-
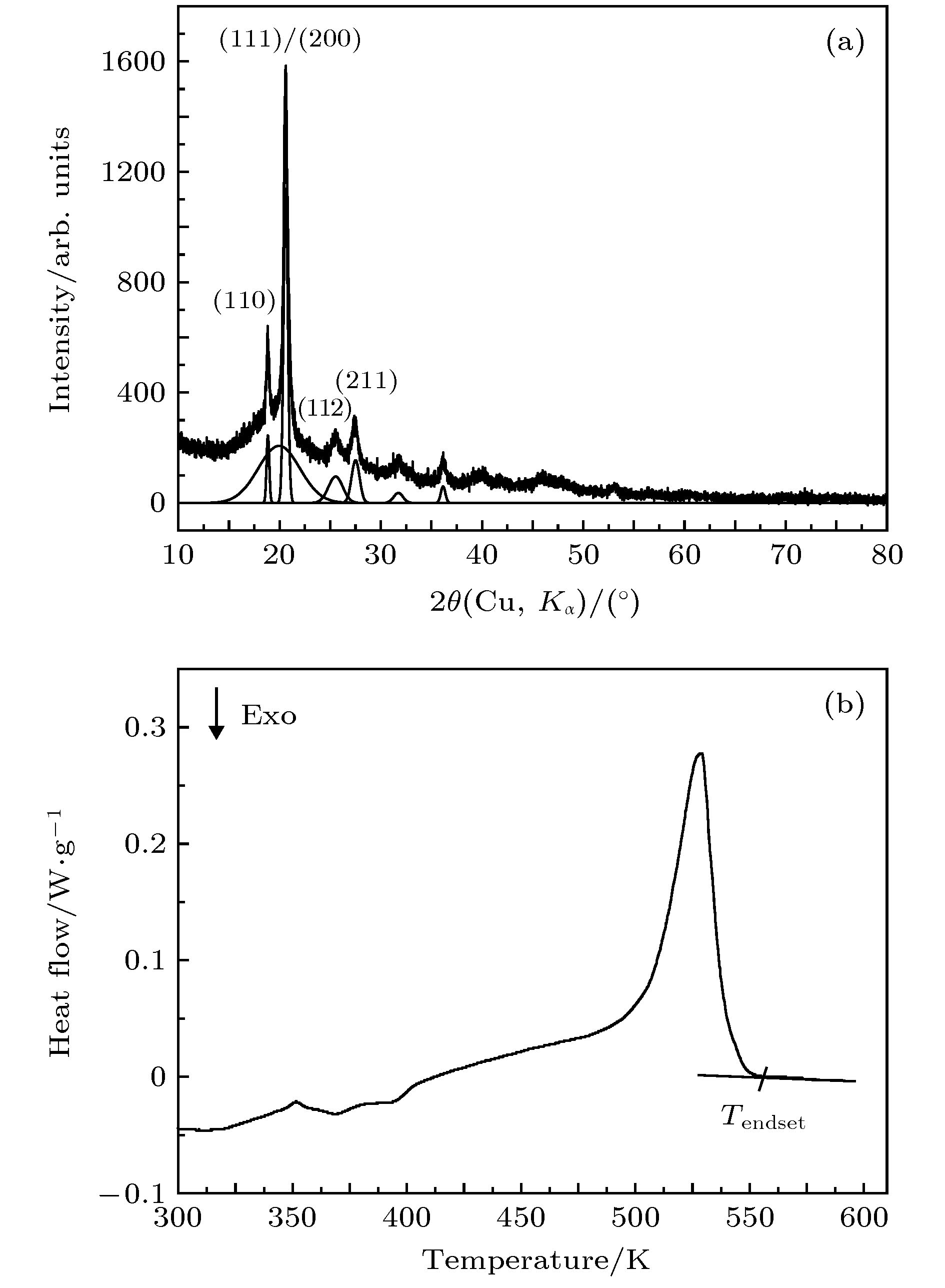
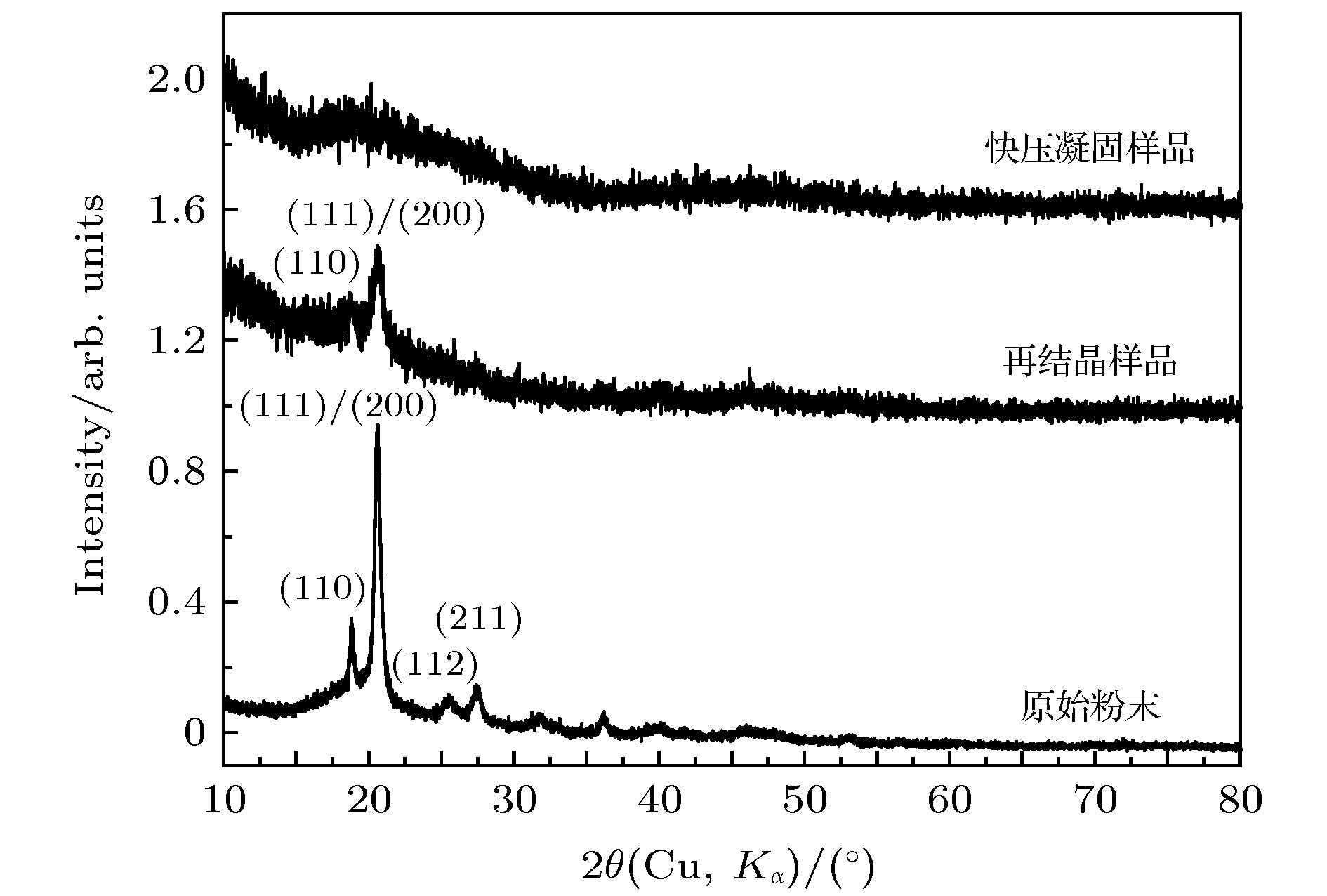
采用施加压力的方法将聚苯硫醚熔体凝固, 凝固后获得的聚苯硫醚样品经过降温和卸压后在常温常压下回收. X射线衍射和差示扫描量热分析表明: 约20 ms时间的快速压缩过程可以抑制熔体结晶, 制备出非晶态聚苯硫醚块材, 样品的表面及中心都是非晶态. 非晶态聚苯硫醚的玻璃化转变温度和晶化温度分别为318和362 K. 常压下的退火实验表明, 非晶态聚苯硫醚在425 K等温结晶的产物为正交相晶型. 压致凝固法中熔体的凝固不是靠温度变化, 而是靠压力变化, 样品表面和内部处在一致的温度下同时受压凝固, 避免了热传导对非晶尺寸的影响, 因此非常有利于获得结构均匀的大尺寸非晶态材料.In this work, pressure-induced rapid solidification of polyphenylene sulfide (PPS) melt is studied on a pressure-jump apparatus. Five PPS samples under a pressure of 0.1 GPa are heated to 563 K, 573 K, 583 K, 603 K and 613 K, respectively. These samples are rapidly compressed to 2.4 GPa in about 20 ms. The solidified samples are quenched to room temperature and then depressured to ambient pressure. The X-ray diffraction (XRD) analyses of the recovered samples indicate that three PPS samples, prepared at 563 K, 573 K and 583 K, contain crystal phases but their crystallinity is lower than that of the original PPS powder. The remaining two PPS samples, prepared at 603 K and 613 K, are in amorphous state but do not sharp crystal diffraction peaks in the XRD patterns. Differential scanning calorimetry curves of the five PPS samples each display an endothermic step of glass transition at about 325 K and an exothermic peak of recrystallization around 360 K. The glass transition temperature decreases roughly with the increase of preparation temperature. The thermal enthalpy of recrystallization process increases with the increase of preparation temperature, indicating that the content of amorphous phase increases. We speculate that the recovered samples are in a “frozen state” of their parent liquid. At 563 K, 573 K and 583 K, the crystalline phases partially melt. More crystal phases melt with the increase of preparation temperature. The molten part is rapidly solidified into amorphous phase. At a temperature higher than 603 K, the crystalline phase fully melts, and after being rapidly compressed, amorphous PPS sample is obtained. For the amorphous PPS sample prepared at 613 K, we investigate whether the interior of this amorphous PPS sample is also in amorphous state. Micro XRD analysis indicates that the central part of the PPS sample is also in amorphous state, which suggests that this PPS sample is of a fully amorphous bulk. For the amorphous PPS sample prepared at 613 K, we investigate its recrystallization product. After being annealed at 425 K for 2 h, the amorphous phase, which is solidified from the melt of crystal phase, is recrystallized into the orthorhombic crystal phase. The results in this work indicate that the rapid compression can inhibit the PPS melt from being crystalized, so, it is a way to prepare amorphous PPS bulk. Since the solidification of polymer melt is realized by increasing pressure instead of quenching and is not limited by polymer thermal conductivity, it is a promising way to prepare amorphous polymer bulks with large size.
-
Keywords:
- pressure-induced solidification /
- amorphous polyphenylene sulfide /
- glass transition /
- crystallization
[1] 吕军 2006 博士学位论文 (成都: 四川大学)
Lü J 2006 Ph. D. Dissertation (Chengdu: Sichuan University) (in Chinese)
[2] Cheng S Z D, Wu Z Q, Wunderlich B 1987 Macromolecules 20 2802
 Google Scholar
Google Scholar
[3] Lu S X, Cebe P 1996 J. Appl. Polym. Sci. 61 473
 Google Scholar
Google Scholar
[4] Mao H K, Hemley R J 2007 Proc. Natl. Acad. Sci. U.S.A. 104 9114
 Google Scholar
Google Scholar
[5] 王彪, 万同, 曾威 2012 高分子通报 10 63
Wang B, Wan T, Zeng W 2012 Polym. Bull. 10 63
[6] Mei Z, Chung D D L 2000 Int. J. Adhes. Adhes. 20 273
 Google Scholar
Google Scholar
[7] Yang Y Q, Duan H J, Zhang G, Long S R, Yang J, Wang X J 2013 J. Polym. Res. 20 198
 Google Scholar
Google Scholar
[8] Lü J, Huang R, Oh I K 2007 Macromol. Chem. Phys. 208 405
 Google Scholar
Google Scholar
[9] Schultze J D, Böhning M, Springer J 1993 Makromol. Chem. 194 339
 Google Scholar
Google Scholar
[10] Lu S X, Cebe P, Capel M 1997 Macromolecules 30 6243
 Google Scholar
Google Scholar
[11] Lu S X, Cebe P 1996 Polymer 37 4857
 Google Scholar
Google Scholar
[12] Yang X T, Tang L, Guo Y Q, Liang C B, Zhang Q Y, Kou K C, Gu J W 2017 Composites Part A 101 237
 Google Scholar
Google Scholar
[13] Huo P, Cebe P 1992 Colloid. Polym. Sci. 270 840
 Google Scholar
Google Scholar
[14] Hong S M, Chen L Y, Liu X R, Wu X H, Su L 2005 Rev. Sci. Instrum. 76 053905
 Google Scholar
Google Scholar
[15] Liu X R, Zhang L J, Yuan C S, Jia R, Shao C G, Wang M Y, Hong S M 2018 Polymers 10 847
 Google Scholar
Google Scholar
[16] Liu X R, Jia R, Zhang D D, Yuan C S, Shao C G, Hong S M 2018 J. Phys. Condens. Matter 30 154001
 Google Scholar
Google Scholar
[17] Hong S M, Liu X R, Su L, Huang D H, Li L B 2006 J. Phys. D: Appl. Phys. 39 3684
 Google Scholar
Google Scholar
[18] Yuan C S, Hong S M, Li X X, Shen R, He Z, Lv S J, Liu X R, Lv J, Xi D K 2011 J. Phys. D: Appl. Phys. 44 165405
 Google Scholar
Google Scholar
[19] Zhang R C, Li R, Lu A, Jin Z J, Liu B Q, Xu Z B 2013 Polym. Int. 62 449
 Google Scholar
Google Scholar
[20] Zhang L J, Ren Y, Liu X R, Han F, Lutterodt K E, Wang H Y, He Y L, Wang J L, Zhao Y, Yang W G 2018 Sci. Rep. 8 4558
 Google Scholar
Google Scholar
-
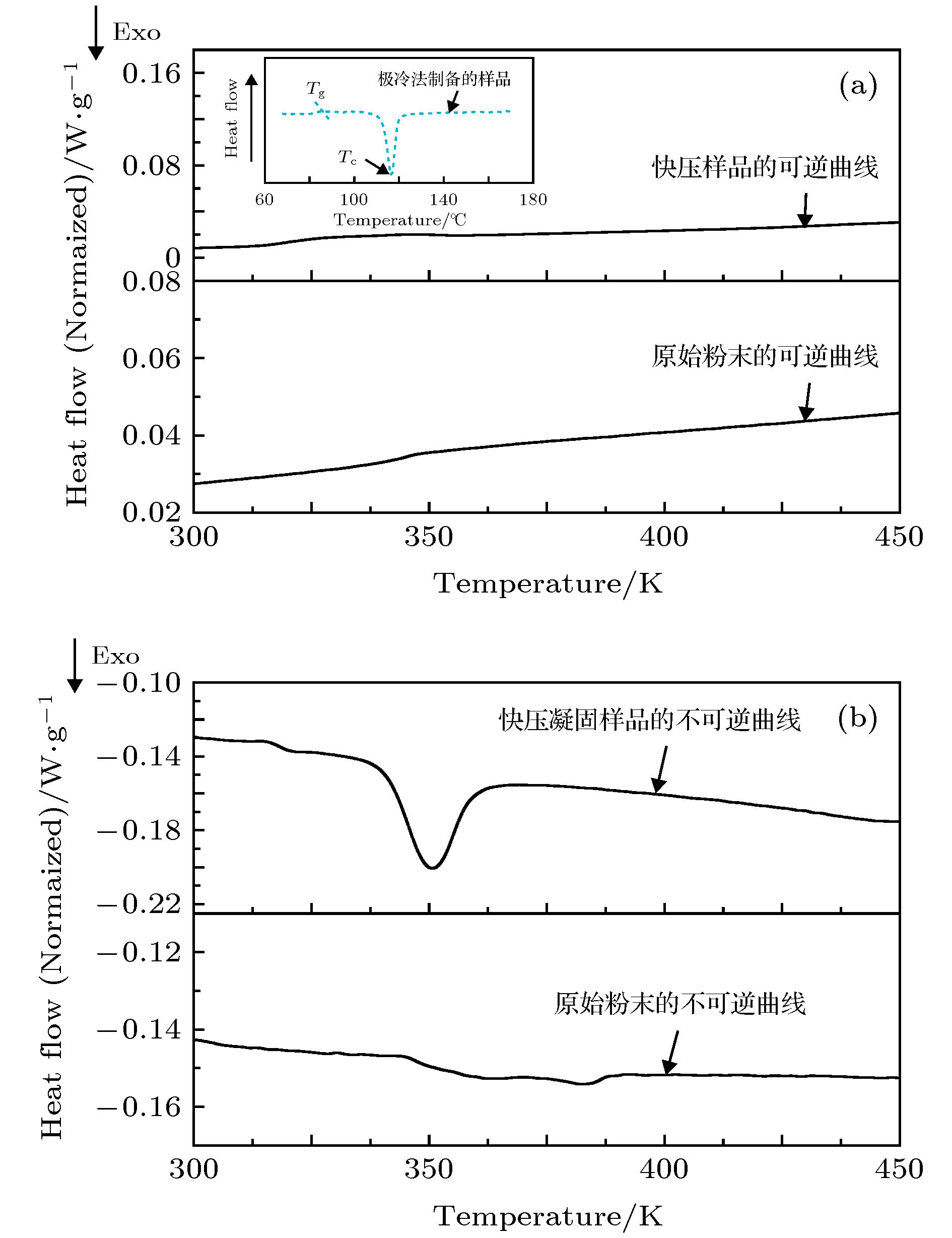
图 7 原始粉末和613 K温度下快压凝固的聚苯硫醚样品的MDSC曲线 (a)反映玻璃化转变的可逆信号(内插图为急冷法制备的聚苯硫醚非晶薄膜的DSC曲线[19]); (b)反映晶化过程的不可逆信号
Fig. 7. MDSC traces of PPS powder and amorphous PPS sample which is rapidly solidified at 613 K: (a) Reversible curve of glass transition; (b) irreversible curve of crystallization. The inset in panel (a) is the DSC trace of amorphous PPS which is solidified by rapidly quenching[19].
表 1 快压凝固的聚苯硫醚样品的热力学参数
Table 1. Thermal dynamics parameters of PPS samples which is solidified by rapid compression.
Sample No. Tg/K Tonset/K Tc/K Tend/K ΔHc/J·g–1 ΔT = Tonset – Tg/K 原始粉末 344.0 — — — — — 563 K快压凝固样品 320.3 354.0 363.0 379.0 10.4 33.7 573 K快压凝固样品 321.0 351.0 360.6 373.8 12.7 30.0 583 K快压凝固样品 320.5 352.0 360.6 368.5 20.8 31.5 603 K快压凝固样品 318.3 352.0 361.2 367.0 24.3 33.7 613 K快压凝固样品 318.0 350.0 362.0 367.8 27.0 32.0 急冷法非晶薄膜[19] 358.0 — 388.0 — — — 注: 玻璃化转变温度Tg; 结晶起始温度Tonset; 晶化峰峰值Tc; 结晶结束温度Tend; 晶化热焓ΔHc; 过冷液相区宽度ΔT. -
[1] 吕军 2006 博士学位论文 (成都: 四川大学)
Lü J 2006 Ph. D. Dissertation (Chengdu: Sichuan University) (in Chinese)
[2] Cheng S Z D, Wu Z Q, Wunderlich B 1987 Macromolecules 20 2802
 Google Scholar
Google Scholar
[3] Lu S X, Cebe P 1996 J. Appl. Polym. Sci. 61 473
 Google Scholar
Google Scholar
[4] Mao H K, Hemley R J 2007 Proc. Natl. Acad. Sci. U.S.A. 104 9114
 Google Scholar
Google Scholar
[5] 王彪, 万同, 曾威 2012 高分子通报 10 63
Wang B, Wan T, Zeng W 2012 Polym. Bull. 10 63
[6] Mei Z, Chung D D L 2000 Int. J. Adhes. Adhes. 20 273
 Google Scholar
Google Scholar
[7] Yang Y Q, Duan H J, Zhang G, Long S R, Yang J, Wang X J 2013 J. Polym. Res. 20 198
 Google Scholar
Google Scholar
[8] Lü J, Huang R, Oh I K 2007 Macromol. Chem. Phys. 208 405
 Google Scholar
Google Scholar
[9] Schultze J D, Böhning M, Springer J 1993 Makromol. Chem. 194 339
 Google Scholar
Google Scholar
[10] Lu S X, Cebe P, Capel M 1997 Macromolecules 30 6243
 Google Scholar
Google Scholar
[11] Lu S X, Cebe P 1996 Polymer 37 4857
 Google Scholar
Google Scholar
[12] Yang X T, Tang L, Guo Y Q, Liang C B, Zhang Q Y, Kou K C, Gu J W 2017 Composites Part A 101 237
 Google Scholar
Google Scholar
[13] Huo P, Cebe P 1992 Colloid. Polym. Sci. 270 840
 Google Scholar
Google Scholar
[14] Hong S M, Chen L Y, Liu X R, Wu X H, Su L 2005 Rev. Sci. Instrum. 76 053905
 Google Scholar
Google Scholar
[15] Liu X R, Zhang L J, Yuan C S, Jia R, Shao C G, Wang M Y, Hong S M 2018 Polymers 10 847
 Google Scholar
Google Scholar
[16] Liu X R, Jia R, Zhang D D, Yuan C S, Shao C G, Hong S M 2018 J. Phys. Condens. Matter 30 154001
 Google Scholar
Google Scholar
[17] Hong S M, Liu X R, Su L, Huang D H, Li L B 2006 J. Phys. D: Appl. Phys. 39 3684
 Google Scholar
Google Scholar
[18] Yuan C S, Hong S M, Li X X, Shen R, He Z, Lv S J, Liu X R, Lv J, Xi D K 2011 J. Phys. D: Appl. Phys. 44 165405
 Google Scholar
Google Scholar
[19] Zhang R C, Li R, Lu A, Jin Z J, Liu B Q, Xu Z B 2013 Polym. Int. 62 449
 Google Scholar
Google Scholar
[20] Zhang L J, Ren Y, Liu X R, Han F, Lutterodt K E, Wang H Y, He Y L, Wang J L, Zhao Y, Yang W G 2018 Sci. Rep. 8 4558
 Google Scholar
Google Scholar
计量
- 文章访问数: 10902
- PDF下载量: 93
- 被引次数: 0














 下载:
下载: