-
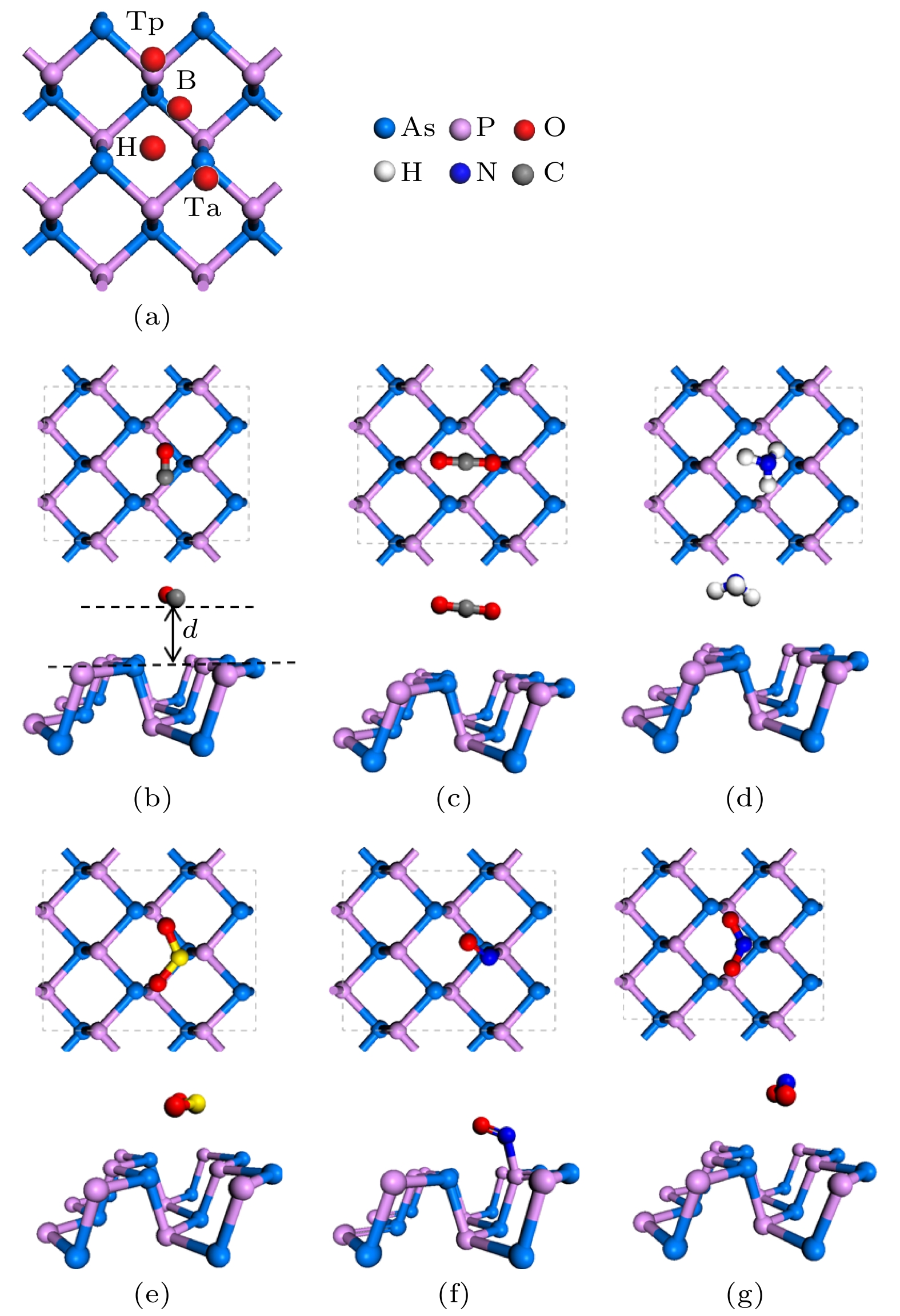
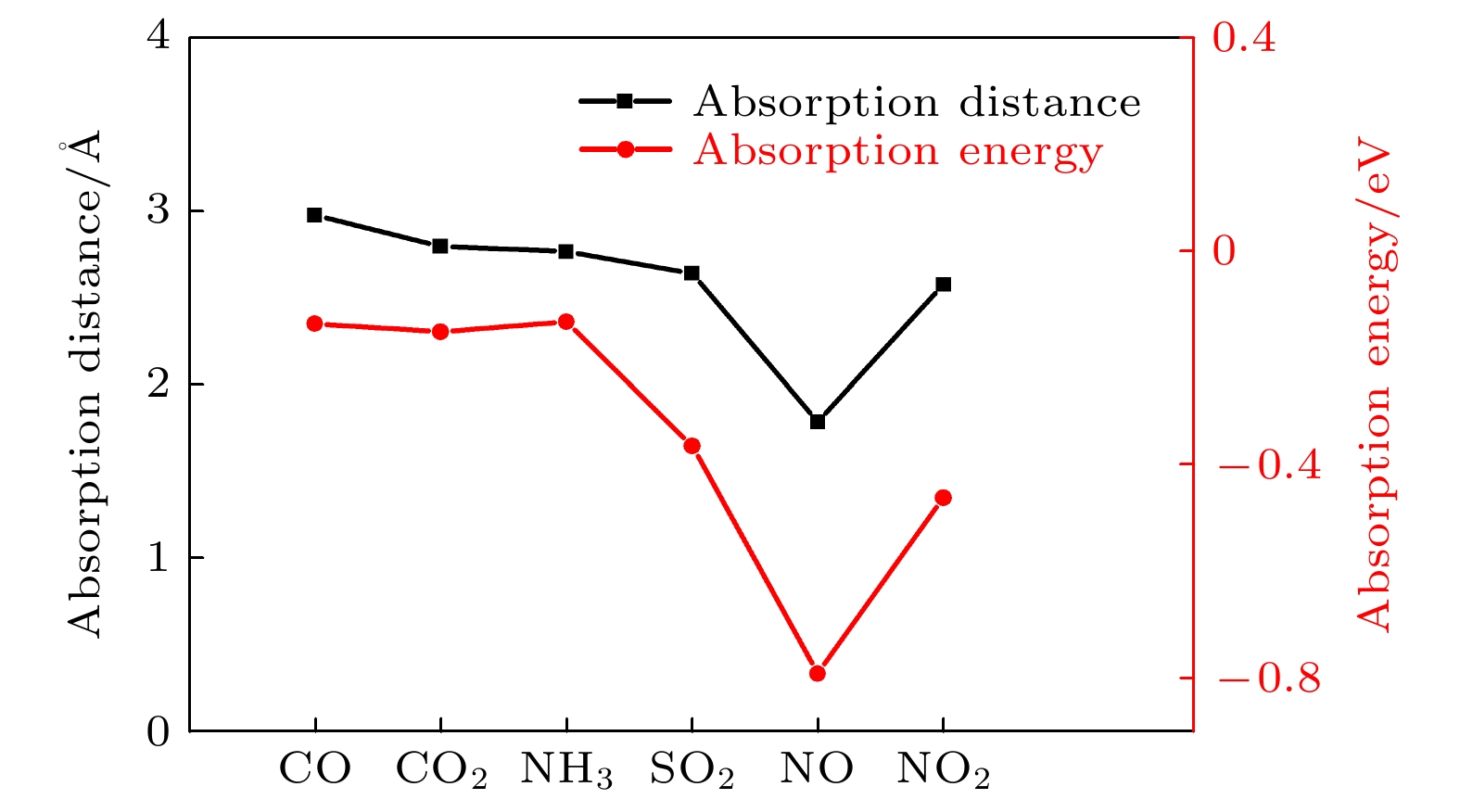
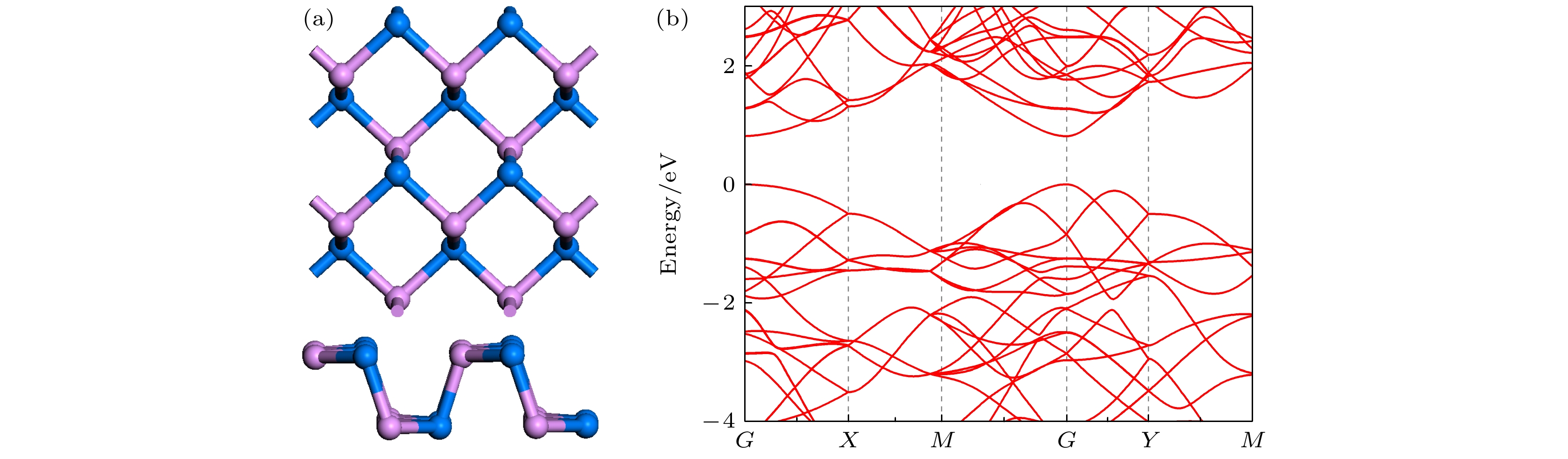
Since the successful synthesis of graphene, two-dimensional materials, including hexagonal boron nitride and transition mental dichalcogenides, have attracted wide attention due to their extraordinary properties and extensive applications. Recent researches have revealed that the sensing system based on graphene or MoS2 can efficiently sense various gas molecules. However, the utility of these materials is limited by their inherent weakness, i.e. the zero bandgap in graphene and the relatively low mobility in MoS2, which impede their applications in electronic devices. This further stimulates the motivation of researchers to find more novel 2D materials. Black arsenic phosphide (AsP) monolayer, a novel two-dimensional nanomaterial with the characteristics of model direct bandgap and superhigh carrier mobility, is an ideal material for gas sensor. Here in this work, we investigate the electronic and magnetic properties of monolayer AsP absorbed with small gas molecules by using first-principle calculations based on density functional theory. Four initial absorption sites are selected to explore the optimal absorption positions of CO, CO2, NH3, SO2, NO and NO2 absorbed on the monolayer AsP. The purpose is to calculate the optimal absorption configurations, the absorption energy, absorption distance, and charge transfer, thereby investigating the absorption types. The results revel that the monolayer AsP is sensitive to NO2 gas and SO2 gas via strong physical absorption, and NO gas by chemical absorption, forming a new bond between N atom and O atom. The CO, CO2 and NH3 gas are absorbed on AsP monolayer with weak van Waals force. From the point of view of charge transfer, the CO, CO2, and NH3 molecules are one order of magnitude smaller than SO2, NO and NO2, approximately 0.03e and the charge transfer of NO gas is 0.21e, highest in all gases. Besides, the effects of absorption on the electrons of AsP are investigated. The results show that the absorption of CO, CO2 and NH3 molecules have little effect on band structure, and that the absorption of SO2 molecule increases the bandgap. The absorption of magnetic gas NO and NO2 reduce the bandgap by introducing impurity level near Fermi level, giving rise to their magnetic moments of 0.83μB and 0.78μB and making the whole system magnetic. Theoretical research shows that monolayer AsP is sensitive to NO, NO2 and SO2 gas molecules, which provides theoretical guidance for the experimental preparation of gas sensors band on black arsenic phosphorus.
-
Keywords:
- black arsenic phosphide /
- first principle /
- gas sensor
[1] Kong J, Franklin N R, Zhou C, Chapline M G, Peng S, Cho K, Dai H 2000 Science 287 622
 Google Scholar
Google Scholar
[2] Bao H, Yu S, Tong D Q 2010 Nature 465 909
 Google Scholar
Google Scholar
[3] Esrafili M D 2019 Phys. Lett. A 383 1607
 Google Scholar
Google Scholar
[4] Chen X, Shen Y, Zhou P, Zhao S, Zhong X, Li T, Han C, Wei D, Meng D 2019 Sens. Actuators, B 280 151
 Google Scholar
Google Scholar
[5] Li W, Ding C, Li J, Ren Q, Bai G, Xu J 2020 Appl. Surf. Sci. 502 144140
 Google Scholar
Google Scholar
[6] 丁超, 李卫, 刘菊燕, 王琳琳, 蔡云, 潘沛锋 2018 67 213102
 Google Scholar
Google Scholar
Ding C, Li W, Liu J Y, Wang L L, Cai Y, Pan P F 2018 Acta Phys. Sin. 67 213102
 Google Scholar
Google Scholar
[7] Manzeli S, Ovchinnikov D, Pasquier D, Yazyev O V, Kis A 2017 Nat. Rev. Mater. 2 17033
 Google Scholar
Google Scholar
[8] Mousavi H 2011 Commun. Theor. Phys. 56 373
 Google Scholar
Google Scholar
[9] Xia W, Hu W, Li Z, Yang J 2014 Phys. Chem. Chem. Phys. 16 22495
 Google Scholar
Google Scholar
[10] Geim A K, Novoselov K S 2007 Nat. Mater. 6 183
 Google Scholar
Google Scholar
[11] Huang B, Li Z, Liu Z, Zhou G, Hao S, Wu J, Gu B L, Duan W 2008 J. Phys. Chem. C 112 13442
 Google Scholar
Google Scholar
[12] Sarkar D, Xie X, Kang J, Zhang H, Liu W, Navarrete J, Moskovits M, Banerjee K 2015 Nano Lett. 15 2852
 Google Scholar
Google Scholar
[13] Tian X Q, Liu L, Wang X R, Wei Y D, Gu J, Du Y, Yakobson B I 2017 J. Mater. Chem. C 5 1463
 Google Scholar
Google Scholar
[14] Zhang Y H, Chen Y B, Zhou K G, Liu C H, Zeng J, Zhang H L, Peng Y 2009 Nanotechnology 20 185504
 Google Scholar
Google Scholar
[15] Yuan W, Shi G 2013 J. Mater. Chem. A 1 10078
 Google Scholar
Google Scholar
[16] Kaasbjerg K, Thygesen K S, Jacobsen K W 2012 Phys. Rev. B 85 115317
 Google Scholar
Google Scholar
[17] Zhou L, Kou L, Sun Y, Felser C, Hu F, Shan G, Smith S C, Yan B, Frauenheim T 2015 Nano Lett. 15 7867
 Google Scholar
Google Scholar
[18] Eswaraiah V, Zeng Q, Long Y, Liu Z 2016 Small 12 3480
 Google Scholar
Google Scholar
[19] Jyothi M S, Nagarajan V, Chandiramouli R 2020 Chem. Phys. 538 110896
 Google Scholar
Google Scholar
[20] Kou L, Frauenheim T, Chen C 2014 J. Phys. Chem. Lett. 5 2675
 Google Scholar
Google Scholar
[21] Osters O, Nilges T, Bachhuber F, Pielnhofer F, Weihrich R, Schöneich M, Schmidt P 2012 Angew. Chem. Int. Ed. 51 2994
 Google Scholar
Google Scholar
[22] Yang A, Wang D, Wang X, Zhang D, Koratkar N, Rong M 2018 Nano Today 20 13
 Google Scholar
Google Scholar
[23] Jing Y, Ma Y, Li Y, Heine T 2017 Nano Lett. 17 1833
 Google Scholar
Google Scholar
[24] Niu F, Cai M, Pang J, Li X, Yang D, Zhang G 2019 Vacuum 168 108823
 Google Scholar
Google Scholar
[25] Zhu Z, Guan J, Liu D, Tománek D 2015 ACS Nano 9 8284
 Google Scholar
Google Scholar
[26] Guo S, Zhang Y, Ge Y, Zhang S, Zeng H, Zhang H 2019 Adv. Mater. 31 e1902352
 Google Scholar
Google Scholar
[27] He Y, Xiong S, Xia F, Shao Z, Zhao J, Zhang X, Jie J, Zhang X 2018 Phys. Rev. B 97 085119
 Google Scholar
Google Scholar
[28] Kocabas T, Cakir D, Gulseren O, Ay F, Kosku Perkgoz N, Sevik C 2018 Nanoscale 10 7803
 Google Scholar
Google Scholar
[29] Liu X, Ni Y X, Wang H Y, Wang H 2020 Chin J. Chem. Phys. 33 311
 Google Scholar
Google Scholar
[30] Tang J P, Xiao W Z, Wang L L 2018 Mater. Sci. Eng., B 228 206
 Google Scholar
Google Scholar
[31] Xiao W Z, Xiao G, Rong Q Y, Wang L L 2018 Mater. Res. Express 5 035903
 Google Scholar
Google Scholar
[32] Liu B, Köpf M, Abbas A N, Wang X, Guo Q, Jia Y, Xia F, Weihrich R, Bachhuber F, Pielnhofer F, Wang H, Dhall R, Cronin S B, Ge M, Fang X, Nilges T, Zhou C 2015 Adv. Mater. 27 4423
 Google Scholar
Google Scholar
[33] Krebs H, Holz W, Worms K H 1957 Chem. Ber. 90 1031
 Google Scholar
Google Scholar
[34] Yu W, Niu C Y, Zhu Z, Wang X, Zhang W B 2016 J. Mater. Chem. C 4 6581
 Google Scholar
Google Scholar
[35] Xie M, Zhang S, Cai B, Huang Y, Zou Y, Guo B, Gu Y, Zeng H 2016 Nano Energy 28 433
 Google Scholar
Google Scholar
[36] Imai Y, Mukaida M, Tsunoda T 2001 Thin Solid Films 381 176
 Google Scholar
Google Scholar
[37] Blöchl P E 1994 Phys. Rev. B 50 17953
 Google Scholar
Google Scholar
[38] Perdew J P, Burke K, Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[39] Setyawan W, Curtarolo S 2010 Comput. Mater. Sci. 49 299
 Google Scholar
Google Scholar
[40] Hirshfeld F L 1977 Theor. Chim. Acta 44 129
 Google Scholar
Google Scholar
[41] Liu C, Liu C S, Yan X 2017 Phys. Lett. A 381 1092
 Google Scholar
Google Scholar
[42] Heyd J, Scuseria G E 2003 J. Chem. Phys. 118 8207
 Google Scholar
Google Scholar
[43] Abbas A N, Liu B, Chen L, Ma Y, Cong S, Aroonyadet N, Köpf M, Nilges T, Zhou C 2015 ACS Nano 9 5618
 Google Scholar
Google Scholar
[44] Bai L, Zhou Z 2007 Carbon 45 2105
 Google Scholar
Google Scholar
[45] Lee S Y, Ito E, Kang H, Hara M, Lee H, Noh J 2014 J. Phys. Chem. C 118 8322
 Google Scholar
Google Scholar
-
图 4 (a) CO, (b) CO2, (c) NH3和(d) SO2吸附在单层AsP表面的能带结构; (e), (f) NO和 (g), (h) NO2吸附在单层AsP表面的能带结构图, 其中黑线和蓝线分别表示自旋向上和自旋向下的能带结构
Figure 4. Band structure of (a) CO, (b) CO2, (c) NH3 and (d) SO2 absorbed on AsP monolayer; the band structure of (e), (f) NO and (g), (h) NO2 absorbed on AsP monolayer, where the black and blue line represent the band structure of spin-up and spin-down, respectively.
图 5 (a) CO, (b) CO2, (c) NH3, (d) SO2, (e) NO和(f) NO2吸附在单层AsP上的态密度和分态密度图, 其中黑线和红线分别是原始AsP的态密度和吸附气体后的态密度图
Figure 5. Density of states (DOS) of (a) CO, (b) CO2, (c) NH3, (d) SO2, (e) NO and (f) NO2 absorbed on AsP monolayer, respectively. The black and red line represent the DOS of pristine AsP and gases absorbed on AsP.
表 1 不同气体吸附的吸附能、转移电荷数量、气体分子的磁矩和恢复时间, 括号里数值是与Mulliken分析法对比的结果
Table 1. The absorption energy (Ead), the charge transfer from basic material to gas molecule (ρ), the local magnetic on gas molecule (Spin) and the recovery time (τ) for gas desorption from material. The values in brackets are the results of comparison with Mulliken analysis.
气体 Ead /eV ρ/e Spin/ μB τ/s CO –0.135 –0.03 (0.02) 0 1.8 × 10–10 CO2 –0.156 –0.01 (–0.04) 0 4.03 × 10–10 NH3 –0.131 –0.02 (–0.04) 0 1.5 × 10–10 SO2 –0.363 –0.15 (0.05) 0 1.2 × 10–6 NO –0.79 –0.21 (–0.20) 0.83 15.7 NO2 –0.46 –0.14 (–0.01) 0.78 4.8 × 10–5 -
[1] Kong J, Franklin N R, Zhou C, Chapline M G, Peng S, Cho K, Dai H 2000 Science 287 622
 Google Scholar
Google Scholar
[2] Bao H, Yu S, Tong D Q 2010 Nature 465 909
 Google Scholar
Google Scholar
[3] Esrafili M D 2019 Phys. Lett. A 383 1607
 Google Scholar
Google Scholar
[4] Chen X, Shen Y, Zhou P, Zhao S, Zhong X, Li T, Han C, Wei D, Meng D 2019 Sens. Actuators, B 280 151
 Google Scholar
Google Scholar
[5] Li W, Ding C, Li J, Ren Q, Bai G, Xu J 2020 Appl. Surf. Sci. 502 144140
 Google Scholar
Google Scholar
[6] 丁超, 李卫, 刘菊燕, 王琳琳, 蔡云, 潘沛锋 2018 67 213102
 Google Scholar
Google Scholar
Ding C, Li W, Liu J Y, Wang L L, Cai Y, Pan P F 2018 Acta Phys. Sin. 67 213102
 Google Scholar
Google Scholar
[7] Manzeli S, Ovchinnikov D, Pasquier D, Yazyev O V, Kis A 2017 Nat. Rev. Mater. 2 17033
 Google Scholar
Google Scholar
[8] Mousavi H 2011 Commun. Theor. Phys. 56 373
 Google Scholar
Google Scholar
[9] Xia W, Hu W, Li Z, Yang J 2014 Phys. Chem. Chem. Phys. 16 22495
 Google Scholar
Google Scholar
[10] Geim A K, Novoselov K S 2007 Nat. Mater. 6 183
 Google Scholar
Google Scholar
[11] Huang B, Li Z, Liu Z, Zhou G, Hao S, Wu J, Gu B L, Duan W 2008 J. Phys. Chem. C 112 13442
 Google Scholar
Google Scholar
[12] Sarkar D, Xie X, Kang J, Zhang H, Liu W, Navarrete J, Moskovits M, Banerjee K 2015 Nano Lett. 15 2852
 Google Scholar
Google Scholar
[13] Tian X Q, Liu L, Wang X R, Wei Y D, Gu J, Du Y, Yakobson B I 2017 J. Mater. Chem. C 5 1463
 Google Scholar
Google Scholar
[14] Zhang Y H, Chen Y B, Zhou K G, Liu C H, Zeng J, Zhang H L, Peng Y 2009 Nanotechnology 20 185504
 Google Scholar
Google Scholar
[15] Yuan W, Shi G 2013 J. Mater. Chem. A 1 10078
 Google Scholar
Google Scholar
[16] Kaasbjerg K, Thygesen K S, Jacobsen K W 2012 Phys. Rev. B 85 115317
 Google Scholar
Google Scholar
[17] Zhou L, Kou L, Sun Y, Felser C, Hu F, Shan G, Smith S C, Yan B, Frauenheim T 2015 Nano Lett. 15 7867
 Google Scholar
Google Scholar
[18] Eswaraiah V, Zeng Q, Long Y, Liu Z 2016 Small 12 3480
 Google Scholar
Google Scholar
[19] Jyothi M S, Nagarajan V, Chandiramouli R 2020 Chem. Phys. 538 110896
 Google Scholar
Google Scholar
[20] Kou L, Frauenheim T, Chen C 2014 J. Phys. Chem. Lett. 5 2675
 Google Scholar
Google Scholar
[21] Osters O, Nilges T, Bachhuber F, Pielnhofer F, Weihrich R, Schöneich M, Schmidt P 2012 Angew. Chem. Int. Ed. 51 2994
 Google Scholar
Google Scholar
[22] Yang A, Wang D, Wang X, Zhang D, Koratkar N, Rong M 2018 Nano Today 20 13
 Google Scholar
Google Scholar
[23] Jing Y, Ma Y, Li Y, Heine T 2017 Nano Lett. 17 1833
 Google Scholar
Google Scholar
[24] Niu F, Cai M, Pang J, Li X, Yang D, Zhang G 2019 Vacuum 168 108823
 Google Scholar
Google Scholar
[25] Zhu Z, Guan J, Liu D, Tománek D 2015 ACS Nano 9 8284
 Google Scholar
Google Scholar
[26] Guo S, Zhang Y, Ge Y, Zhang S, Zeng H, Zhang H 2019 Adv. Mater. 31 e1902352
 Google Scholar
Google Scholar
[27] He Y, Xiong S, Xia F, Shao Z, Zhao J, Zhang X, Jie J, Zhang X 2018 Phys. Rev. B 97 085119
 Google Scholar
Google Scholar
[28] Kocabas T, Cakir D, Gulseren O, Ay F, Kosku Perkgoz N, Sevik C 2018 Nanoscale 10 7803
 Google Scholar
Google Scholar
[29] Liu X, Ni Y X, Wang H Y, Wang H 2020 Chin J. Chem. Phys. 33 311
 Google Scholar
Google Scholar
[30] Tang J P, Xiao W Z, Wang L L 2018 Mater. Sci. Eng., B 228 206
 Google Scholar
Google Scholar
[31] Xiao W Z, Xiao G, Rong Q Y, Wang L L 2018 Mater. Res. Express 5 035903
 Google Scholar
Google Scholar
[32] Liu B, Köpf M, Abbas A N, Wang X, Guo Q, Jia Y, Xia F, Weihrich R, Bachhuber F, Pielnhofer F, Wang H, Dhall R, Cronin S B, Ge M, Fang X, Nilges T, Zhou C 2015 Adv. Mater. 27 4423
 Google Scholar
Google Scholar
[33] Krebs H, Holz W, Worms K H 1957 Chem. Ber. 90 1031
 Google Scholar
Google Scholar
[34] Yu W, Niu C Y, Zhu Z, Wang X, Zhang W B 2016 J. Mater. Chem. C 4 6581
 Google Scholar
Google Scholar
[35] Xie M, Zhang S, Cai B, Huang Y, Zou Y, Guo B, Gu Y, Zeng H 2016 Nano Energy 28 433
 Google Scholar
Google Scholar
[36] Imai Y, Mukaida M, Tsunoda T 2001 Thin Solid Films 381 176
 Google Scholar
Google Scholar
[37] Blöchl P E 1994 Phys. Rev. B 50 17953
 Google Scholar
Google Scholar
[38] Perdew J P, Burke K, Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[39] Setyawan W, Curtarolo S 2010 Comput. Mater. Sci. 49 299
 Google Scholar
Google Scholar
[40] Hirshfeld F L 1977 Theor. Chim. Acta 44 129
 Google Scholar
Google Scholar
[41] Liu C, Liu C S, Yan X 2017 Phys. Lett. A 381 1092
 Google Scholar
Google Scholar
[42] Heyd J, Scuseria G E 2003 J. Chem. Phys. 118 8207
 Google Scholar
Google Scholar
[43] Abbas A N, Liu B, Chen L, Ma Y, Cong S, Aroonyadet N, Köpf M, Nilges T, Zhou C 2015 ACS Nano 9 5618
 Google Scholar
Google Scholar
[44] Bai L, Zhou Z 2007 Carbon 45 2105
 Google Scholar
Google Scholar
[45] Lee S Y, Ito E, Kang H, Hara M, Lee H, Noh J 2014 J. Phys. Chem. C 118 8322
 Google Scholar
Google Scholar
Catalog
Metrics
- Abstract views: 9093
- PDF Downloads: 213
- Cited By: 0














 DownLoad:
DownLoad: