-
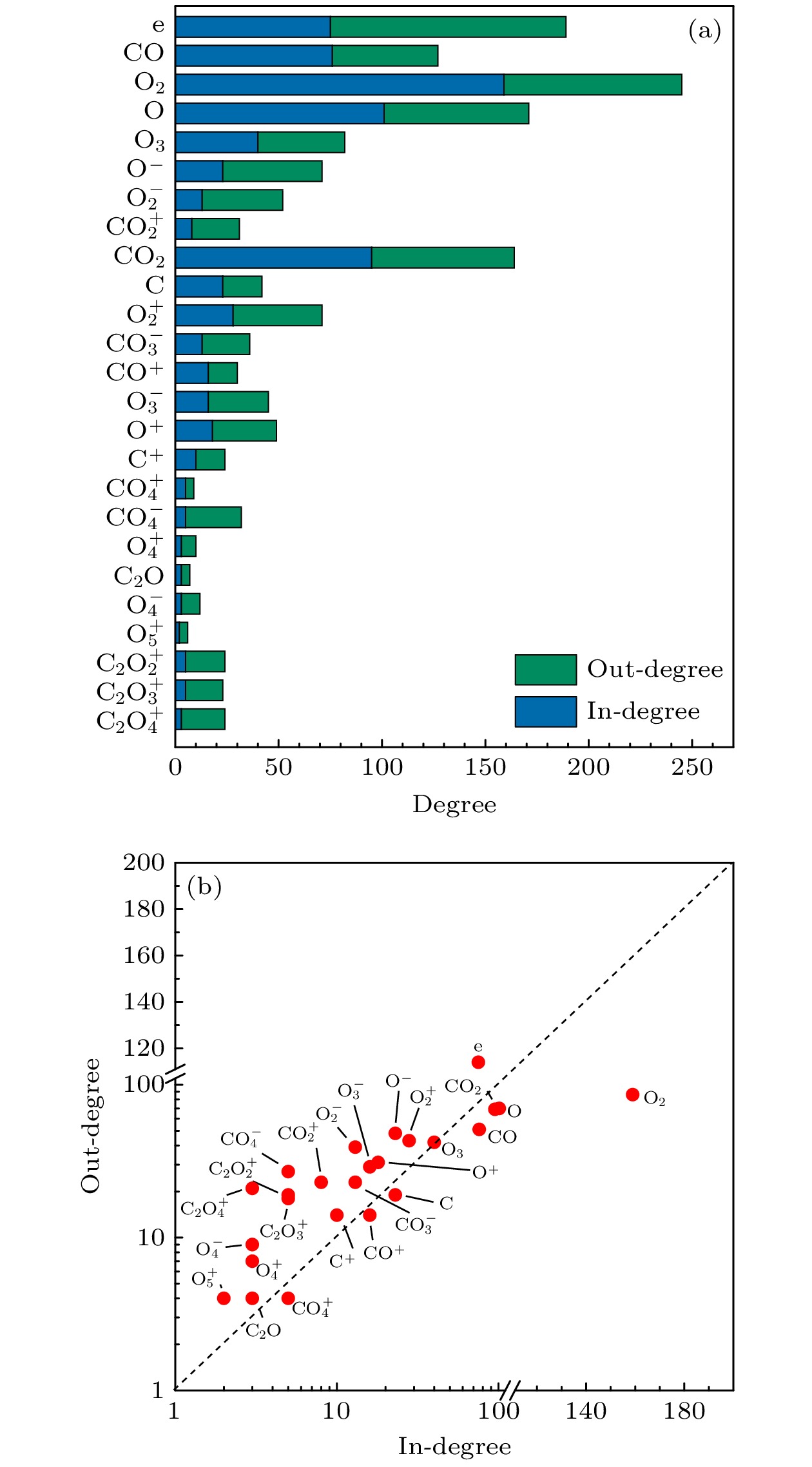
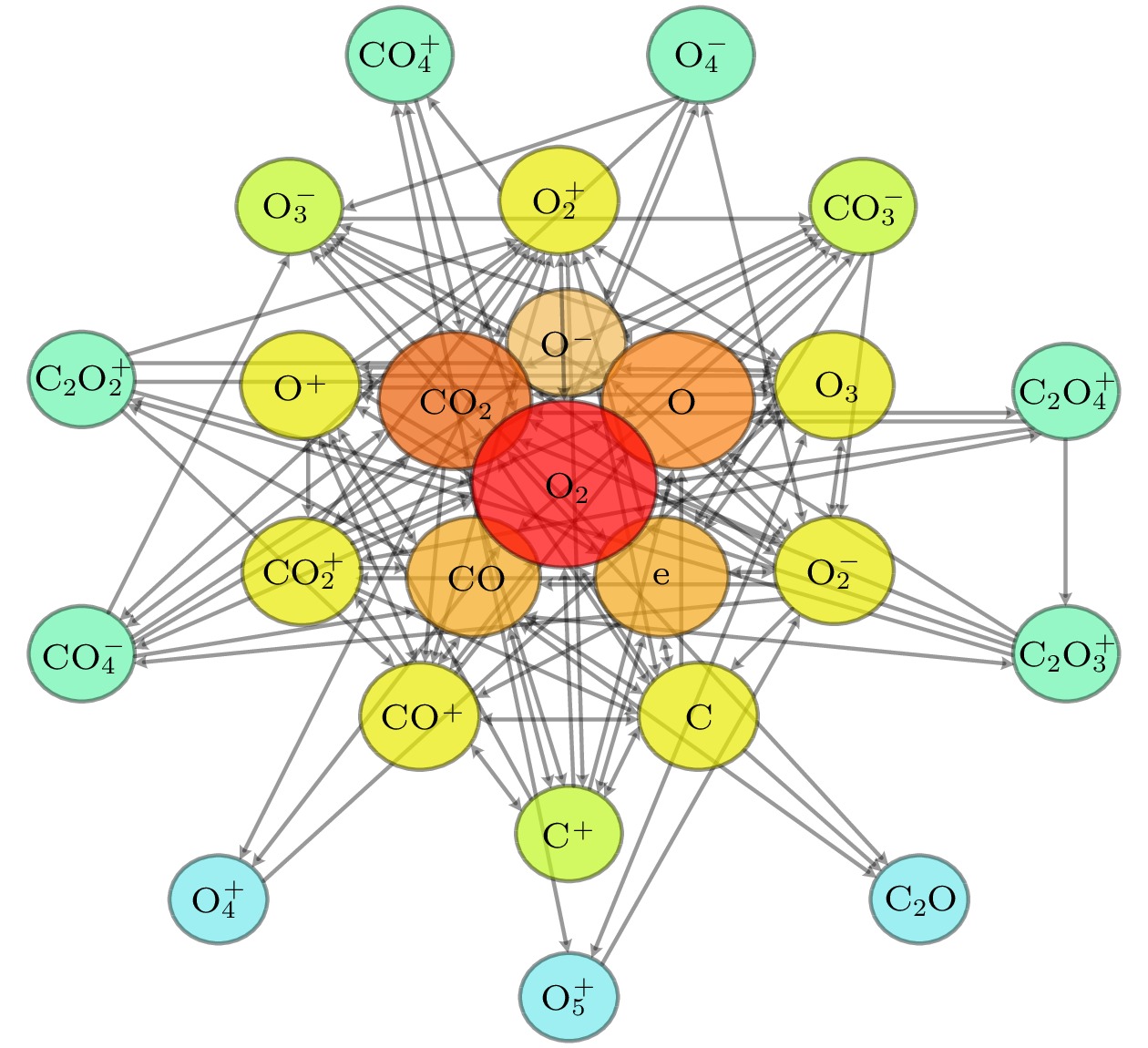
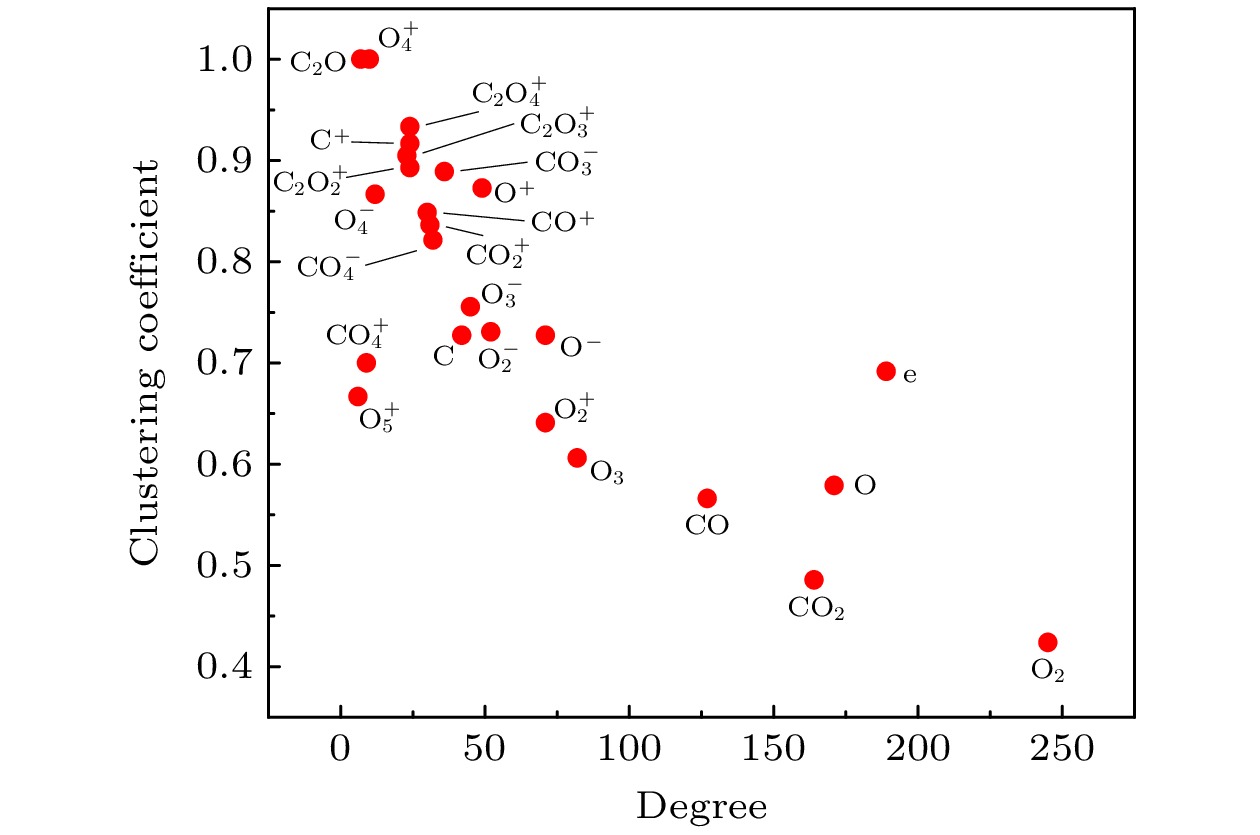
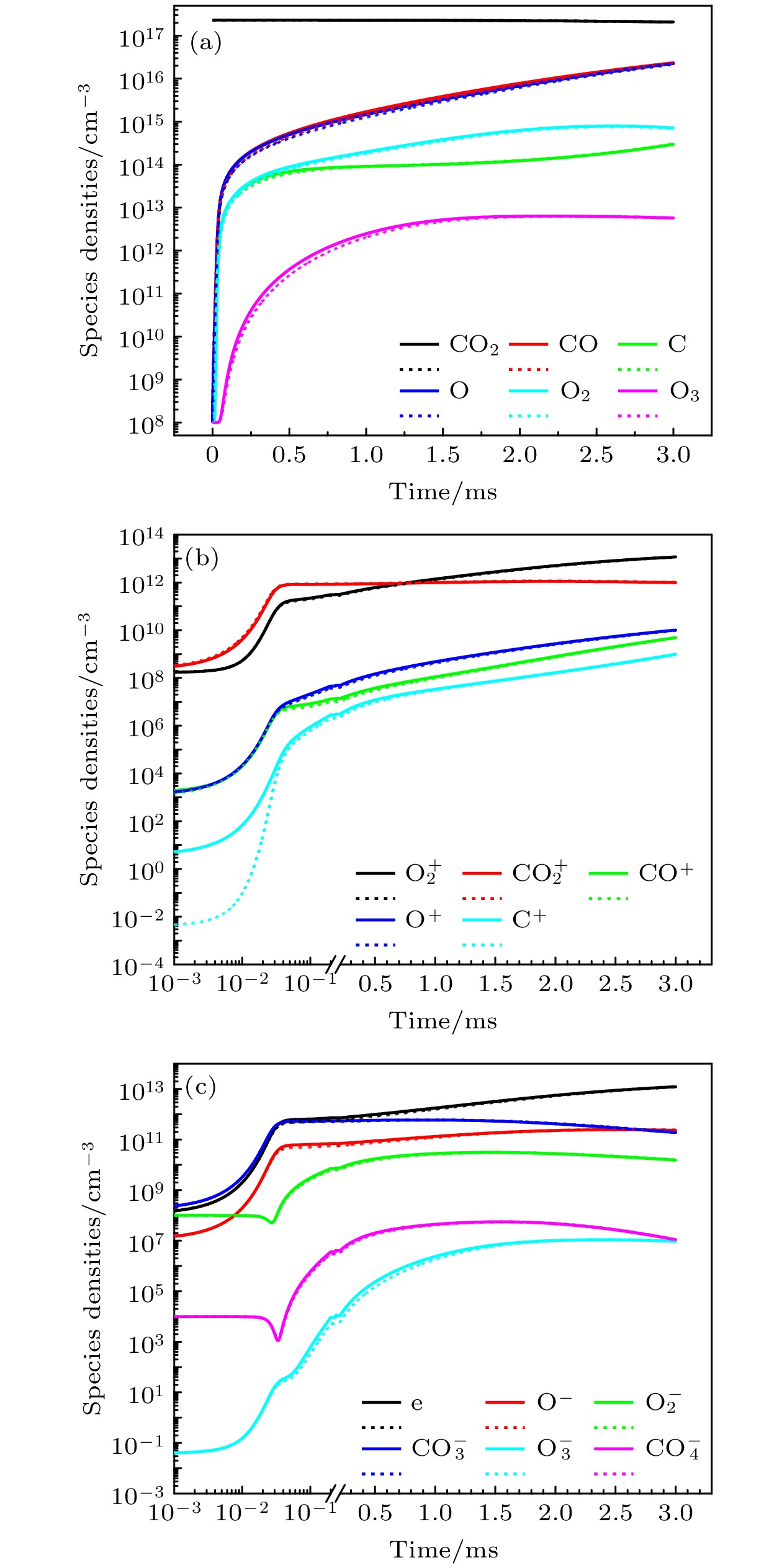
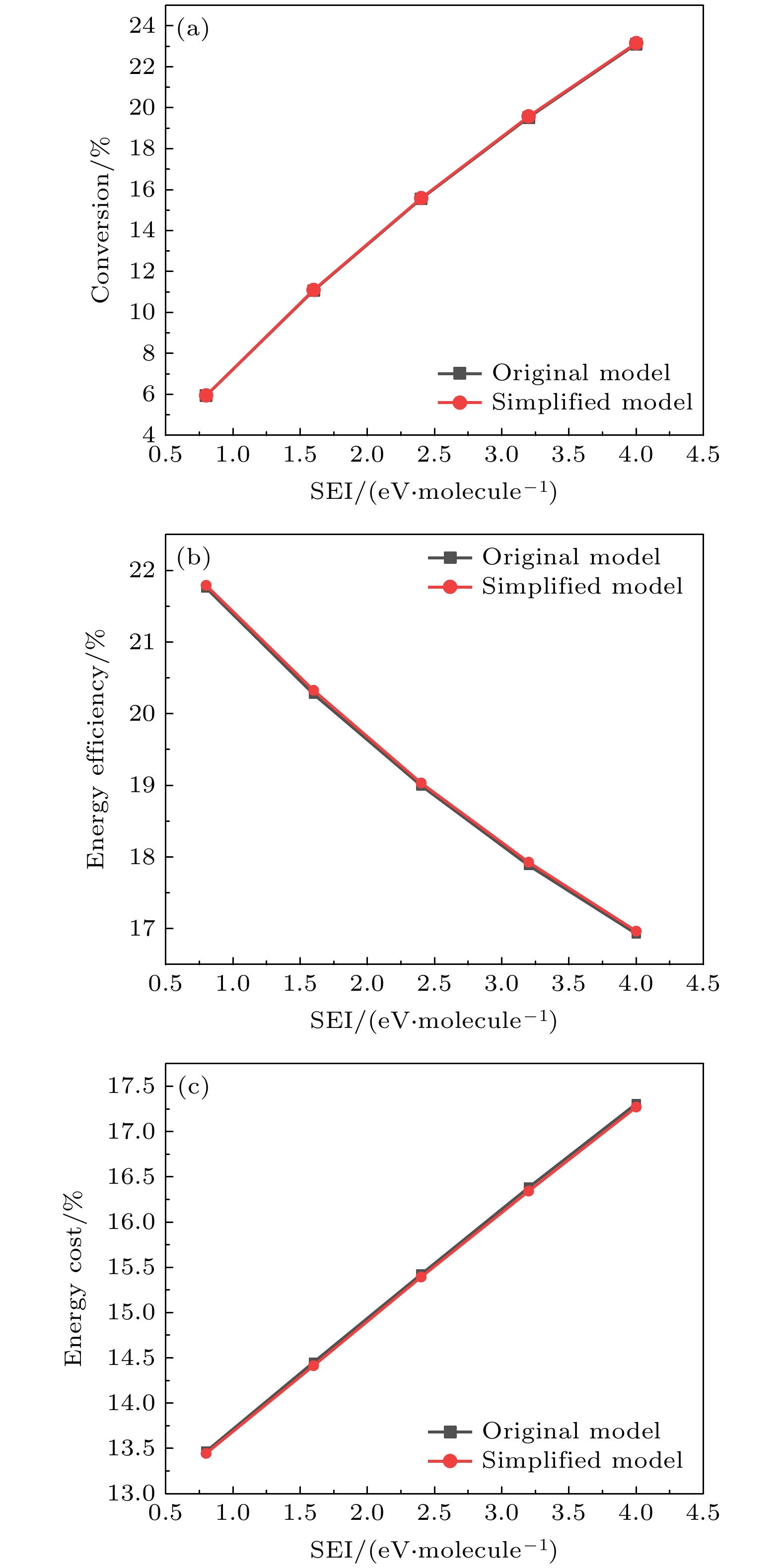
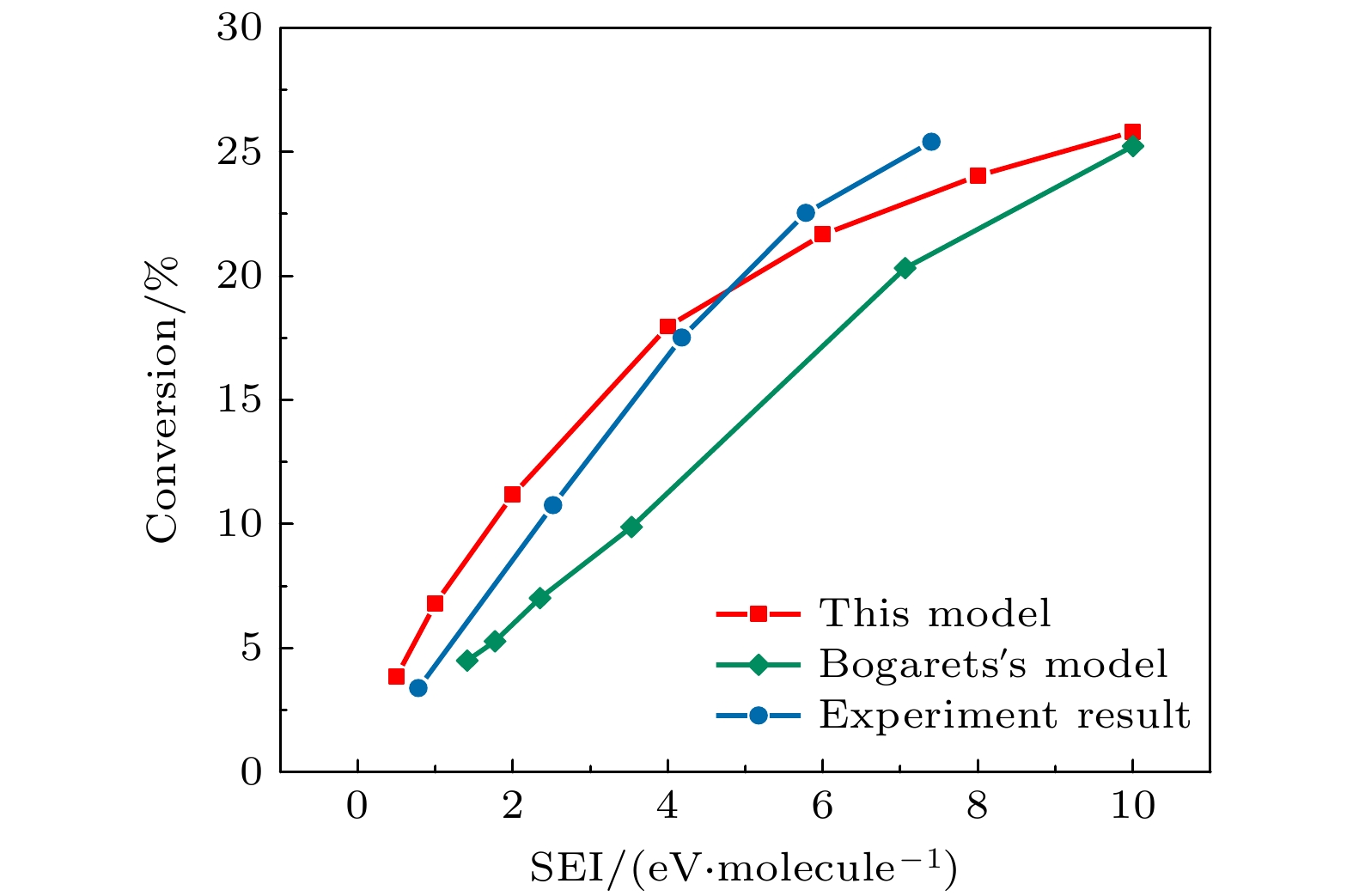
The exploration of Mars has attracted increasing interest in these years. The experiments and simulations show that strong electric field triggered by the dust storms in the Martian atmosphere may cause CO2 discharge. Studies on this phenomenon will not only help deepen our comprehension on the evolution of Martian surface, but also provide a possibility to realize the in-situ oxygen generation on Mars based on plasma chemistry. In this paper, a zero-dimensional global model is used to simplify the complicated description of CO2 chemical kinetics, therefore a reduced chemistry can be obtained for detailed numerical simulation in the near future. At the beginning of simplification, the graph theoretical analysis is used to pre-treat the original model by exploring the relationship between reacting species. Through the study of connectivity and the topological network, species such as O2, e, and CO prove to be important in the information transmission of the network. While gaining a clearer understanding of the chemistry model, dependence analysis will be used to investigate numerical simulation results. In this way a directed relation diagram can be obtained, where the influence between different species is quantitively explained in terms of numerical solutions. Users could keep different types of species correspondingly according to their own needs, and in this paper, some species with low activeness such as C2O,
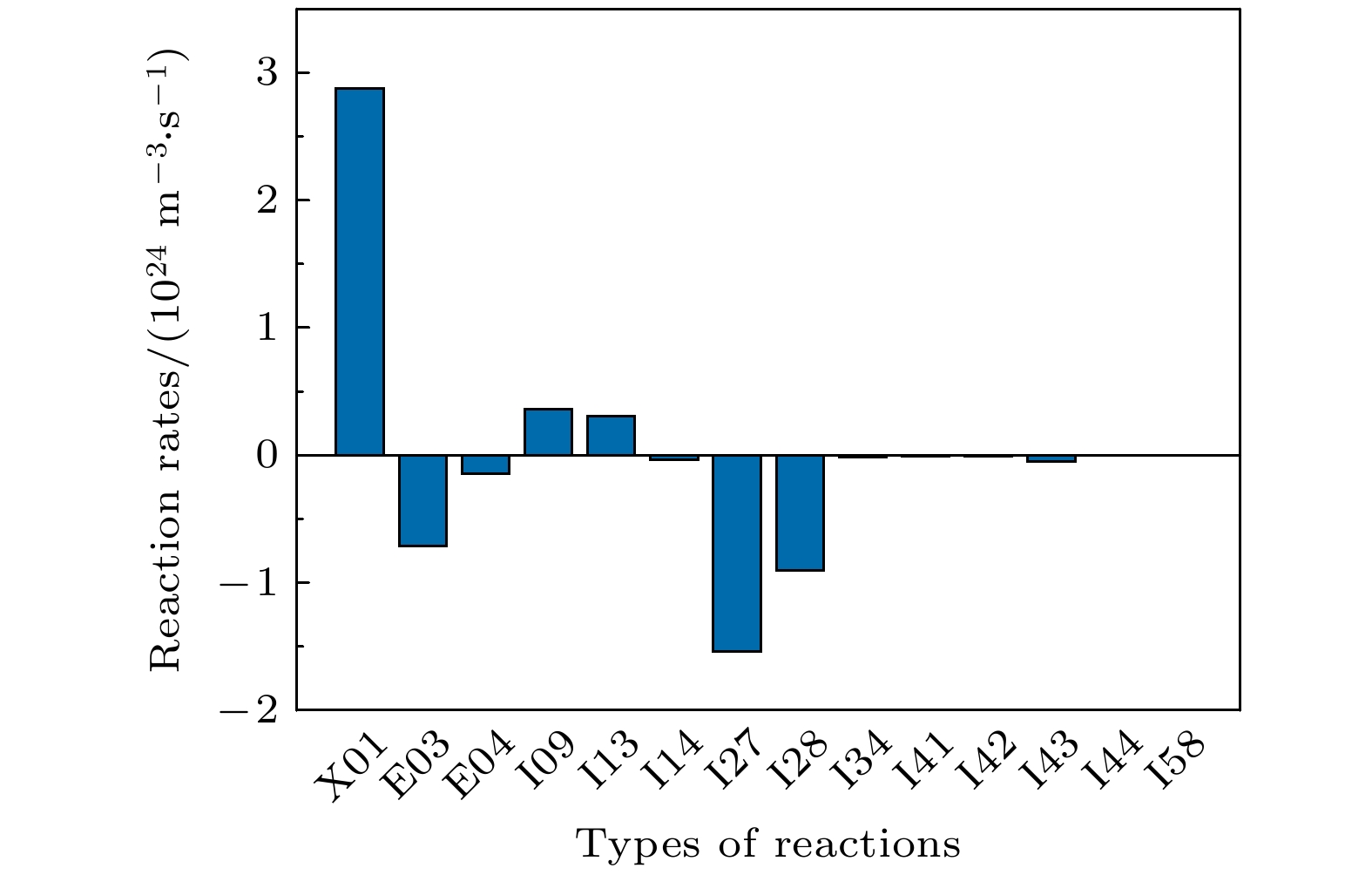
$ {\mathrm{O}}_{5}^{+} $ ,$ {\mathrm{O}}_{4}^{-} $ and species with uncertainties such as$ {\mathrm{C}}_{2}{\mathrm{O}}_{2}^{+} $ ,$ {\mathrm{C}\mathrm{O}}_{4}^{+} $ are removed from the original model. As for the reduction of specific reactions among species, the reaction proportion analysis based on the calculation of reaction rates is used to obtain the contribution of each reaction to the entire process of CO2 discharge, through which the important reactions can be selected. Finally, a simplified chemistry model of CO2 discharge based on Martian atmospheric conditions, including 16 species and 67 reactions, is established. The numerical simulations show that the trends of species densities based on the simplified chemistry model are highly consistent with those based on the original one, and considerations about the CO2 conversion and the energy efficiency are also in line with expectations, which can help deepen the understanding of the essential process of CO2 discharge under Martian atmospheric conditions. In addition, the quantitative results of the relationship between reactive species will lay a theoretical foundation for the accurate analysis of various products in Martian dust storm discharges and the realization of Mars in-situ oxygen generation technology based on plasma chemistry.[1] 史建魁, 张仲谋, 刘振兴 1997 地球物理学进展 04 98
 Google Scholar
Google Scholar
Shi J K, Zhang Z M, Liu Z X 1997 Prog. Geophys. 04 98
 Google Scholar
Google Scholar
[2] Thomas P, Gierasch P J 1985 Science 230 175
 Google Scholar
Google Scholar
[3] Sullivan R, Banfield D, Bell J F 2005 Nature 436 58
 Google Scholar
Google Scholar
[4] Bougher S W, Murphy J, Haberle R M 1997 Adv. Space Res. 19 1255
 Google Scholar
Google Scholar
[5] Lämmel M, Kroy K 2017 Phys. Rev. E 96 052906
 Google Scholar
Google Scholar
[6] Read P L, Lewis S R, Mulholland D P 2015 Rep. Prog. Phys. 78 125901
 Google Scholar
Google Scholar
[7] Barth E L, Farrell W M, Rafkin S C R 2016 Icarus 268 253
 Google Scholar
Google Scholar
[8] Esposito F, Molinaro R, Popa C I 2016 Geophys. Res. Lett. 43 5501
 Google Scholar
Google Scholar
[9] Schmidt D S, Schmidt R A, Dent J D 1998 J. Geophys. Res. 103 8997
 Google Scholar
Google Scholar
[10] Melnik O, Parrot M 1998 J. Geophys. Res. 103 29107
 Google Scholar
Google Scholar
[11] Eden H F, Vonnegut B 1973 Science 180 962
 Google Scholar
Google Scholar
[12] Farrell W M, McLain J L, Collier M R 2015 Icarus 254 333
 Google Scholar
Google Scholar
[13] Farrell W M, McLainb J L, Collier M R 2017 Icarus 297 90
 Google Scholar
Google Scholar
[14] Hecht M, Hoffman J, Rapp D 2021 Space Sci. Rev. 217 9
 Google Scholar
Google Scholar
[15] Keudell A V, Volker S 2017 Plasma Sources Sci. Technol. 26 113001
 Google Scholar
Google Scholar
[16] Guerra V, Silva T, Ogloblina P 2017 Plasma Sources Sci. Technol. 26 11LT01
 Google Scholar
Google Scholar
[17] Bogaerts A, Kozak T, Laer K V 2015 Faraday Discuss. 183 217
 Google Scholar
Google Scholar
[18] Kozák T, Bogaerts A 2014 Plasma Sources Sci. Technol. 23 045004
 Google Scholar
Google Scholar
[19] Aerts R, Martens T, Bogaerts A 2012 J. Phys. Chem. C 116 23257
 Google Scholar
Google Scholar
[20] Berthelot A, Bogaerts A 2017 J. Phys. Chem. C 121 8236
 Google Scholar
Google Scholar
[21] Sun R, Wang H X, Bogaerts A 2020 Plasma Sources Sci. Technol. 29 025012
 Google Scholar
Google Scholar
[22] Hurlbatt A, Gibson A, Schröter S 2017 Plasma Processes Polym. 14 1600138
 Google Scholar
Google Scholar
[23] Lazarou C, Belmonte T, Chiper A 2016 Plasma Sources Sci. Technol. 25 055023
 Google Scholar
Google Scholar
[24] Wang W, Berthelot A, Kolev S 2016 Plasma Sources Sci. Technol. 25 065012
 Google Scholar
Google Scholar
[25] Takana H, Nishiyama H 2014 Plasma Sources Sci. Technol. 23 034001
 Google Scholar
Google Scholar
[26] Dubin D H E, Jin D Z 2003 Phys. Plasmas 10 1338
 Google Scholar
Google Scholar
[27] Kelly S, Golda J, Turner M 2015 J. Phys. D:Appl. Phys. 48 444002
 Google Scholar
Google Scholar
[28] Iqbal M M, Stallard C P, Dowling D P, Turner M M 2014 Plasma Processes Polym. 12 201
 Google Scholar
Google Scholar
[29] Stagni A, Frassoldati A, Cuoci A 2015 Combust. Flame 163 382
 Google Scholar
Google Scholar
[30] Rabitz H, Kramer M, Dacol D 2003 Annu. Rev. Phys. Chem. 34 419
 Google Scholar
Google Scholar
[31] Tomlin A S, Pilling M J, Meriting J H 1995 Ind. Eng. Chem. Res. 34 3749
 Google Scholar
Google Scholar
[32] Brown N J, And G L, Koszykowski M L 2015 Int. J. Chem. Kinet. 29 393
 Google Scholar
Google Scholar
[33] Lehmann R 2004 J. Atmos. Chem. 47 45
 Google Scholar
Google Scholar
[34] Strogatz S, Steven H 2003 SIAM Rev. 45 167
 Google Scholar
Google Scholar
[35] Kolaczyk E D 2009 Statistical Analysis of Network Data: Methods and Models (New York: Springer) pp79−120
[36] Boccaletti S, Bianconi G, Criado R 2014 Phys. Rep. 544 1
 Google Scholar
Google Scholar
[37] Estrada E, Hatano N, Benzi M 2012 Phys. Rep. 514 89
 Google Scholar
Google Scholar
[38] Klamt S, Hädicke O, Kamp A V 2014 Large-Scale Networks in Engineering and Life Sciences (Cham: Birkhäuser Basel) pp263−316
[39] Sakai O, Nobuto K, Miyagi S 2015 AIP Adv. 5 107140
 Google Scholar
Google Scholar
[40] Mizui Y, Kojima T, Miyagi S 2017 Symmetry 9 309
 Google Scholar
Google Scholar
[41] Lu T F, Law C K 2005 Proc. Combust. Inst. 30 1333
 Google Scholar
Google Scholar
[42] Lu T F, Law C K 2006 Combust. Flame 146 472
 Google Scholar
Google Scholar
[43] Snoeckx R, Bogaerts A 2017 Chem. Soc. Rev. 46 5805
 Google Scholar
Google Scholar
[44] Aerts R, Somers W, Bogaerts A 2015 ChemSusChem 8 702
 Google Scholar
Google Scholar
[45] Zhang Y T, Wang Y H 2018 Phys. Plasmas 25 023509
 Google Scholar
Google Scholar
[46] 高书涵, 王绪成, 张远涛 2020 69 115204
 Google Scholar
Google Scholar
Gao S H, Wang X C, Zhang Y T 2020 Acta Phys. Sin. 69 115204
 Google Scholar
Google Scholar
[47] Bogaerts A, Wang W Z 2016 Plasma Sources Sci. Technol. 25 055016
 Google Scholar
Google Scholar
[48] Ponduri S, Becker M M, Welzel S 2016 J. Appl. Phys. 119 093301
 Google Scholar
Google Scholar
[49] Stijn H, Luca M M, Giorgio D 2019 J. Phys. Chem. C 123 12104
 Google Scholar
Google Scholar
[50] Phelps A V www.lxcat.net [2020-3-21]
[51] Lowke J J, Phelps A V, Irwin B W 1973 J. Appl. Phys. 44 4664
 Google Scholar
Google Scholar
[52] Beuthe T G, Chang J S 1997 Jpn. J. Appl. Phys. 36 4997
 Google Scholar
Google Scholar
[53] Eliasson B, Kogelschatz U 1986 Brown Boveri Research Report KLR 86-11C
[54] Roberson G, Roberto M, Verboncoeur J 2015 Braz. J. Phys. 37 457
 Google Scholar
Google Scholar
[55] Spencer L F 2012 Ph. D. Dissertation (Ann Arbor: University of Michigan)
[56] Berthelot A, Bogaerts A 2017 Plasma Sources Sci. Technol. 26 115002
 Google Scholar
Google Scholar
[57] Wang W, Snoeckx R, Zhang X 2018 J. Phys. Chem. C 122 8704
 Google Scholar
Google Scholar
[58] Berthelot A, Bogaerts A 2016 Plasma Sources Sci. Technol. 25 045022
 Google Scholar
Google Scholar
[59] Moss M S 2017 Plasma Sources Sci. Technol. 26 035009
 Google Scholar
Google Scholar
[60] Harder N D, Bekerom D C M V D, Al R S 2017 Plasma Processes Polym. 14 1600120
 Google Scholar
Google Scholar
[61] Vermeiren V, Bogaerts A 2018 J. Phys. Chem. C 122 25869
 Google Scholar
Google Scholar
[62] Cheng C, Ma M, Zhang Y, Liu D 2020 J. Phys. D: Appl. Phys. 53 144001
 Google Scholar
Google Scholar
[63] Li J, Fang C, Chen J, Li H P 2021 J. Appl. Phys. 129 133302
 Google Scholar
Google Scholar
-
表 A1 初始反应集合中的粒子构成
Table A1. Composition of particles in the original model.
初始集合粒子构成 中性粒子 CO2, CO, C, O, O2, O3, C2O 正离子 $ {\mathrm{C}\mathrm{O}}_{4}^{+} $, $ {\mathrm{C}\mathrm{O}}_{2}^{+} $, CO+, C+, O+, $ {\mathrm{O}}_{2}^{+} $, $ {\mathrm{O}}_{4}^{+} $,
$ {\mathrm{O}}_{5}^{+} $, $ {\mathrm{C}}_{2}{\mathrm{O}}_{2}^{+} $, $ {\mathrm{C}}_{2}{\mathrm{O}}_{3}^{+} $, $ {\mathrm{C}}_{2}{\mathrm{O}}_{4}^{+} $负离子 $ {\mathrm{C}\mathrm{O}}_{4}^{-} $, $ {\mathrm{C}\mathrm{O}}_{3}^{-} $, O–, $ {\mathrm{O}}_{2}^{-} $, $ {\mathrm{O}}_{3}^{-} $, $ {\mathrm{O}}_{4}^{-} $ 表 A2 初始集合中由碰撞截面描述的电子碰撞反应
Table A2. Electron impact reactions described by collision cross sections in the original model.
序号 反应 文献 (X01) e + CO2 $ \Rightarrow $ $ {\mathrm{C}\mathrm{O}}_{2}^{+} $ + 2e [50] (X02) e + CO2 $ \Rightarrow $ CO + O + e [50] (X03) e + CO2 $ \Rightarrow $ CO + O– [50] (X04) e + CO2 $ \Rightarrow $ 2e + O + CO+ [50] (X05) e + CO2 $ \Rightarrow $ 2e + CO + O+ [50] (X06) e + CO2 $ \Rightarrow $ 2e + C+ + O2 [50] (X07) e + CO2 $ \Rightarrow $ $ {\mathrm{O}}_{2}^{+} $ + C + 2e [51, 52] (X08) e + CO $ \Rightarrow $ C + O– [50] (X09) e + CO $ \Rightarrow $ e + C + O [50] (X10) e + CO $ \Rightarrow $ 2e + CO+ [50] (X11) e + CO $ \Rightarrow $ 2e + C + O+ [50] (X12) e + CO $ \Rightarrow $ 2e + C+ + O [50] (X13) e + O3 $ \Rightarrow $ $ {{\mathrm{O}}_{2}^{-}}^{-} $ + O [50] (X14) e + O3 $ \Rightarrow $ O2 + O– [50] (X15) e + O3 $ \Rightarrow $ O + O2 + e [53] (X16) e + O3 $ \Rightarrow $ $ {\mathrm{O}}_{2}^{+} $ + O + 2e [53] (X17) e + O3 $ \Rightarrow $ O+ + O– + O + e [53] (X18) e + O2 $ \Rightarrow $ 2 O + e [50] (X19) e + O2 $ \Rightarrow $ O + O– [50] (X20) e + O2 $ \Rightarrow $ 2e + $ {\mathrm{O}}_{2}^{+} $ [50] (X21) e + O2 $ \Rightarrow $ 2e + O + O+ [50] (X22) e + O2 $ \Rightarrow $ e + O+ + O– [50, 54] (X23) e + O $ \Rightarrow $ 2e + O+ [50] (X24) e + C $ \Rightarrow $ 2e + C+ [50] 表 A5 初始集合中的离子-中性和离子-离子反应, 其中Tg为气体温度, 单位是K, 速率系数的单位在二体或三体反应中分别是m3/s或m6/s
Table A5. Ion-neutral and ion-ion reactions in the original model. Tg is the gas temperature in K. The rate coefficients are in m3/s or m6/s for binary or ternary reactions.
序号 反应 反应速率系数 文献 (I01) O– + CO2 $ \Rightarrow$ O + CO2 + e 4.0 × 10–18 [48] (I02) O– + CO2 + CO $ \Rightarrow$ $ {\mathrm{C}\mathrm{O}}_{3}^{-} $ + CO 1.5 × 10–40 [61] (I03) O– + CO2 + O2 $ \Rightarrow$ $ {\mathrm{C}\mathrm{O}}_{3}^{-} $ + O2 3.1 × 10–40 [61] (I04) O– + CO2 + CO2 $ \Rightarrow$ $ {\mathrm{C}\mathrm{O}}_{3}^{-} $ + CO2 9.0 × 10–41 [52] (I05) $ {\mathrm{O}}_{2}^{-} $ + CO2 + O2 $ \Rightarrow$ $ {\mathrm{C}\mathrm{O}}_{4}^{-} $ + O2 4.7 × 10–41 [52] (I06) $ {\mathrm{O}}_{3}^{-} $+ CO2 $ \Rightarrow$ $ {\mathrm{C}\mathrm{O}}_{3}^{-} $ + O2 5.5 × 10–16 [18] (I07) $ {\mathrm{O}}_{4}^{-} $ + CO2 $ \Rightarrow$ O2 + $ {\mathrm{C}\mathrm{O}}_{4}^{-} $ 4.8 × 10–16 [18] (I08) O+ + CO2 $ \Rightarrow$ $ {\mathrm{O}}_{2}^{+} $ + CO 9.4 × 10–16 [18] (I09) O+ + CO2 $ \Rightarrow$ O + $ {\mathrm{C}\mathrm{O}}_{2}^{+} $ 4.5 × 10–16 [18] (I10) C+ + CO2 $ \Rightarrow$ CO+ + CO 1.1 × 10–15 [18] (I11) $ {\mathrm{O}}_{2}^{+} $ + CO2 + M $ \Rightarrow$ $ {\mathrm{C}\mathrm{O}}_{4}^{+} $ + M 2.3 × 10–41 [18] (I12) $ {\mathrm{O}}_{5}^{+} $ + CO2 $ \Rightarrow$ $ {\mathrm{C}\mathrm{O}}_{4}^{+} $ + O3 1.0 × 10–17 [52] (I13) CO+ + CO2 $ \Rightarrow$ $ {\mathrm{C}\mathrm{O}}_{2}^{+} $ + CO 1.0 × 10–15 [56] (I14) $ {\mathrm{C}\mathrm{O}}_{2}^{+} $ + CO2 + M $ \Rightarrow$ $ {\mathrm{C}}_{2}{\mathrm{O}}_{4}^{+} $ + M 3.0 × 10–40 [18] (I15) O+ + CO $ \Rightarrow$ O + CO+ 4.9 × 10–18(Tg/298)0.5exp(–4580/Tg) [18] (I16) C+ + CO $ \Rightarrow$ CO+ + C 5.0 × 10–19 [18] (I17) $ {\mathrm{C}}_{2}{\mathrm{O}}_{3}^{+} $ + CO $ \Rightarrow$ CO2 + $ {\mathrm{C}}_{2}{\mathrm{O}}_{2}^{+} $ 1.1 × 10–15 [18] (I18) $ {\mathrm{C}}_{2}{\mathrm{O}}_{4}^{+} $ + CO $ \Rightarrow$ CO2 + $ {\mathrm{C}}_{2}{\mathrm{O}}_{3}^{+} $ 9.0 × 10–16 [18] (I19) $ {\mathrm{C}}_{2}{\mathrm{O}}_{3}^{+} $ + CO + M $ \Rightarrow$ CO2 + $ {\mathrm{C}}_{2}{\mathrm{O}}_{2}^{+} $ + M 2.6 × 10–38 [18] (I20) $ {\mathrm{C}}_{2}{\mathrm{O}}_{4}^{+} $ + CO + M $ \Rightarrow$ CO2 + $ {\mathrm{C}}_{2}{\mathrm{O}}_{3}^{+} $ + M 4.2 × 10–38 [18] (I21) O– + CO $ \Rightarrow$ CO2 + e 5.5 × 10–16 [44] (I22) $ {\mathrm{C}\mathrm{O}}_{3}^{-} $ + CO $ \Rightarrow$ 2CO2 + e 5.0 × 10–19 [58] (I23) $ {\mathrm{O}}_{2}^{+} $ + C $ \Rightarrow$ CO+ + O 5.2 × 10–17 [18] (I24) $ {\mathrm{O}}_{2}^{+} $ + C $ \Rightarrow$ C+ + O2 5.2 × 10–17 [18] (I25) CO+ + C $ \Rightarrow$ CO + C+ 1.1 × 10–16 [18] (I26) O– + C $ \Rightarrow$ CO + e 5.0 × 10–16 [57] (I27) $ {\mathrm{C}\mathrm{O}}_{2}^{+} $ + O $ \Rightarrow$ $ {\mathrm{O}}_{2}^{+} $ + CO 1.638 × 10–16 [52] (I28) $ {\mathrm{C}\mathrm{O}}_{2}^{+} $ + O $ \Rightarrow$ CO2 + O+ 9.62 × 10–17 [18] (I29) CO+ + O $ \Rightarrow$ CO + O+ 1.4 × 10–16 [18] (I30) $ {\mathrm{C}\mathrm{O}}_{4}^{-} $ + O $ \Rightarrow$ $ {\mathrm{C}\mathrm{O}}_{3}^{-} $ + O2 1.1 × 10–16 [18] (I31) $ {\mathrm{C}\mathrm{O}}_{4}^{-} $ + O $ \Rightarrow$ CO2 + O2 + O– 1.4 × 10–17 [18] (I32) $ {\mathrm{C}\mathrm{O}}_{4}^{-} $ + O $ \Rightarrow$ CO2 + $ {\mathrm{O}}_{3}^{-} $ 1.4 × 10–16 [18] (I33) $ {\mathrm{C}\mathrm{O}}_{3}^{-} $ + O $ \Rightarrow$ CO2 + $ {\mathrm{O}}_{2}^{-} $ 8.0 × 10–17 [58] (I34) $ {\mathrm{C}\mathrm{O}}_{2}^{+} $ + O2 $ \Rightarrow$ $ {\mathrm{O}}_{2}^{+} $ + CO2 6.4 × 10–17 [52] (I35) CO+ + O2 $ \Rightarrow$ $ {\mathrm{O}}_{2}^{+} $ + CO 1.2 × 10–16 [18] (I36) C+ + O2 $ \Rightarrow$ CO + O+ 6.2 × 10–16 [18] (I37) C+ + O2 $ \Rightarrow$ CO+ + O 3.8 × 10–16 [18] (I38) $ {\mathrm{C}}_{2}{\mathrm{O}}_{2}^{+} $ + O2 $ \Rightarrow$ 2CO + $ {\mathrm{O}}_{2}^{+} $ 5.0 × 10–18 [18] (I39) $ {\mathrm{C}\mathrm{O}}_{4}^{-} $ + O3 $ \Rightarrow$ CO2 + $ {\mathrm{O}}_{3}^{-} $ + O2 1.3 × 10–16 [18] (I40) $ {\mathrm{C}\mathrm{O}}_{4}^{+} $ + O3 $ \Rightarrow$ $ {\mathrm{O}}_{5}^{+} $ + CO2 1.0 × 10–16 [52] (I41) $ {\mathrm{C}\mathrm{O}}_{2}^{+} $ + O– $ \Rightarrow$ O + CO2 1.0 × 10–13 [62] (I42) $ {\mathrm{C}\mathrm{O}}_{2}^{+} $ + $ {\mathrm{O}}_{2}^{-} $ $ \Rightarrow$ CO + O2 + O 6.0 × 10–13 [44] (I43) $ {\mathrm{C}\mathrm{O}}_{2}^{+} $ + $ {\mathrm{C}\mathrm{O}}_{3}^{-} $ $ \Rightarrow$ 2CO2 + O 5.0 × 10–13 [58] (I44) $ {\mathrm{C}\mathrm{O}}_{2}^{+} $ + $ {\mathrm{C}\mathrm{O}}_{4}^{-} $ $ \Rightarrow$ 2CO2 + O2 5.0 × 10–13 [18] (I45) CO+ + $ {\mathrm{O}}_{2}^{-} $ $ \Rightarrow$ CO + O2 2.0 × 10–13 [55] (I46) C+ + O– $ \Rightarrow$ C + O 5.0 × 10–14 [55] (I47) C+ + $ {\mathrm{O}}_{2}^{-} $ $ \Rightarrow$ C + O2 5.0 × 10–14 [55] (I48) O–+ CO+ $ \Rightarrow$ CO + O 1.0 × 10–13 [62] (I49) $ {\mathrm{C}\mathrm{O}}_{3}^{-} $ + $ {\mathrm{O}}_{2}^{+} $ $ \Rightarrow$ CO2 + O2 + O 3.0 × 10–13 [18] (I50) $ {\mathrm{O}}_{2}^{+} $ + $ {\mathrm{C}\mathrm{O}}_{4}^{-} $ $ \Rightarrow$ CO2 + 2O2 3.0 × 10–13 [18] (I51) $ {\mathrm{C}}_{2}{\mathrm{O}}_{2}^{+} $ + M $ \Rightarrow$ CO+ + CO + M 1.0 × 10–18 [18] (I52) $ {\mathrm{C}}_{2}{\mathrm{O}}_{2}^{+} $ + $ {\mathrm{C}\mathrm{O}}_{3}^{-} $$ \Rightarrow$ CO2 + 2CO + O 5.0 × 10–13 [18] (I53) $ {\mathrm{C}}_{2}{\mathrm{O}}_{2}^{+} $ + $ {\mathrm{C}\mathrm{O}}_{4}^{-} $ $ \Rightarrow$ CO2 + 2CO + O2 5.0 × 10–13 [18] (I54) $ {\mathrm{C}}_{2}{\mathrm{O}}_{2}^{+} $ + $ {\mathrm{O}}_{2}^{-} $$ \Rightarrow$ 2CO + O2 6.0 × 10–13 [18] (I55) $ {\mathrm{C}}_{2}{\mathrm{O}}_{3}^{+} $ + $ {\mathrm{C}\mathrm{O}}_{3}^{-} $ $ \Rightarrow$ 2CO2 + CO + O 5.0 × 10–13 [18] (I56) $ {\mathrm{C}}_{2}{\mathrm{O}}_{3}^{+} $ + $ {\mathrm{C}\mathrm{O}}_{4}^{-} $ $ \Rightarrow$ 2CO2 + CO + O2 5.0 × 10–13 [18] (I57) $ {\mathrm{C}}_{2}{\mathrm{O}}_{3}^{+} $ + $ {\mathrm{O}}_{2}^{-} $ $ \Rightarrow$ CO2 + CO + O2 6.0 × 10–13 [18] (I58) $ {\mathrm{C}}_{2}{\mathrm{O}}_{4}^{+} $ + M $ \Rightarrow$ $ {\mathrm{C}\mathrm{O}}_{2}^{+} $ + CO2 + M 1.0 × 10–20 [18] (I59) $ {\mathrm{C}}_{2}{\mathrm{O}}_{4}^{+} $ + $ {\mathrm{C}\mathrm{O}}_{3}^{-} $$ \Rightarrow$ 3CO2 + O 5.0 × 10–13 [18] (I60) $ {\mathrm{C}}_{2}{\mathrm{O}}_{4}^{+} $ + $ {\mathrm{C}\mathrm{O}}_{4}^{-} $ $ \Rightarrow$ 3CO2 + O2 5.0 × 10–13 [18] (I61) $ {\mathrm{C}}_{2}{\mathrm{O}}_{4}^{+} $ + $ {\mathrm{O}}_{2}^{-} $ $ \Rightarrow$ 2CO2 + O2 6.0 × 10–13 [18] (I62) O–+ O3 $ \Rightarrow$ 2O2 + e 3.0 × 10–16 [44] (I63) O–+ O3 $ \Rightarrow$ $ {\mathrm{O}}_{3}^{-} $ + O 8.0 × 10–16 [18] (I64) $ {\mathrm{O}}_{2}^{-} $ + O3 $ \Rightarrow$ $ {\mathrm{O}}_{3}^{-} $ + O2 4.0 × 10–16 [18] (I65) $ {\mathrm{O}}_{3}^{-} $ + O3 $ \Rightarrow$ 3O2 + e 3.0 × 10–16 [18] (I66) O+ + O3 $ \Rightarrow$ $ {\mathrm{O}}_{2}^{+} $ + O2 1.0 × 10–16 [18] (I67) O– + O2 $ \Rightarrow$ O3 + e 1.0 × 10–18 [44] (I68) O– + O2 $ \Rightarrow$ $ {\mathrm{O}}_{2}^{-} $ + O 1.5 × 10–18 [55] (I69) O– + O2 $ \Rightarrow$ O + e + O2 6.9 × 10–16 [52] (I70) O– + O2 + O2 $ \Rightarrow$ $ {\mathrm{O}}_{3}^{-} $ + O2 1.1 × 10–42(Tg/300) [52] (I71) $ {\mathrm{O}}_{2}^{-} $ + O2 $ \Rightarrow$ 2O2 + e 2.7 × 10–16(Tg/300)0.5exp(–5590/Tg) [52] (I72) $ {\mathrm{O}}_{2}^{-} $ + O2 + M $ \Rightarrow$ $ {\mathrm{O}}_{4}^{-} $ + M 3.5 × 10–43 [18] (I73) $ {\mathrm{O}}_{3}^{-} $ + O2 $ \Rightarrow$ O2 + O3 + e 2.3 × 10–17 [18] (I74) O+ + O2 $ \Rightarrow$ O + $ {\mathrm{O}}_{2}^{+} $ 1.9 × 10–17(Tg/298)–0.5 [18] (I75) $ {\mathrm{O}}_{2}^{+} $ + O2 + O2 $ \Rightarrow$ $ {\mathrm{O}}_{4}^{+} $ + O2 4.0 × 10–42(Tg/300)–2.93 [52] (I76) $ {\mathrm{O}}_{4}^{+} $ + O2 $ \Rightarrow$ $ {\mathrm{O}}_{2}^{+} $ + O2 + O2 1.8 × 10–19 [52] (I77) O+ + O + O2 $ \Rightarrow$ $ {\mathrm{O}}_{2}^{+} $ + O2 1.0 × 10–41 [52] (I78) O– + O $ \Rightarrow$ O2 + e 2.3 × 10–16 [21] (I79) $ {\mathrm{O}}_{2}^{-} $ + O $ \Rightarrow$ O2 + O– 3.31 × 10–16 [21] (I80) $ {\mathrm{O}}_{2}^{-} $ + O $ \Rightarrow$ O3 + e 3.3 × 10–16 [18] (I81) $ {\mathrm{O}}_{3}^{-} $ + O $ \Rightarrow$ O3 + O– 1.0 × 10–19 [18] (I82) $ {\mathrm{O}}_{3}^{-} $ + O $ \Rightarrow$ 2O2 + e 1.0 × 10–19 [18] (I83) $ {\mathrm{O}}_{3}^{-} $ + O $ \Rightarrow$ $ {\mathrm{O}}_{2}^{-} $ + O2 2.5 × 10–16 [18] (I84) $ {\mathrm{O}}_{4}^{-} $ + O $ \Rightarrow$ $ {\mathrm{O}}_{3}^{-} $ + O2 4.0 × 10–16 [18] (I85) $ {\mathrm{O}}_{4}^{-} $ + O $ \Rightarrow$ O– + 2O2 3.0 × 10–16 [18] (I86) $ {\mathrm{O}}_{4}^{+} $ + O $ \Rightarrow$ $ {\mathrm{O}}_{2}^{+} $ + O3 3.0 × 10–16 [18] (I87) O– + $ {\mathrm{O}}_{2}^{+} $ $ \Rightarrow$ O2 + O 2.6 × 10–14(300/Tg)0.44 [21] (I88) O– + $ {\mathrm{O}}_{2}^{+} $ $ \Rightarrow$ 3O 4.2 × 10–13(300/Tg)0.44 [21] (I89) O– + $ {\mathrm{O}}_{2}^{+} $ + O2 $ \Rightarrow$ O3 + O2 2.0 × 10–37 [57] (I90) O– + O+ + O $ \Rightarrow$ O2 + O 2.0 × 10–37 [57] (I91) O– + O+ + O2 $ \Rightarrow$ 2O2 2.0 × 10–37 [57] (I92) O– + O+ $ \Rightarrow$ 2O 4.0 × 10–14 [18] (I93) $ {\mathrm{O}}_{2}^{-} $ + O+ $ \Rightarrow$ O + O2 2.7 × 10–13 [18] (I94) $ {\mathrm{O}}_{2}^{-} $ + O+ + O2 $ \Rightarrow$ O3 + O2 2.0 × 10–37 [57] (I95) $ {\mathrm{O}}_{2}^{-} $ + $ {\mathrm{O}}_{2}^{+} $ $ \Rightarrow$ 2O2 2.01 × 10–13(300/Tg)0.5 [21] (I96) $ {\mathrm{O}}_{2}^{-} $ + $ {\mathrm{O}}_{2}^{+} $ $ \Rightarrow$ O2 + 2O 4.2 × 10–13 [21] (I97) $ {\mathrm{O}}_{2}^{-} $ + $ {\mathrm{O}}_{2}^{+} $ + O2 $ \Rightarrow$ 3O2 2.0 × 10–37 [57] (I98) $ {\mathrm{O}}_{3}^{-} $ + O + M $ \Rightarrow$ O3 + O + M 2.0 × 10–37(Tg/300)–2.5 [57] (I99) $ {\mathrm{O}}_{3}^{-} $ + $ {\mathrm{O}}_{2}^{+} $ + M $ \Rightarrow$ O3 + O2 + M 2.0 × 10–37(Tg/300)–2.5 [57] (I100) $ {\mathrm{O}}_{3}^{-} $ + O+ $ \Rightarrow$ O3 + O 1.0 × 10–13 [18] (I101) $ {\mathrm{O}}_{3}^{-} $+ $ {\mathrm{O}}_{2}^{+} $ $ \Rightarrow$ O2 + O3 2.0 × 10–13 [18] (I102) $ {\mathrm{O}}_{3}^{-} $ + $ {\mathrm{O}}_{2}^{+} $ $ \Rightarrow$ 2O + O3 1.0 × 10–13 [18] (I103) $ {\mathrm{O}}_{4}^{-} $ + M $ \Rightarrow$ $ {\mathrm{O}}_{2}^{-} $ + O2 + M 4.0 × 10–18 [18] 表 B1 简化集合中的粒子构成
Table B1. Composition of particles in the simplified model.
简化集合粒子构成 中性粒子 CO2, CO, C, O, O2, O3 正离子 $ {\mathrm{C}\mathrm{O}}_{2}^{+} $, CO+, C+, O+, $ {\mathrm{O}}_{2}^{+} $ 负离子 $ {\mathrm{C}\mathrm{O}}_{4}^{-} $, $ {\mathrm{C}\mathrm{O}}_{3}^{-} $, O–, $ {\mathrm{O}}_{2}^{-} $, $ {\mathrm{O}}_{3}^{-} $ 表 A3 初始集合中由反应速率系数描述的电子碰撞反应, 其中Te为电子温度, 单位是eV; Tg为气体温度, 单位是K; 速率系数的单位在二体或三体反应中分别是m3/s或m6/s
Table A3. Electron impact reactions described by rate coefficients in the original model. Te is the electron temperature in eV and Tg is the gas temperature in K. The rate coefficients are in m3/s or m6/s for binary or ternary reactions.
序号 反应 反应速率系数 文献 (E01) e + e + C+ $\Rightarrow $ C + e 5.0 × 10–39 [55] (E02) e + CO+ $\Rightarrow $ C + O 3.46 × 10–14Te–0.48 [56] (E03) e + $ {\mathrm{C}\mathrm{O}}_{2}^{+} $ $\Rightarrow $ C + O2 3.94 × 10–13Te–0.4 [21] (E04) e + $ {\mathrm{C}\mathrm{O}}_{2}^{+} $ $\Rightarrow $ CO + O 2.0 × 10–11Te–0.5Tg–1 [18] (E05) e + $ {\mathrm{C}\mathrm{O}}_{4}^{+} $ $\Rightarrow $ CO2 + O2 1.61 × 10–13Te–0.5 [18] (E06) e + $ {\mathrm{C}}_{2}{\mathrm{O}}_{2}^{+} $ $\Rightarrow $ 2CO 4.0 × 10–13Te–0.34 [18] (E07) e + $ {\mathrm{C}}_{2}{\mathrm{O}}_{3}^{+} $ $\Rightarrow $ CO2 + CO 5.4 × 10–14Te–0.7 [18] (E08) e + $ {\mathrm{C}}_{2}{\mathrm{O}}_{4}^{+} $ $\Rightarrow $ 2CO2 2.0 × 10–11Te–0.5Tg–1 [18] (E09) e + O2 + O2 $\Rightarrow $ $ {\mathrm{O}}_{2}^{-} $ + O2 2.2 × 10–41(300/Tg)1.5exp(-600/Tg) [52] (E10) e + O + O2 $\Rightarrow $ O– + O2 1.0 × 10–43exp(300/Tg) [52] (E11) e + O3 + O2 $\Rightarrow $ $ {\mathrm{O}}_{3}^{-} $ + O2 4.6 × 10–40 [52] (E12) e + O+ + M $\Rightarrow $ O + M 6.0 × 10–39(Te × 38.67)–1.5 [57] (E13) e + $ {\mathrm{O}}_{2}^{+} $ $\Rightarrow $ 2O 6.0 × 10–13Te–0.5(1/Tg)0.5 [21] (E14) e + $ {\mathrm{O}}_{2}^{+} $ + M $\Rightarrow $ O2 + M 6.0 × 10–39(Te × 38.67)–1.5 [57] (E15) e + $ {\mathrm{O}}_{2}^{+} $ + e $\Rightarrow $ O2 + e 1.0 × 10–31(Te × 38.67)–4.5 [57] (E16) e + O+ + e $\Rightarrow $ O + e 7.2 × 10–32(Te × 38.67)–4.5 [57] (E17) e + $ {\mathrm{O}}_{4}^{+} $ $\Rightarrow $ 2O2 2.25 × 10–13Te–0.5 [18] (E18) e + $ {\mathrm{O}}_{5}^{+} $ $\Rightarrow $ O2 + O3 5.0 × 10–12(Te × 38.67)–0.6 [52] 表 A4 初始集合中的中性粒子反应, 其中 Tg 为气体温度, 单位是 K; 速率系数的单位在二体或三体反应中分别是 m3/s 或 m6/s
Table A4. Reaction of neutrals in the original model. Tg is the gas temperature in K. The rate coefficients are in m3/s or m6/s for binary or ternary reactions.
序号 反应 反应速率系数 文献 (N01) CO2 + O $ \Rightarrow$ CO + O2 2.8 × 10–17exp(–26500/Tg) [21] (N02) CO + O2 $ \Rightarrow$ CO2 + O 4.2 × 10–18exp(–24000/Tg) [21] (N03) CO2 + C $ \Rightarrow$ 2CO 1.0 × 10–21 [21] (N04) C + O2 $ \Rightarrow$ O + CO 3.0 × 10–17 [21] (N05) C + O + M $ \Rightarrow$ M + CO 2.14 × 10–41(Tg/300)–3.08exp(–2114/Tg) [21] (N06) CO + M $ \Rightarrow$ O + C + M 1.52 × 10–10(Tg/298)–3.1exp(–129000/Tg) [58] (N07) CO + O3 $ \Rightarrow$ CO2 + O2 4.0 × 10–31 [52] (N08) CO2 + CO2 $ \Rightarrow$ CO + O + CO2 3.91 × 10–16exp(–49430/Tg) [59] (N09) C + CO + CO2 $ \Rightarrow$ C2O + CO2 6.3 × 10–44 [59] (N10) C2O + O $ \Rightarrow$ 2CO 5.0 × 10–17 [18] (N11) C2O + O2 $ \Rightarrow$ CO2 + CO 3.3 × 10–19 [18] (N12) O + O2 + CO2 $ \Rightarrow$ O3 + CO2 1.7 × 10–42Tg–1.2 [48] (N13) O + O + CO2 $ \Rightarrow$ O2 + CO2 3.81 × 10–42Tg–1exp(–170/Tg) [48] (N14) O + CO + CO2 $ \Rightarrow$ 2CO2 1.6 × 10–45exp(–1510/Tg) [48] (N15) O + CO + CO $ \Rightarrow$ CO2 + CO 6.54 × 10–45 [60] (N16) O + O2 + CO $ \Rightarrow$ CO2 + O2 6.51 × 10–48 [60] (N17) O + O + CO $ \Rightarrow$ O2 + CO 2.76 × 10–46 [60] (N18) O + O + O $ \Rightarrow$ O2 + O 6.2 × 10–44exp(–750/Tg) [52] (N19) O + O + O2 $ \Rightarrow$ 2O2 1.3 × 10–44(Tg/300)–1exp(–170/Tg) [52] (N20) O + O3 $ \Rightarrow$ 2O2 3.1 × 10–20Tg0.75exp(–1575/Tg) [18] (N21) O2 + O3 $ \Rightarrow$ 2O2 + O 7.26 × 10–16exp(–11400/Tg) [48] (N22) O2 + O + O2 $ \Rightarrow$ O3 + O2 8.61 × 10–43Tg–1.25 [48] (N23) O2 + O2 $ \Rightarrow$ O + O3 2.1 × 10–17exp(–498000/Tg) [57] (N24) O2 + M $ \Rightarrow$ O + O + M 3.0 × 10–12Tg–1exp(–59380/Tg) [57] 表 B2 简化集合中由碰撞截面描述的电子碰撞反应
Table B2. Electron impact reactions described by collision cross sections in the simplified model.
序号 反应 文献 序号 反应 文献 (Y01) e + CO2 $\Rightarrow $ $ {\mathrm{C}\mathrm{O}}_{2}^{+} $ + 2e [50] (Y11) e + O3 $\Rightarrow $ $ {\mathrm{O}}_{2}^{-} $ + O [50] (Y02) e + CO2 $\Rightarrow $ CO + O + e [50] (Y12) e + O3 $\Rightarrow $ O2 + O– [50] (Y03) e + CO2 $\Rightarrow $ CO + O– [50] (Y13) e + O3 $\Rightarrow $ O + O2 + e [53] (Y04) e + CO2 $\Rightarrow $ 2e + O + CO+ [50] (Y14) e + O3 $\Rightarrow $ $ {\mathrm{O}}_{2}^{+} $ + O + 2e [53] (Y05) e + CO2 $\Rightarrow $ 2e + CO + O+ [50] (Y15) e + O2 $\Rightarrow $ 2 O + e [50] (Y06) e + CO2 $\Rightarrow $ $ {\mathrm{O}}_{2}^{+} $ + C + 2e [51, 52] (Y16) e + O2 $\Rightarrow $ O + O– [50] (Y07) e + CO $\Rightarrow $ C + O– [50] (Y17) e + O2 $\Rightarrow $ 2e + $ {\mathrm{O}}_{2}^{+} $ [50] (Y08) e + CO $\Rightarrow $ e + C + O [50] (Y18) e + O $\Rightarrow $ 2e + O+ [50] (Y09) e + CO $\Rightarrow $ 2e + CO+ [50] (Y19) e + C $\Rightarrow $ 2e + C+ [50] (Y10) e + CO $\Rightarrow $ 2e + C+ + O [50] 表 B3 简化集合中由反应速率系数描述的电子碰撞反应, 其中Te为电子温度, 单位是eV; Tg为气体温度, 单位是K; 速率系数的单位在二体或三体反应中分别是m3/s或m6/s
Table B3. Electron impact reactions described by rate coefficients in the simplified model. Te is the electron temperature in eV and Tg is the gas temperature in K. The rate coefficients are in m3/s or m6/s for binary or ternary reactions.
序号 反应 反应速率系数 文献 (F01) e + $ {\mathrm{C}\mathrm{O}}_{2}^{+} $ $\Rightarrow $ C + O2 3.94 × 10–13Te–0.4 [21] (F02) e + $ {\mathrm{C}\mathrm{O}}_{2}^{+} $ $\Rightarrow $ CO + O 2.0 × 10–11Te–0.5Tg–1 [18] (F03) e + O3 + O2 $\Rightarrow $ $ {\mathrm{O}}_{3}^{-} $ + O2 4.6 × 10–40 [52] (F04) e + $ {\mathrm{O}}_{2}^{+} $ $\Rightarrow $ 2O 6.0 × 10–13Te–0.5(1/Tg)0.5 [21] 表 B4 简化集合中的中性粒子反应, 其中, Tg 为气体温度, 单位是 K; 速率系数的单位在二体或三体反应中分别是 m3/s 或 m6/s
Table B4. Reaction of neutrals in the simplified model. Tg is the gas temperature in K. The rate coefficients are in m3/s or m6/s for binary or ternary reactions.
表 B5 简化集合中的离子-中性和离子-离子反应, 其中Tg为气体温度, 单位是K; 速率系数的单位在二体或三体反应中分别是m3/s或m6/s
Table B5. Ion-neutral and ion-ion reactions in the simplified model. Tg is the gas temperature in K. The rate coefficients are in m3/s or m6/s for binary or ternary reactions.
序号 反应 反应速率系数 文献 (H01) O– + CO2 $ \Rightarrow $ O + CO2 + e 4.0 × 10–18 [48] (H02) O– + CO2 + CO $ \Rightarrow $ $ {\mathrm{C}\mathrm{O}}_{3}^{-} $ + CO 1.5 × 10–40 [61] (H03) O– + CO2 + O2 $ \Rightarrow $ $ {\mathrm{C}\mathrm{O}}_{3}^{-} $ + O2 3.1 × 10–40 [61] (H04) O– + CO2 + CO2 $ \Rightarrow $ $ {\mathrm{C}\mathrm{O}}_{3}^{-} $ + CO2 9.0 × 10–41 [52] (H05) $ {\mathrm{O}}_{2}^{-} $ + CO2 + O2 $ \Rightarrow $ $ {\mathrm{C}\mathrm{O}}_{4}^{-} $ + O2 4.7 × 10–41 [52] (H06) $ {\mathrm{O}}_{3}^{-} $ + CO2 $ \Rightarrow $ $ {\mathrm{C}\mathrm{O}}_{3}^{-} $ + O2 5.5 × 10–16 [18] (H07) O+ + CO2 $ \Rightarrow $ $ {\mathrm{O}}_{2}^{+} $ + CO 9.4 × 10–16 [18] (H08) O+ + CO2 $ \Rightarrow $ O + $ {\mathrm{C}\mathrm{O}}_{2}^{+} $ 4.5 × 10–16 [18] (H09) C+ + CO2 $ \Rightarrow $ CO+ + CO 1.1 × 10–15 [18] (H10) CO+ + CO2 $ \Rightarrow $ $ {\mathrm{C}\mathrm{O}}_{2}^{+} $ + CO 1.0 × 10–15 [56] (H11) O– + CO $ \Rightarrow $ CO2 + e 5.5 × 10–16 [44] (H12) $ {\mathrm{C}\mathrm{O}}_{3}^{-} $ + CO $ \Rightarrow $ 2CO2 + e 5.0 × 10–19 [58] (H13) $ {\mathrm{O}}_{2}^{+} $ + C $ \Rightarrow $ CO+ + O 5.2 × 10–17 [18] (H14) $ {\mathrm{O}}_{2}^{+} $ + C $ \Rightarrow $ C+ + O2 5.2 × 10–17 [18] (H15) O– + C $ \Rightarrow $ CO + e 5.0 × 10–16 [57] (H16) $ {\mathrm{C}\mathrm{O}}_{2}^{+} $ + O $ \Rightarrow $ $ {\mathrm{O}}_{2}^{+} $ + CO 1.638 × 10–16 [52] (H17) $ {\mathrm{C}\mathrm{O}}_{2}^{+} $ + O $ \Rightarrow $ CO2 + O+ 9.62 × 10–17 [18] (H18) $ {\mathrm{C}\mathrm{O}}_{4}^{-} $ + O $ \Rightarrow $ $ {\mathrm{C}\mathrm{O}}_{3}^{-} $ + O2 1.1 × 10–16 [18] (H19) $ {\mathrm{C}\mathrm{O}}_{4}^{-} $ + O $ \Rightarrow $ CO2 + O2 + O– 1.4 × 10–17 [18] (H20) $ {\mathrm{C}\mathrm{O}}_{4}^{-} $ + O $ \Rightarrow $ CO2 + $ {\mathrm{O}}_{3}^{-} $ 1.4 × 10–16 [18] (H21) $ {\mathrm{C}\mathrm{O}}_{3}^{-} $ + O $ \Rightarrow $ CO2 + $ {\mathrm{O}}_{2}^{-} $ 8.0 × 10–17 [58] (H22) $ {\mathrm{C}\mathrm{O}}_{2}^{+} $ + O2 $ \Rightarrow $ $ {\mathrm{O}}_{2}^{+} $ + CO2 6.4 × 10–17 [52] (H23) $ {\mathrm{C}\mathrm{O}}_{2}^{+} $ + O– $ \Rightarrow $ O + CO2 1.0 × 10–13 [62] (H24) $ {\mathrm{C}\mathrm{O}}_{2}^{+} $ + $ {\mathrm{O}}_{2}^{-} $ $ \Rightarrow $ CO + O2 + O 6.0 × 10–13 [44] (H25) $ {\mathrm{C}\mathrm{O}}_{2}^{+} $ + $ {\mathrm{C}\mathrm{O}}_{3}^{-} $ $ \Rightarrow $ 2CO2 + O 5.0 × 10–13 [58] (H26) $ {\mathrm{C}\mathrm{O}}_{2}^{+} $ + $ {\mathrm{C}\mathrm{O}}_{4}^{-} $ $ \Rightarrow $ 2CO2 + O2 5.0 × 10–13 [18] (H27) $ {\mathrm{C}\mathrm{O}}_{3}^{-} $ + $ {\mathrm{O}}_{2}^{+} $ $ \Rightarrow $ CO2 + O2 + O 3.0 × 10–13 [18] (H28) $ {\mathrm{O}}_{2}^{+} $ + $ {\mathrm{C}\mathrm{O}}_{4}^{-} $ $ \Rightarrow $ CO2 + 2O2 3.0 × 10–13 [18] (H29) O– + O3 $ \Rightarrow $ 2O2 + e 3.0 × 10–16 [44] (H30) O– + O3 $ \Rightarrow $ $ {\mathrm{O}}_{3}^{-} $ + O 8.0 × 10–16 [18] (H31) $ {\mathrm{O}}_{2}^{-} $ + O3 $ \Rightarrow $ $ {\mathrm{O}}_{3}^{-} $ + O2 4.0 × 10–16 [18] (H32) O– + O2 $ \Rightarrow $ O + e + O2 6.9 × 10–16 [52] (H33) O– + O $ \Rightarrow $ O2 + e 2.3 × 10–16 [21] (H34) $ {\mathrm{O}}_{2}^{-} $ + O $ \Rightarrow $ O2 + O– 3.31 × 10–16 [21] (H35) $ {\mathrm{O}}_{2}^{-} $ + O $ \Rightarrow $ O3 + e 3.3 × 10–16 [18] (H36) $ {\mathrm{O}}_{3}^{-} $ + O $ \Rightarrow $ $ {\mathrm{O}}_{2}^{-} $ + O2 2.5 × 10–16 [18] (H37) O–+ $ {\mathrm{O}}_{2}^{+} $ $ \Rightarrow $ O2 + O 2.6 × 10–14(300/Tg)0.44 [21] (H38) O– + $ {\mathrm{O}}_{2}^{+} $ $ \Rightarrow $ 3O 4.2 × 10–13(300/Tg)0.44 [21] (H39) $ {\mathrm{O}}_{2}^{-} $ + $ {\mathrm{O}}_{2}^{+} $ $ \Rightarrow $ 2O2 2.01 × 10–13(300/Tg)0.5 [21] (H40) $ {\mathrm{O}}_{2}^{-} $ + $ {\mathrm{O}}_{2}^{+} $ $ \Rightarrow $ O2 + 2O 4.2 × 10–13 [21] -
[1] 史建魁, 张仲谋, 刘振兴 1997 地球物理学进展 04 98
 Google Scholar
Google Scholar
Shi J K, Zhang Z M, Liu Z X 1997 Prog. Geophys. 04 98
 Google Scholar
Google Scholar
[2] Thomas P, Gierasch P J 1985 Science 230 175
 Google Scholar
Google Scholar
[3] Sullivan R, Banfield D, Bell J F 2005 Nature 436 58
 Google Scholar
Google Scholar
[4] Bougher S W, Murphy J, Haberle R M 1997 Adv. Space Res. 19 1255
 Google Scholar
Google Scholar
[5] Lämmel M, Kroy K 2017 Phys. Rev. E 96 052906
 Google Scholar
Google Scholar
[6] Read P L, Lewis S R, Mulholland D P 2015 Rep. Prog. Phys. 78 125901
 Google Scholar
Google Scholar
[7] Barth E L, Farrell W M, Rafkin S C R 2016 Icarus 268 253
 Google Scholar
Google Scholar
[8] Esposito F, Molinaro R, Popa C I 2016 Geophys. Res. Lett. 43 5501
 Google Scholar
Google Scholar
[9] Schmidt D S, Schmidt R A, Dent J D 1998 J. Geophys. Res. 103 8997
 Google Scholar
Google Scholar
[10] Melnik O, Parrot M 1998 J. Geophys. Res. 103 29107
 Google Scholar
Google Scholar
[11] Eden H F, Vonnegut B 1973 Science 180 962
 Google Scholar
Google Scholar
[12] Farrell W M, McLain J L, Collier M R 2015 Icarus 254 333
 Google Scholar
Google Scholar
[13] Farrell W M, McLainb J L, Collier M R 2017 Icarus 297 90
 Google Scholar
Google Scholar
[14] Hecht M, Hoffman J, Rapp D 2021 Space Sci. Rev. 217 9
 Google Scholar
Google Scholar
[15] Keudell A V, Volker S 2017 Plasma Sources Sci. Technol. 26 113001
 Google Scholar
Google Scholar
[16] Guerra V, Silva T, Ogloblina P 2017 Plasma Sources Sci. Technol. 26 11LT01
 Google Scholar
Google Scholar
[17] Bogaerts A, Kozak T, Laer K V 2015 Faraday Discuss. 183 217
 Google Scholar
Google Scholar
[18] Kozák T, Bogaerts A 2014 Plasma Sources Sci. Technol. 23 045004
 Google Scholar
Google Scholar
[19] Aerts R, Martens T, Bogaerts A 2012 J. Phys. Chem. C 116 23257
 Google Scholar
Google Scholar
[20] Berthelot A, Bogaerts A 2017 J. Phys. Chem. C 121 8236
 Google Scholar
Google Scholar
[21] Sun R, Wang H X, Bogaerts A 2020 Plasma Sources Sci. Technol. 29 025012
 Google Scholar
Google Scholar
[22] Hurlbatt A, Gibson A, Schröter S 2017 Plasma Processes Polym. 14 1600138
 Google Scholar
Google Scholar
[23] Lazarou C, Belmonte T, Chiper A 2016 Plasma Sources Sci. Technol. 25 055023
 Google Scholar
Google Scholar
[24] Wang W, Berthelot A, Kolev S 2016 Plasma Sources Sci. Technol. 25 065012
 Google Scholar
Google Scholar
[25] Takana H, Nishiyama H 2014 Plasma Sources Sci. Technol. 23 034001
 Google Scholar
Google Scholar
[26] Dubin D H E, Jin D Z 2003 Phys. Plasmas 10 1338
 Google Scholar
Google Scholar
[27] Kelly S, Golda J, Turner M 2015 J. Phys. D:Appl. Phys. 48 444002
 Google Scholar
Google Scholar
[28] Iqbal M M, Stallard C P, Dowling D P, Turner M M 2014 Plasma Processes Polym. 12 201
 Google Scholar
Google Scholar
[29] Stagni A, Frassoldati A, Cuoci A 2015 Combust. Flame 163 382
 Google Scholar
Google Scholar
[30] Rabitz H, Kramer M, Dacol D 2003 Annu. Rev. Phys. Chem. 34 419
 Google Scholar
Google Scholar
[31] Tomlin A S, Pilling M J, Meriting J H 1995 Ind. Eng. Chem. Res. 34 3749
 Google Scholar
Google Scholar
[32] Brown N J, And G L, Koszykowski M L 2015 Int. J. Chem. Kinet. 29 393
 Google Scholar
Google Scholar
[33] Lehmann R 2004 J. Atmos. Chem. 47 45
 Google Scholar
Google Scholar
[34] Strogatz S, Steven H 2003 SIAM Rev. 45 167
 Google Scholar
Google Scholar
[35] Kolaczyk E D 2009 Statistical Analysis of Network Data: Methods and Models (New York: Springer) pp79−120
[36] Boccaletti S, Bianconi G, Criado R 2014 Phys. Rep. 544 1
 Google Scholar
Google Scholar
[37] Estrada E, Hatano N, Benzi M 2012 Phys. Rep. 514 89
 Google Scholar
Google Scholar
[38] Klamt S, Hädicke O, Kamp A V 2014 Large-Scale Networks in Engineering and Life Sciences (Cham: Birkhäuser Basel) pp263−316
[39] Sakai O, Nobuto K, Miyagi S 2015 AIP Adv. 5 107140
 Google Scholar
Google Scholar
[40] Mizui Y, Kojima T, Miyagi S 2017 Symmetry 9 309
 Google Scholar
Google Scholar
[41] Lu T F, Law C K 2005 Proc. Combust. Inst. 30 1333
 Google Scholar
Google Scholar
[42] Lu T F, Law C K 2006 Combust. Flame 146 472
 Google Scholar
Google Scholar
[43] Snoeckx R, Bogaerts A 2017 Chem. Soc. Rev. 46 5805
 Google Scholar
Google Scholar
[44] Aerts R, Somers W, Bogaerts A 2015 ChemSusChem 8 702
 Google Scholar
Google Scholar
[45] Zhang Y T, Wang Y H 2018 Phys. Plasmas 25 023509
 Google Scholar
Google Scholar
[46] 高书涵, 王绪成, 张远涛 2020 69 115204
 Google Scholar
Google Scholar
Gao S H, Wang X C, Zhang Y T 2020 Acta Phys. Sin. 69 115204
 Google Scholar
Google Scholar
[47] Bogaerts A, Wang W Z 2016 Plasma Sources Sci. Technol. 25 055016
 Google Scholar
Google Scholar
[48] Ponduri S, Becker M M, Welzel S 2016 J. Appl. Phys. 119 093301
 Google Scholar
Google Scholar
[49] Stijn H, Luca M M, Giorgio D 2019 J. Phys. Chem. C 123 12104
 Google Scholar
Google Scholar
[50] Phelps A V www.lxcat.net [2020-3-21]
[51] Lowke J J, Phelps A V, Irwin B W 1973 J. Appl. Phys. 44 4664
 Google Scholar
Google Scholar
[52] Beuthe T G, Chang J S 1997 Jpn. J. Appl. Phys. 36 4997
 Google Scholar
Google Scholar
[53] Eliasson B, Kogelschatz U 1986 Brown Boveri Research Report KLR 86-11C
[54] Roberson G, Roberto M, Verboncoeur J 2015 Braz. J. Phys. 37 457
 Google Scholar
Google Scholar
[55] Spencer L F 2012 Ph. D. Dissertation (Ann Arbor: University of Michigan)
[56] Berthelot A, Bogaerts A 2017 Plasma Sources Sci. Technol. 26 115002
 Google Scholar
Google Scholar
[57] Wang W, Snoeckx R, Zhang X 2018 J. Phys. Chem. C 122 8704
 Google Scholar
Google Scholar
[58] Berthelot A, Bogaerts A 2016 Plasma Sources Sci. Technol. 25 045022
 Google Scholar
Google Scholar
[59] Moss M S 2017 Plasma Sources Sci. Technol. 26 035009
 Google Scholar
Google Scholar
[60] Harder N D, Bekerom D C M V D, Al R S 2017 Plasma Processes Polym. 14 1600120
 Google Scholar
Google Scholar
[61] Vermeiren V, Bogaerts A 2018 J. Phys. Chem. C 122 25869
 Google Scholar
Google Scholar
[62] Cheng C, Ma M, Zhang Y, Liu D 2020 J. Phys. D: Appl. Phys. 53 144001
 Google Scholar
Google Scholar
[63] Li J, Fang C, Chen J, Li H P 2021 J. Appl. Phys. 129 133302
 Google Scholar
Google Scholar
Catalog
Metrics
- Abstract views: 5849
- PDF Downloads: 105
- Cited By: 0


















 DownLoad:
DownLoad: