-
With the depletion of fossil fuels and the environmental problems, the development and utilization of new energy resources is imminent. Hydrogen energy is one of the main new energy sources in the 21st century. Finding stable and efficient hydrogen storage materials is the key to achieving the hydrogen economy. Transition metal (TM)-decorated graphenes have been widely studied as hydrogen storage materials theoretically, but they suffer metal agglomeration and H2 dissociation. Our calculations show that the reconstruction energy of Sc, Ti, V decorated pristine graphenes in the process of adsorption and desorption of hydrogen molecules are only 0.00, 0.12 and 0.08 eV, respectively. The adsorption energy values of the first H2 dissociation adsorption on the Sc, Ti, V decorated pristine graphenes are –1.34, –1.34, and –1.16 eV, respectively. So, some hydrogen molecules are difficult to desorb at room temperature and medium pressure. In this paper, the stability and hydrogen storage properties of Sc, Ti, V decorated monovacancy graphene are also investigated based on density functional theory. The results show that the binding energy values between Sc, Ti, V and themonovacancy graphene are –6.93, –8.82, –9.30 eV, respectively, which indicate monovacancy can effectively avoid metal aggregation. The Sc, Ti and V atoms decorated on the monovacancy graphene would transfer more electrons to the carbon material with charge of +1.24|e|–+1.37|e|. They can adsorb 7, 3 and 4 hydrogen molecules through electrostatic interaction. When a monovacancy is introduced, all of the hydrogen molecules are adsorbed in molecular form. The average adsorption energy values of H2 are –0.13, –0.20 and –0.18 eV, respectively, which are in the best energy range for the adsorption/desorption process at room temperature and medium pressure. The most important thing is that their deformations in the adsorption/desorption process are very small, which is conducive to the rapid hydrogen adsorption/desorption. The calculated results show that the monovacancy introduction can effectively solve the two major problems, i.e. metal agglomeration and hydrogen molecular dissociation during hydrogen storage on Sc, Ti, V decorated pristine graphenes. The research in this paper will be helpful to further understand the hydrogen storage mechanism of 3d TM-decorated carbon nanomaterials.
[1] Gomes I L R, Pousinho H M I, Melício R, Mendes V M F 2017 Energy 124 310
 Google Scholar
Google Scholar
[2] Niaz S, Manzoor T, Pandith A H 2015 Renew. Sust. Energy Rev. 50 457
 Google Scholar
Google Scholar
[3] Møller K T, Jensen T R, Akiba E, Li H W 2017 Prog. Nat. Sci. :Mater. Int. 27 34
 Google Scholar
Google Scholar
[4] Hirscher M, Yartys V A, Baricco M, et al. 2020 J. Alloys Compd. 827 153548
 Google Scholar
Google Scholar
[5] Hanley E S, Deane J P, Gallachóir B P Ó 2018 Renew. Sust. Energy Rev. 82 3027
 Google Scholar
Google Scholar
[6] Satyapal S, Petrovic J, Read C, Thomas G, Ordaz G 2007 Catal. Today 120 246
 Google Scholar
Google Scholar
[7] Bellosta von Colbe J, Ares J R, Barale J, et al. 2019 Int. J. Hydrog. Energy 44 7780
 Google Scholar
Google Scholar
[8] Moradi R, Groth K M 2019 Int. J. Hydrog. Energy 44 12254
 Google Scholar
Google Scholar
[9] Shiraz H G, Tavakoli O 2017 Renew. Sust. Energ. Rev. 74 104
 Google Scholar
Google Scholar
[10] Kubas G J 2001 J. Organomet. Chem. 635 37
 Google Scholar
Google Scholar
[11] Niu J, Rao B K, Jena P 1992 Phys. Rev. Lett. 68 2277
 Google Scholar
Google Scholar
[12] Jena P 2011 J. Phys. Chem. Lett. 2 206
 Google Scholar
Google Scholar
[13] Mananghaya M, Yu D, Santos G N, Rodulfo E 2016 Sci. Rep. 6 27370
 Google Scholar
Google Scholar
[14] Guo Y, Lan X, Cao J, Xu B, Xia Y, Yin J, Liu Z 2013 Int. J. Hydrog. Energy 38 3987
 Google Scholar
Google Scholar
[15] Sun Q, Wang Q, Jena P, Kawazoe Y 2005 J. Am. Chem. Soc. 127 14582
 Google Scholar
Google Scholar
[16] Valencia H, Gil A, Frapper G 2015 J. Phys. Chem. C 119 5506
 Google Scholar
Google Scholar
[17] Ma L J, Wang J, Han M, Jia J, Wu H S, Zhang X 2019 Energy 171 315
 Google Scholar
Google Scholar
[18] Dixit M, AditMaark T, Ghatak K, Ahuja R, Pal S 2012 J. Phys. Chem. C 116 17336
 Google Scholar
Google Scholar
[19] Manade M, Vines F, Gil A, Illas F 2018 Phys. Chem. Chem. Phys. 20 3819
[20] Guo J, Liu Z, Liu S, Zhao X, HuangK 2011 Appl. Phys. Lett. 98 023107
 Google Scholar
Google Scholar
[21] Zhai Y, Dou Y, Zhao D, Fulvio P F, Mayes R T, Dai S 2011 Adv. Mater. 23 4828
 Google Scholar
Google Scholar
[22] Seenithurai S, Pandyan R K, Kumar S V, Saranya C, Mahendran M 2014 Int. J. Hydrog. Energy 39 11016
 Google Scholar
Google Scholar
[23] Zhou Y, Chu W, Jing F, Zheng J, Sun W, Xue Y 2017 Appl. Surf. Sci. 410 166
 Google Scholar
Google Scholar
[24] Choudhary A, Malakkal L, Siripurapu R K, Szpunar B, Szpunar J 2016 Int. J. Hydrog. Energy 41 17652
 Google Scholar
Google Scholar
[25] Luo Z, Fan X, Pan R, An Y 2017 Int. J. Hydrog. Energy 42 3106
 Google Scholar
Google Scholar
[26] KimG, Jhi S H, Lim S, Park N 2009 Appl. Phys. Lett. 94 173102
 Google Scholar
Google Scholar
[27] Baby A, Trovato L, Valentin C D 2021 Carbon 174 772
 Google Scholar
Google Scholar
[28] Ma S, Chen J, Wang L, Jiao Z 2020 Appl. Surf. Sci. 504 144413
 Google Scholar
Google Scholar
[29] Kresse G, FurthmüLler J 1996 Phys. Rev. B 54 11169
 Google Scholar
Google Scholar
[30] Perdew J P, Burke K, Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[31] Blochl P E 1994 Phys. Rev. B 50 17953
 Google Scholar
Google Scholar
[32] Chadi D J 1977 Phys. Rev. B 16 1746
 Google Scholar
Google Scholar
[33] Grimme S, Antony J, Ehrlich S, Krieg H 2010 J. Chem. Phys. 132 154104
 Google Scholar
Google Scholar
[34] Grimme S, Ehrlich S, Goerigk L 2011 J. Comput. Chem. 32 1456
 Google Scholar
Google Scholar
[35] Manadé M, Viñes F, Illas F 2015 Carbon 95 525
 Google Scholar
Google Scholar
[36] Krasheninnikov A V, Lehtinen P O, Foster A S, Pyykko P, Nieminen R M 2009 Phys. Rev. Lett. 102 126807
 Google Scholar
Google Scholar
[37] Charles K 2019 Introduction To Solid State Physics (8th Ed.) (Hoboken: Wiley)
[38] Mashoff T, Takamura M, Tanabe S, Hibino H, Beltram F, Heun S 2013 Appl. Phys. Lett. 103 013903
 Google Scholar
Google Scholar
[39] Yildirim T, Ciraci S 2005 Phys. Rev. Lett. 94 175501
 Google Scholar
Google Scholar
[40] Yildirim T, Ĩñiguez Jorge, Ciraci S 2005 Phys. Rev. B 72 153403
 Google Scholar
Google Scholar
[41] Chu S, Hu L, Hu X, Yang M, Deng J 2011 Int. J. Hydrog. Energy 36 12324
 Google Scholar
Google Scholar
[42] Ma L J, Jia J, Wu H S 2015 Int. J. Hydrog. Energy 40 420
 Google Scholar
Google Scholar
[43] Granja-DelRío A, Alonso J A, Lopez M J 2017 J. Phys. Chem. C 121 10843
 Google Scholar
Google Scholar
[44] Zhu J, Huang Y, Mei W, Zhao C, Zhang C, Zhang J, Amiinu I S, Mu S 2019 Angew. Chem. Int. Ed. 58 3859
 Google Scholar
Google Scholar
[45] Qiao B, Wang A, Yang X, Allard L F, Jiang Z, Cui Y, Liu J, Li J, Zhang T 2011 Nat. Chem. 3 634
 Google Scholar
Google Scholar
-
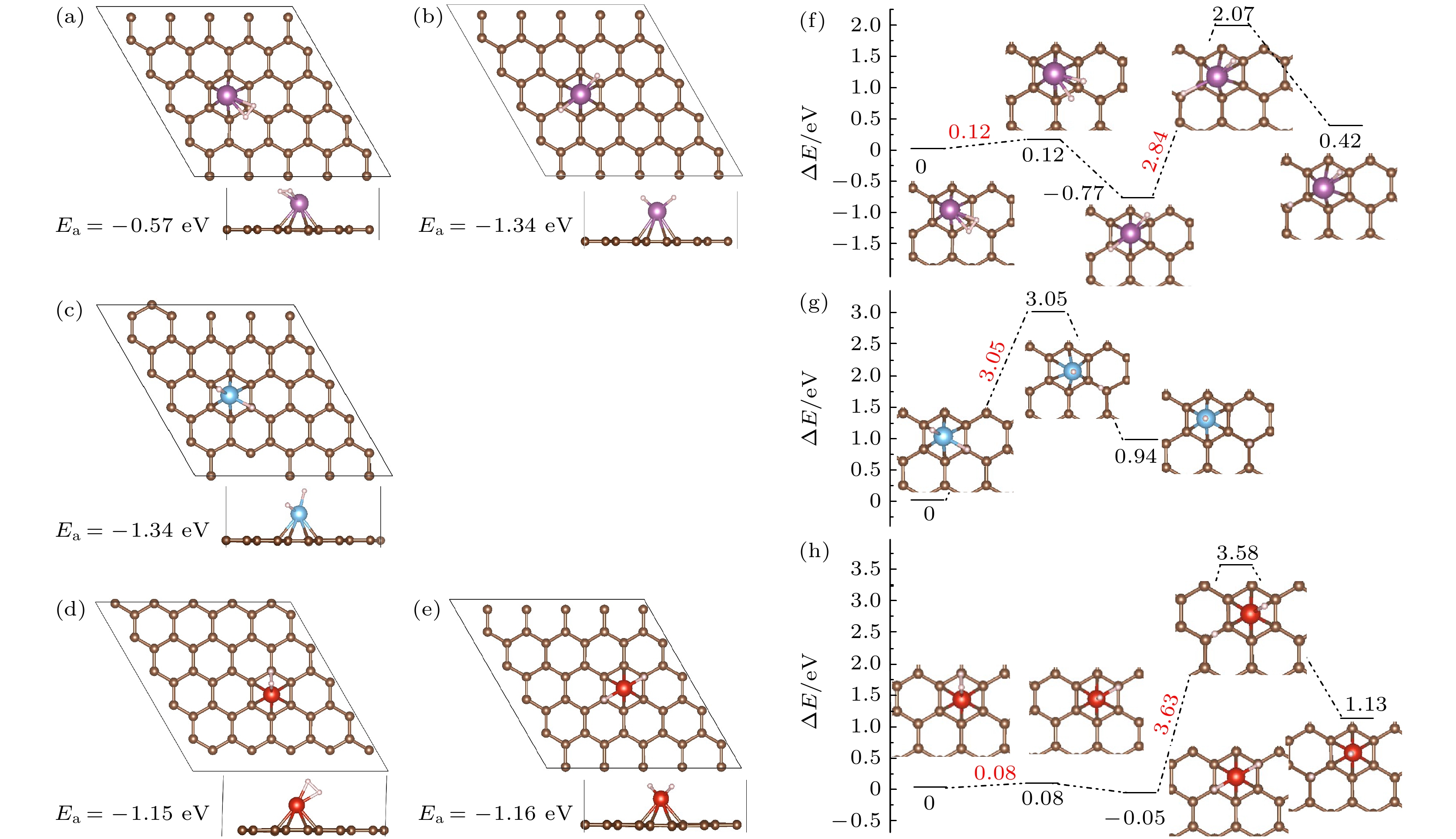
图 3 Sc, Ti, V修饰PG吸附1个氢分子的优化结构(a)−(e)及H2解离、H原子迁移势能面图(f)−(h) (紫色小球代表Sc, 蓝色小球代表Ti, 红色小球代表V) (a), (b), (f) Sc/PG; (c), (g) Ti/PG; (d), (e), (h) Sc/PG
Figure 3. (a)−(e) Optimized structures of 1H2-TM/PG (TM = Sc, Ti, V) and (f)−(h) the schematic diagrams of dissociation of H2 and the H migration process (Sc, Ti, and V atoms are represented by purple, blue and red balls): (a), (b), (f) Sc/PG; (c), (g) Ti/PG; (d), (e), (h) Sc/PG.
图 5 (a) H2-Sc/MVG, (b) 2H-Sc/MVG, (c) H2-V/MVG, (d) 2H-V/MVG的电荷差分密度图, 黄色部分表示电荷增加, 青色部分表示电荷减少, 界面值为0.003 e/Å3
Figure 5. Charge density difference of (a) H2-Sc/MVG, (b) 2H-Sc/MVG, (c) H-V/MVG, and (d) 2H-V/MVG. The charge accumulation and depletion regions are indicated by yellow and blue, respectively. The isosurface value is 0.003 e/Å3 .
图 6 (a) H2-Sc/MVG, (b) 2H-Sc/MVG, (c) H2-V/MVG, (d) 2H-V/MVG的DOS图和主要的轨道图(黄色部分表示正相位, 青色部分表示负相位, 等值面为1.5 × 10–7)
Figure 6. Main orbitals and PDOS of (a) H2-Sc/PG, (b) 2H-Sc/PG, (c) H2-V/PG, (d) 2H-V/PG. The positive and negative phases are indicated by yellow and blue regions, respectively. The isosurface is 1.5 × 10–7.
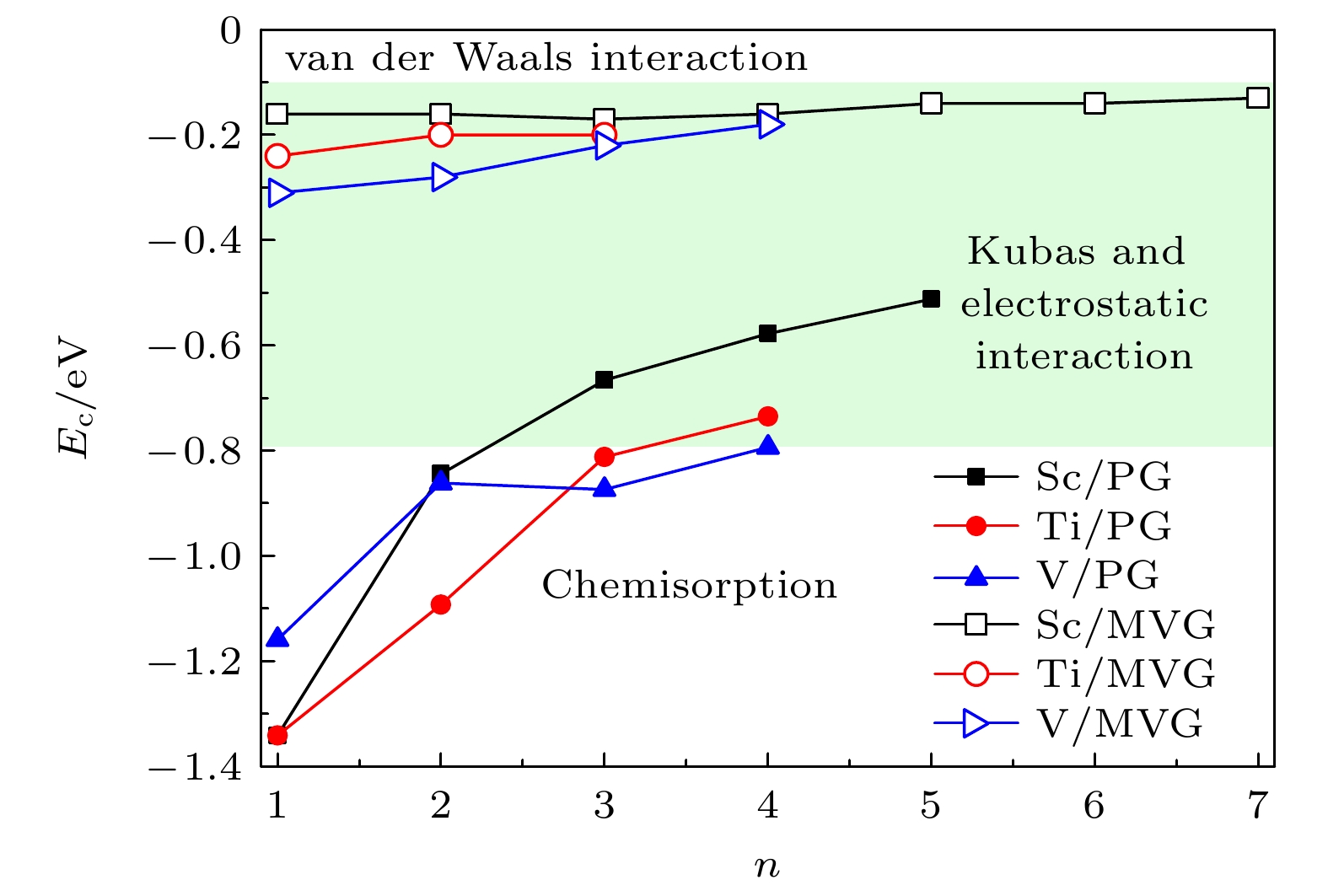
图 8 Sc, Ti, V修饰完整和MVG的平均氢分子吸附能随H2分子数量的变化曲线, 其中–0.10 eV至–0.80 eV之间的绿色区域为最佳H2存储的能量窗口
Figure 8. Variation in the average binding energies per H2 molecule for TM/MVG (TM = Sc, Ti, V) as a function of the number of H2 molecules. The green region from –0.10 eV to –0.80 eV represents the energetic window for an optimal H2 storage.
表 1 Sc, Ti, V修饰PG和MVG的相关参数 (d为TM与石墨烯平面的距离; Δz为TM修饰前后, 基底在z方向的最大 形变量)
Table 1. Relevant parameters of Sc, Ti and V decorated PG and MVG (d is distance between TM and graphene plane; Δz is the maximum deformation in the z direction of the substrate before and after TM modification).
System TM cohesive nenergy[37]
(eV/atom)Eb/eV dTM—C/Å d/Å Δz/Å Charge/|e| Sc/PG –3.90 –1.60 2.35—2.36 1.78 +1.09 Ti/PG –4.85 –1.81 2.12—2.37 1.63 +1.06 V/PG –5.31 –2.40 2.08—2.31 1.52 +1.05 Sc/MVG –3.90 –6.93 2.07—2.08 1.96 0.58 +1.37 Ti/MVG –4.85 –8.82 1.84—2.14 1.67 0.52 +1.37 V/MVG –5.31 –9.30 1.77—2.07 1.47 0.41 +1.24 表 2 1H2-TM/MVG (TM = Sc, Ti, V)的氢分子吸附能(Ea)、重构能(ER)、化学键能(E1)及TM的电荷
Table 2. Hydrogen adsorption (Ea), structure reconstruction energy (ER), the chemical bond energy (E1) of 1H2-TM/MVG (TM = Sc, Ti, V), and chargesof TM(Q|).
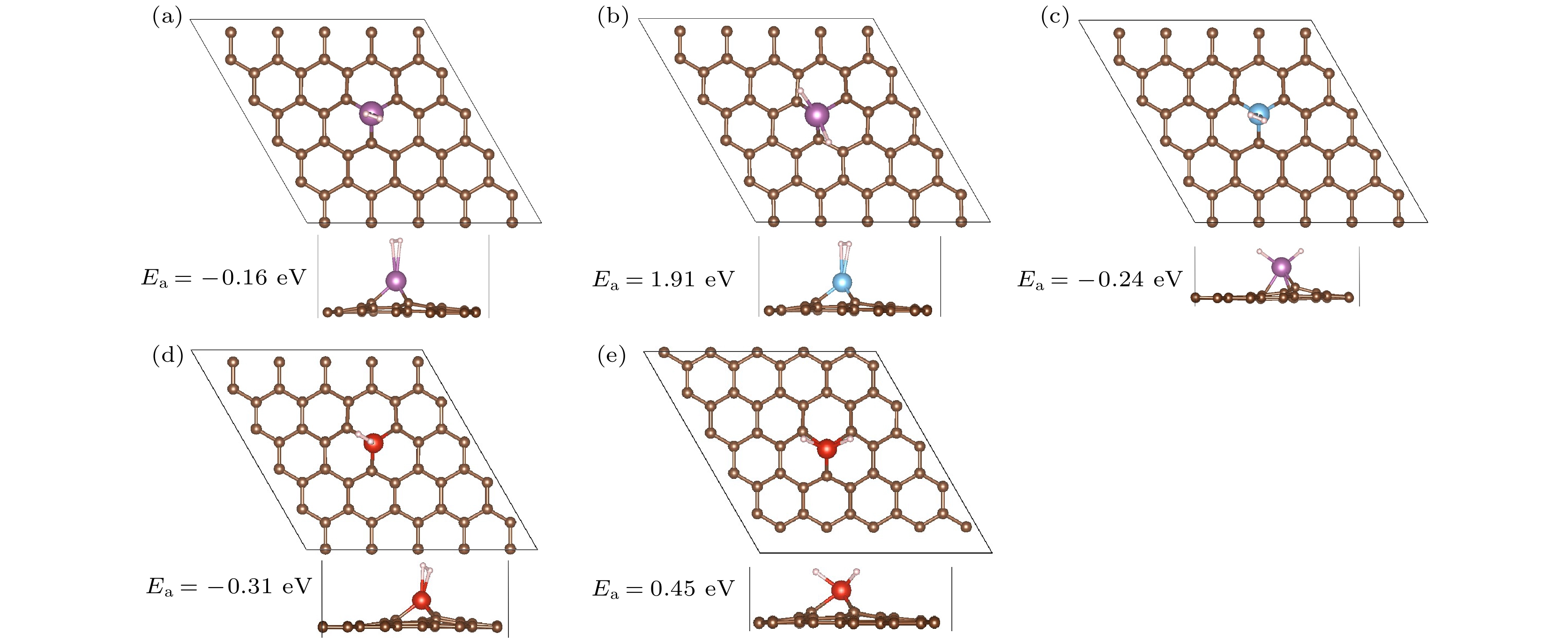
Syestem Ea/eV ER/eV E1/eV Q/|e| System Ea/eV ER/eV E1/eV Q/|e| 1H2-Sc/MVG –0.16 0.00 –0.16 1.38 2H-Sc/MVG 1.91 2.55 –0.64 1.58 1H2-Ti/MVG –0.24 0.51 –0.75 1.37 1H2-V/MVG –0.31 0.03 –0.34 1.23 2H-V/MVG 0.45 1.28 –0.83 1.39 表 3 TM/MVG (TM = Sc, Ti, V)吸附多个氢分子的连续吸附能(Ec/eV)、平均吸附能(Ea/eV)以及H—H/Sc—H键长
Table 3. Stepwise H2 adsorption energy (Ec in eV), the average H2 binding energies (Ea in eV), and bond lengths of H—H and Sc—H in TM/MVG (TM = Sc, Ti, V).
nH2-Sc/MVG Ec/eV Ea/eV 键长/Å H—H Sc—H n = 1 –0.16 –0.16 0.76 2.53—2.54 n = 2 –0.16 –0.16 0.75—0.76 2.42—2.58 n = 3 –0.20 –0.17 0.76—0.77 2.39—2.57 n = 4 –0.12 –0.16 0.75—0.76 2.45—2.96 n = 5 –0.08 –0.14 0.75—0.76 2.67—3.19 n = 6 –0.13 –0.14 0.75—0.76 2.67—3.68 n = 7 –0.09 –0.13 0.75—0.76 2.60—3.89 nH2-Ti/MVG Ec/eV Ea/eV 键长/Å H—H Ti—H n = 1 –0.24 –0.24 0.78 2.30—2.31 n = 2 –0.16 –0.20 0.75—0.78 2.14—2.32 n = 3 –0.20 –0.20 0.75—0.81 2.10—2.52 nH2-V/MVG Ec/eV Ea/eV 键长/Å H—H V—H n = 1 –0.31 –0.31 0.79 1.93—2.01 n = 2 –0.15 –0.24 0.76—0.82 2.07—2.18 n = 3 –0.10 –0.19 0.77—0.82 1.93—3.36 n = 4 –0.06 –0.18 0.75—0.86 2.14—4.03 -
[1] Gomes I L R, Pousinho H M I, Melício R, Mendes V M F 2017 Energy 124 310
 Google Scholar
Google Scholar
[2] Niaz S, Manzoor T, Pandith A H 2015 Renew. Sust. Energy Rev. 50 457
 Google Scholar
Google Scholar
[3] Møller K T, Jensen T R, Akiba E, Li H W 2017 Prog. Nat. Sci. :Mater. Int. 27 34
 Google Scholar
Google Scholar
[4] Hirscher M, Yartys V A, Baricco M, et al. 2020 J. Alloys Compd. 827 153548
 Google Scholar
Google Scholar
[5] Hanley E S, Deane J P, Gallachóir B P Ó 2018 Renew. Sust. Energy Rev. 82 3027
 Google Scholar
Google Scholar
[6] Satyapal S, Petrovic J, Read C, Thomas G, Ordaz G 2007 Catal. Today 120 246
 Google Scholar
Google Scholar
[7] Bellosta von Colbe J, Ares J R, Barale J, et al. 2019 Int. J. Hydrog. Energy 44 7780
 Google Scholar
Google Scholar
[8] Moradi R, Groth K M 2019 Int. J. Hydrog. Energy 44 12254
 Google Scholar
Google Scholar
[9] Shiraz H G, Tavakoli O 2017 Renew. Sust. Energ. Rev. 74 104
 Google Scholar
Google Scholar
[10] Kubas G J 2001 J. Organomet. Chem. 635 37
 Google Scholar
Google Scholar
[11] Niu J, Rao B K, Jena P 1992 Phys. Rev. Lett. 68 2277
 Google Scholar
Google Scholar
[12] Jena P 2011 J. Phys. Chem. Lett. 2 206
 Google Scholar
Google Scholar
[13] Mananghaya M, Yu D, Santos G N, Rodulfo E 2016 Sci. Rep. 6 27370
 Google Scholar
Google Scholar
[14] Guo Y, Lan X, Cao J, Xu B, Xia Y, Yin J, Liu Z 2013 Int. J. Hydrog. Energy 38 3987
 Google Scholar
Google Scholar
[15] Sun Q, Wang Q, Jena P, Kawazoe Y 2005 J. Am. Chem. Soc. 127 14582
 Google Scholar
Google Scholar
[16] Valencia H, Gil A, Frapper G 2015 J. Phys. Chem. C 119 5506
 Google Scholar
Google Scholar
[17] Ma L J, Wang J, Han M, Jia J, Wu H S, Zhang X 2019 Energy 171 315
 Google Scholar
Google Scholar
[18] Dixit M, AditMaark T, Ghatak K, Ahuja R, Pal S 2012 J. Phys. Chem. C 116 17336
 Google Scholar
Google Scholar
[19] Manade M, Vines F, Gil A, Illas F 2018 Phys. Chem. Chem. Phys. 20 3819
[20] Guo J, Liu Z, Liu S, Zhao X, HuangK 2011 Appl. Phys. Lett. 98 023107
 Google Scholar
Google Scholar
[21] Zhai Y, Dou Y, Zhao D, Fulvio P F, Mayes R T, Dai S 2011 Adv. Mater. 23 4828
 Google Scholar
Google Scholar
[22] Seenithurai S, Pandyan R K, Kumar S V, Saranya C, Mahendran M 2014 Int. J. Hydrog. Energy 39 11016
 Google Scholar
Google Scholar
[23] Zhou Y, Chu W, Jing F, Zheng J, Sun W, Xue Y 2017 Appl. Surf. Sci. 410 166
 Google Scholar
Google Scholar
[24] Choudhary A, Malakkal L, Siripurapu R K, Szpunar B, Szpunar J 2016 Int. J. Hydrog. Energy 41 17652
 Google Scholar
Google Scholar
[25] Luo Z, Fan X, Pan R, An Y 2017 Int. J. Hydrog. Energy 42 3106
 Google Scholar
Google Scholar
[26] KimG, Jhi S H, Lim S, Park N 2009 Appl. Phys. Lett. 94 173102
 Google Scholar
Google Scholar
[27] Baby A, Trovato L, Valentin C D 2021 Carbon 174 772
 Google Scholar
Google Scholar
[28] Ma S, Chen J, Wang L, Jiao Z 2020 Appl. Surf. Sci. 504 144413
 Google Scholar
Google Scholar
[29] Kresse G, FurthmüLler J 1996 Phys. Rev. B 54 11169
 Google Scholar
Google Scholar
[30] Perdew J P, Burke K, Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[31] Blochl P E 1994 Phys. Rev. B 50 17953
 Google Scholar
Google Scholar
[32] Chadi D J 1977 Phys. Rev. B 16 1746
 Google Scholar
Google Scholar
[33] Grimme S, Antony J, Ehrlich S, Krieg H 2010 J. Chem. Phys. 132 154104
 Google Scholar
Google Scholar
[34] Grimme S, Ehrlich S, Goerigk L 2011 J. Comput. Chem. 32 1456
 Google Scholar
Google Scholar
[35] Manadé M, Viñes F, Illas F 2015 Carbon 95 525
 Google Scholar
Google Scholar
[36] Krasheninnikov A V, Lehtinen P O, Foster A S, Pyykko P, Nieminen R M 2009 Phys. Rev. Lett. 102 126807
 Google Scholar
Google Scholar
[37] Charles K 2019 Introduction To Solid State Physics (8th Ed.) (Hoboken: Wiley)
[38] Mashoff T, Takamura M, Tanabe S, Hibino H, Beltram F, Heun S 2013 Appl. Phys. Lett. 103 013903
 Google Scholar
Google Scholar
[39] Yildirim T, Ciraci S 2005 Phys. Rev. Lett. 94 175501
 Google Scholar
Google Scholar
[40] Yildirim T, Ĩñiguez Jorge, Ciraci S 2005 Phys. Rev. B 72 153403
 Google Scholar
Google Scholar
[41] Chu S, Hu L, Hu X, Yang M, Deng J 2011 Int. J. Hydrog. Energy 36 12324
 Google Scholar
Google Scholar
[42] Ma L J, Jia J, Wu H S 2015 Int. J. Hydrog. Energy 40 420
 Google Scholar
Google Scholar
[43] Granja-DelRío A, Alonso J A, Lopez M J 2017 J. Phys. Chem. C 121 10843
 Google Scholar
Google Scholar
[44] Zhu J, Huang Y, Mei W, Zhao C, Zhang C, Zhang J, Amiinu I S, Mu S 2019 Angew. Chem. Int. Ed. 58 3859
 Google Scholar
Google Scholar
[45] Qiao B, Wang A, Yang X, Allard L F, Jiang Z, Cui Y, Liu J, Li J, Zhang T 2011 Nat. Chem. 3 634
 Google Scholar
Google Scholar
Catalog
Metrics
- Abstract views: 8054
- PDF Downloads: 136
- Cited By: 0















 DownLoad:
DownLoad: