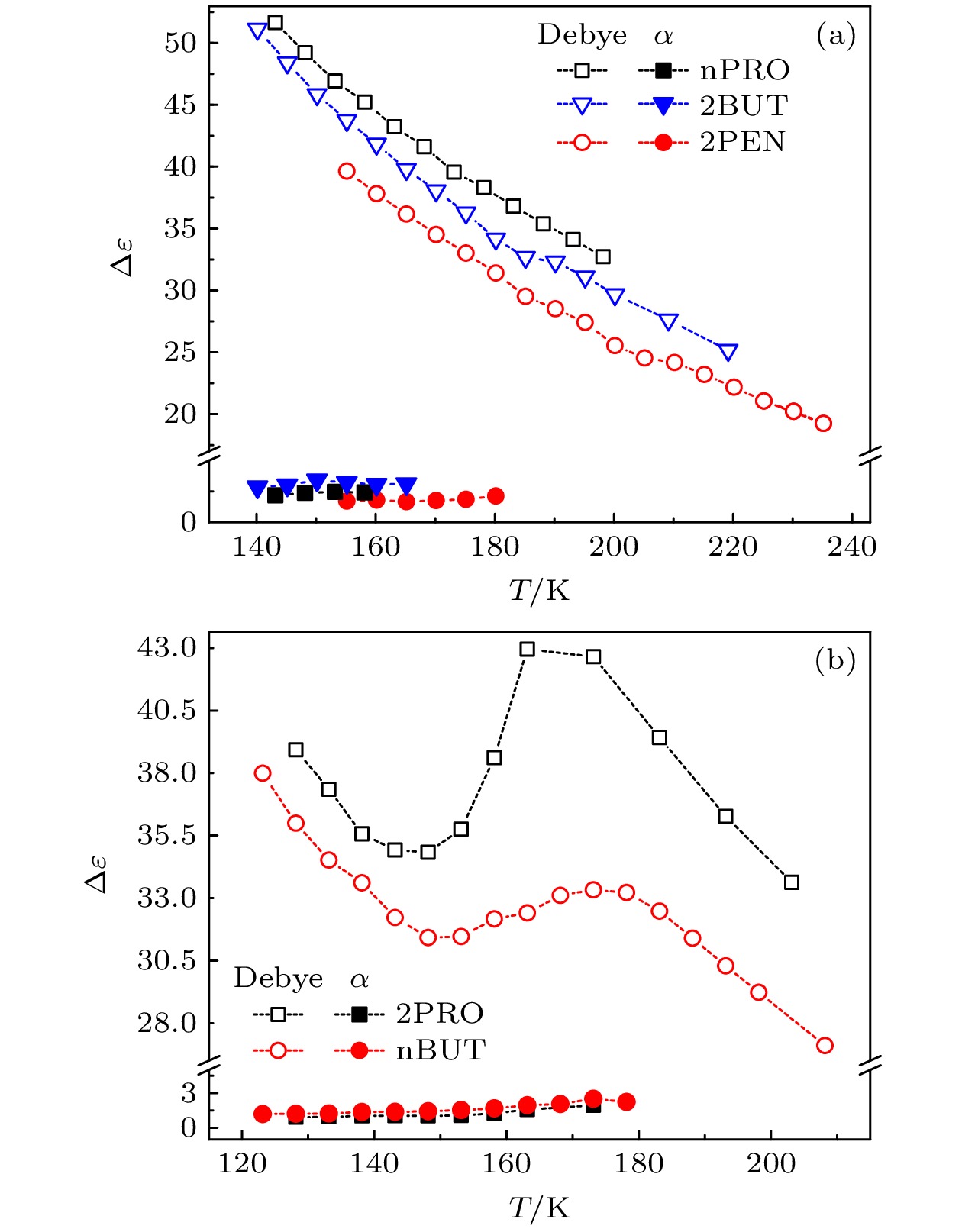
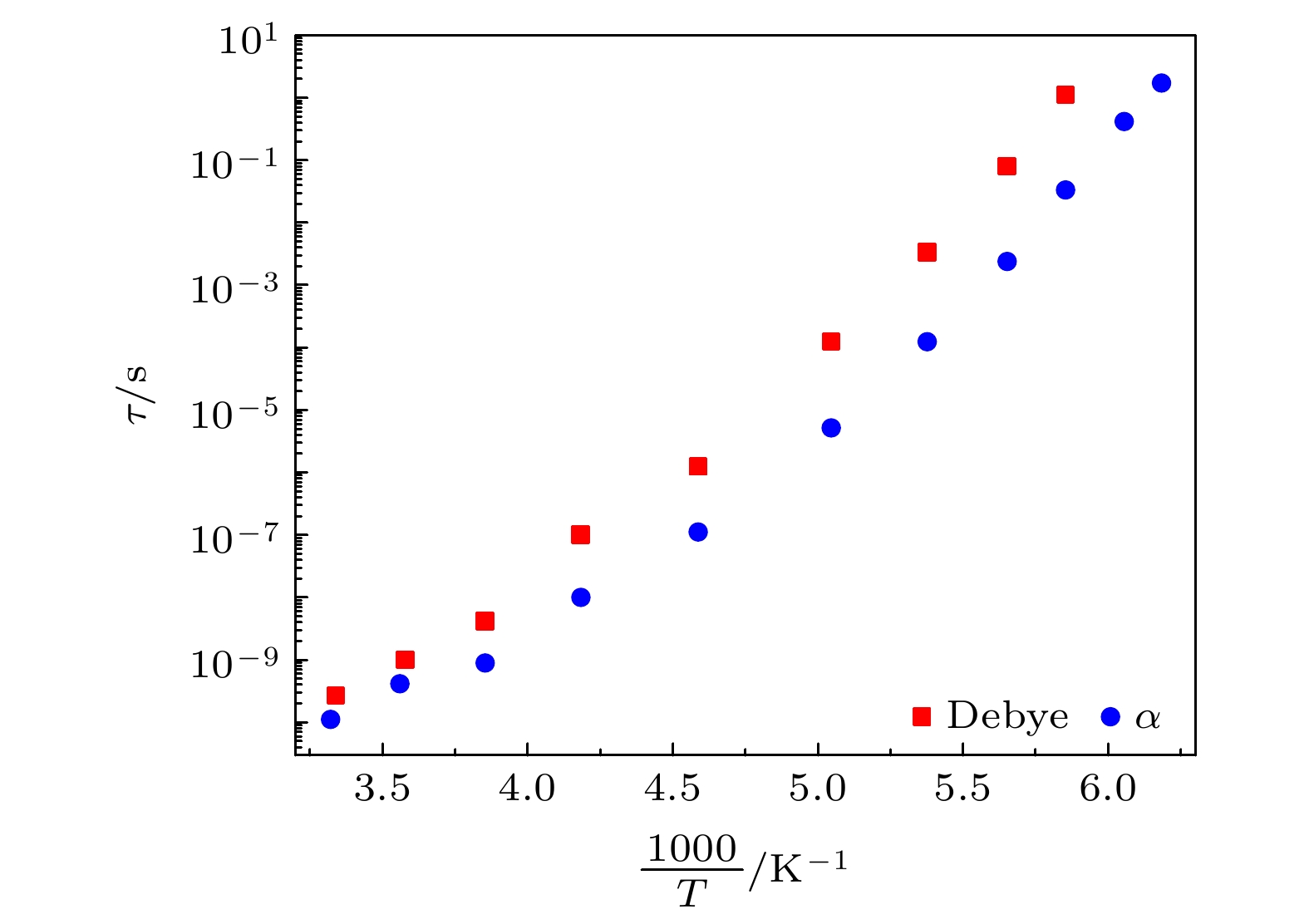
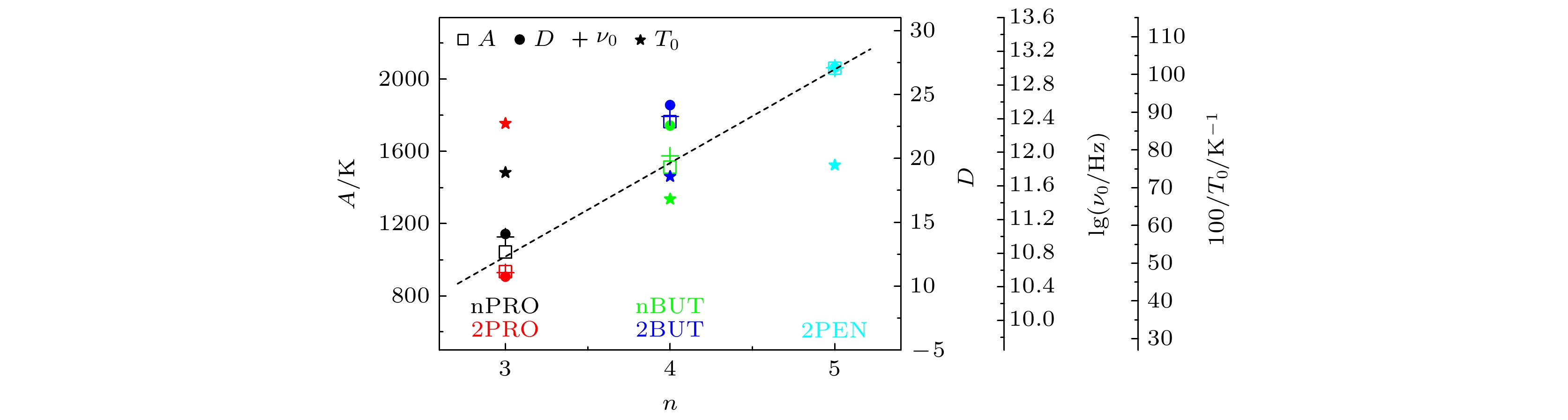
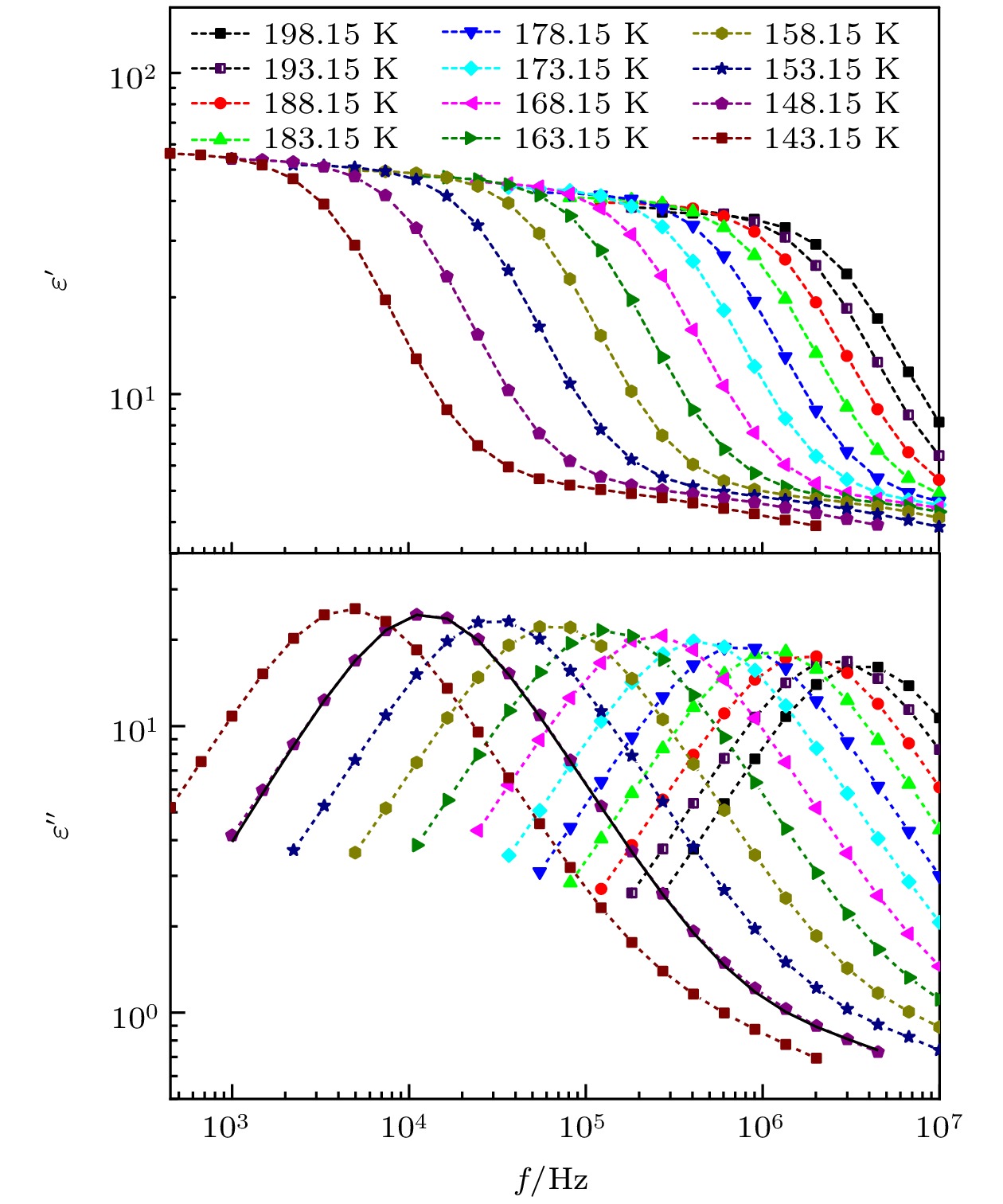
对正丙醇、异丙醇、正丁醇、仲丁醇和仲戊醇5种链长相近、结构又略有差异的线型伯醇和仲醇进行了介电谱测量, 发现异丙醇和正丁醇的介电谱存在异常变化的情况, 即介电谱中强度最大的弛豫峰约在145—175 K的范围内, 随着温度的升高出现了逐渐升高的趋势. 经过分析发现该异常变化来源于单羟基醇中的德拜(Debye)介电弛豫强度在上述温区内的异常变化. 结合单羟基醇德拜介电弛豫强度的理论模型进行分析, 认为该异常变化是温度导致的德拜介电弛豫强度减小与分子运动性增加所致使的氢键分子链结构变化而引起的弛豫强度改变的共同结果; 通过将这5种单羟基醇的弛豫时间进行对比, 发现产生上述异常变化所要求的条件比较苛刻. 另外, 研究结果还表明仲醇中德拜弛豫过程的强度参量、弛豫单元的固有振动频率和高温极限下的激活能也具有随着碳原子数的增加而增大的特征, 与伯醇中的规律是类似的. 这些结果不仅能为单羟基醇奇异性质的研究提供新的切入点, 而且也可以为分子链长对单羟基醇动力学影响的研究提供参考.The five linear primary and secondary alcohols, i.e. n-propanol, isopropanol, n-butanol, 2-butanol and 2-pentanol, have similar chain lengths and slightly different structures. In this work, dielectric spectroscopy is used to investigate the properties of monohydroxy alcohols. The dielectric spectra of isopropanol and n-butanol show an abnormal change. i.e. the relaxation peaks with the highest strength gradually increases with temperature rising in a range of about 145–175 K. The analyses indicate that the abnormal variation originates from that of the Debye dielectric relaxation strength (DDRS) in the monohydroxy alcohols at above temperatures. According to the theoretical model of the DDRS for the monohydroxy alcohol, the abnormal variation is believed to be the result of the combined effects of decrease and increase of the DDRS caused by temperature, and the transformation of the structure of the hydrogen bonding molecular chain caused by the variation of the mobility of molecules. By comparing the relaxation times of the five monohydroxy alcohols, it is found that the conditions should be more stringent to cause the above-mentioned abnormal variation. In addition, the results also show that strength parameter of Debye processes, intrinsic vibration frequency of the relaxation units and their activation energy in the high-temperature limit in secondary alcohols also rise with the increase of the number of carbon atoms, similar to the scenario in the case of primary alcohols. These results can not only provide a new breakthrough point for the investigation of exotic properties in monohydroxy alcohols but also give a reference to explore the effect of molecular chain length on their dynamics.
-
Keywords:
- Debye relaxation /
- monohydroxy alcohol /
- dielectric relaxation /
- abnormal variation
[1] Kennedy D, Norman C 2005 Science 309 75
 Google Scholar
Google Scholar
[2] Böhmer R, Gainaru C, Richert R 2014 Phys. Rep. 545 125
 Google Scholar
Google Scholar
[3] Tsai S D, Yao H Y, Chang T H 2024 J. Mol. Liq. 405 125043
 Google Scholar
Google Scholar
[4] Arrese-Igor S, Alegría A, Colmenero J 2018 Phys. Chem. Chem. Phys. 20 27758
 Google Scholar
Google Scholar
[5] Fragiadakis D, Roland C M, Casalini R 2010 J. Chem. Phys. 132 144505
 Google Scholar
Google Scholar
[6] Bergman R, Jansson H, Swenson J 2010 J. Chem. Phys. 132 044504
 Google Scholar
Google Scholar
[7] Huth H, Wang L M, Schick C, Richert R 2007 J. Chem. Phys. 126 104503
 Google Scholar
Google Scholar
[8] Mandanici A, Huang W, Cutroni M, Richert R 2008 J. Chem. Phys. 128 124505
 Google Scholar
Google Scholar
[9] Wang L M, Tian Y, Liu R, Richert R 2008 J. Chem. Phys. 128 084503
 Google Scholar
Google Scholar
[10] Hu L N, Zhang C Z, Yue Y Z, Bian X F 2010 Chin. Sci. Bull. 55 457
 Google Scholar
Google Scholar
[11] Gainaru C, Kastner S, Mayr F, et al. 2011 Phys. Rev. Lett. 107 118304
 Google Scholar
Google Scholar
[12] Wikarek M, Pawlus S, Tripathy S N, Szulc A, Paluch M 2016 J. Phys. Chem. B 120 5744
 Google Scholar
Google Scholar
[13] Gainaru C, Meier R, Schildmann S, Lederle C, Hiller W, Rössler E A, Böhmer R 2010 Phys. Rev. Lett. 105 258303
 Google Scholar
Google Scholar
[14] Ananiadou A, Papamokos G, Steinhart M, Floudas G 2021 J. Chem. Phys. 155 184504
 Google Scholar
Google Scholar
[15] Sillrén P, Matic A, Karlsson M, et al. 2014 J. Chem. Phys. 140 124501
 Google Scholar
Google Scholar
[16] Bauer S, Burlafinger K, Gainaru C, Lunkenheimer P, Hiller W, Loidl A, Böhmer R 2013 J. Chem. Phys. 138 094505
 Google Scholar
Google Scholar
[17] Wang L N, Zhao X Y, Huang Y N 2019 Int. J. Mod. Phys. B 33 1950313
 Google Scholar
Google Scholar
[18] Wang L N, Zhao X Y, Huang Y N 2019 Chin. Phys. Lett. 36 097701
 Google Scholar
Google Scholar
[19] Wang L N, Zhao X Y, Shang J Y, Zhou H W 2023 Acta Phys. Sin. 72 037701
 Google Scholar
Google Scholar
[20] Zhao X Y, Wang L N, He Y F, Zhou H W, Huang Y N 2020 Chem. Phys. 528 110473
 Google Scholar
Google Scholar
[21] 赵兴宇, 王丽娜, 韩宏博, 尚洁莹 2024 73 147701
 Google Scholar
Google Scholar
Zhao X Y, Wang L N, Han H B, Shang J Y 2024 Acta Phys. Sin. 73 147701
 Google Scholar
Google Scholar
[22] Havriliak S, Negami S 1966 J. Polym. Sci. 14 99
 Google Scholar
Google Scholar
[23] Fulcher G S 1925 J. Am. Ceram. Soc. 8 339
 Google Scholar
Google Scholar
[24] Tammann G, Hesse W 1926 Z. Anorg. Allg. Chem. 156 245
 Google Scholar
Google Scholar
[25] Wang L M, Richert R 2005 J. Chem. Phys. 123 054516
 Google Scholar
Google Scholar
[26] Xu D, Feng S, Wang J Q, Wang L M, Richert R 2020 J. Phys. Chem. Lett. 11 5792
 Google Scholar
Google Scholar
[27] Hu L N, Zhang C Z, Yue Y Z, Bian X F 2010 Chin. Sci. Bull. 55 115
 Google Scholar
Google Scholar
-
表 1 单羟基醇的分子结构
Table 1. Molecular structures of monohydroxy alcohols.
Monohydroxy alcohols Molecular structures n-propanol (nPRO) 
Isopropanol (2PRO) 
n-butanol (nBUT) 
2-Butanol (2BUT) 
2-Pentanol (2PEN) 
-
[1] Kennedy D, Norman C 2005 Science 309 75
 Google Scholar
Google Scholar
[2] Böhmer R, Gainaru C, Richert R 2014 Phys. Rep. 545 125
 Google Scholar
Google Scholar
[3] Tsai S D, Yao H Y, Chang T H 2024 J. Mol. Liq. 405 125043
 Google Scholar
Google Scholar
[4] Arrese-Igor S, Alegría A, Colmenero J 2018 Phys. Chem. Chem. Phys. 20 27758
 Google Scholar
Google Scholar
[5] Fragiadakis D, Roland C M, Casalini R 2010 J. Chem. Phys. 132 144505
 Google Scholar
Google Scholar
[6] Bergman R, Jansson H, Swenson J 2010 J. Chem. Phys. 132 044504
 Google Scholar
Google Scholar
[7] Huth H, Wang L M, Schick C, Richert R 2007 J. Chem. Phys. 126 104503
 Google Scholar
Google Scholar
[8] Mandanici A, Huang W, Cutroni M, Richert R 2008 J. Chem. Phys. 128 124505
 Google Scholar
Google Scholar
[9] Wang L M, Tian Y, Liu R, Richert R 2008 J. Chem. Phys. 128 084503
 Google Scholar
Google Scholar
[10] Hu L N, Zhang C Z, Yue Y Z, Bian X F 2010 Chin. Sci. Bull. 55 457
 Google Scholar
Google Scholar
[11] Gainaru C, Kastner S, Mayr F, et al. 2011 Phys. Rev. Lett. 107 118304
 Google Scholar
Google Scholar
[12] Wikarek M, Pawlus S, Tripathy S N, Szulc A, Paluch M 2016 J. Phys. Chem. B 120 5744
 Google Scholar
Google Scholar
[13] Gainaru C, Meier R, Schildmann S, Lederle C, Hiller W, Rössler E A, Böhmer R 2010 Phys. Rev. Lett. 105 258303
 Google Scholar
Google Scholar
[14] Ananiadou A, Papamokos G, Steinhart M, Floudas G 2021 J. Chem. Phys. 155 184504
 Google Scholar
Google Scholar
[15] Sillrén P, Matic A, Karlsson M, et al. 2014 J. Chem. Phys. 140 124501
 Google Scholar
Google Scholar
[16] Bauer S, Burlafinger K, Gainaru C, Lunkenheimer P, Hiller W, Loidl A, Böhmer R 2013 J. Chem. Phys. 138 094505
 Google Scholar
Google Scholar
[17] Wang L N, Zhao X Y, Huang Y N 2019 Int. J. Mod. Phys. B 33 1950313
 Google Scholar
Google Scholar
[18] Wang L N, Zhao X Y, Huang Y N 2019 Chin. Phys. Lett. 36 097701
 Google Scholar
Google Scholar
[19] Wang L N, Zhao X Y, Shang J Y, Zhou H W 2023 Acta Phys. Sin. 72 037701
 Google Scholar
Google Scholar
[20] Zhao X Y, Wang L N, He Y F, Zhou H W, Huang Y N 2020 Chem. Phys. 528 110473
 Google Scholar
Google Scholar
[21] 赵兴宇, 王丽娜, 韩宏博, 尚洁莹 2024 73 147701
 Google Scholar
Google Scholar
Zhao X Y, Wang L N, Han H B, Shang J Y 2024 Acta Phys. Sin. 73 147701
 Google Scholar
Google Scholar
[22] Havriliak S, Negami S 1966 J. Polym. Sci. 14 99
 Google Scholar
Google Scholar
[23] Fulcher G S 1925 J. Am. Ceram. Soc. 8 339
 Google Scholar
Google Scholar
[24] Tammann G, Hesse W 1926 Z. Anorg. Allg. Chem. 156 245
 Google Scholar
Google Scholar
[25] Wang L M, Richert R 2005 J. Chem. Phys. 123 054516
 Google Scholar
Google Scholar
[26] Xu D, Feng S, Wang J Q, Wang L M, Richert R 2020 J. Phys. Chem. Lett. 11 5792
 Google Scholar
Google Scholar
[27] Hu L N, Zhang C Z, Yue Y Z, Bian X F 2010 Chin. Sci. Bull. 55 115
 Google Scholar
Google Scholar
计量
- 文章访问数: 1836
- PDF下载量: 62
- 被引次数: 0













 下载:
下载: