-
With the advantages of non-radiation and low-cost, ultrasound imaging has been widely used in clinical diagnosis. However, due to the compromise between temporal and spatial resolution, the traditional ultrasound imaging method which collects images line by line fails to obtain the images at a high frame rate. Ultrafast ultrasound imaging method based on the plane-wave emission can achieve a high frame rate with the capability for instantaneous hemodynamic imaging of neurovascular response. Besides, by the coherent compounding of the echo signals received from emitting a set of tilting plane-waves, the image quality can be improved in terms of resolution, contrast and signal-to-noise ratio (SNR). Considering the fact that received signals are the mixture of echo signals from the low-speed soft tissue and high-speed blood flow, a clutter filtering method is used to remove the signals of soft tissue. In this study, the ultrafast ultrasound sequence of emission and reception is designed to image the spinal cord by using the groups of multiple steering-angle plane-waves. The so-called f-k migration algorithm based on an exploding reflector model (ERM) is used for coherent plane-wave compounding. Eigenvalue decomposition (EVD) is then applied to tissue and blood signal extraction. The static tissue signals correspond to eigenvectors with low Doppler frequency shift and large magnitude eigenvalue while the high-speed blood flow signals correspond to eigenvectors with high Doppler frequency shift and small eigenvalue. Therefore, frequency and amplitude thresholds can be applied to the accurate separation of the blood component and the tissue component. After the signal extraction, the hemodynamic imaging of blood vessels can thus be obtained from the power Doppler results. The experiments are carried out by using a programmable ultrasonic array system and a high-frequency linear array transducer L22-14vX with a central frequency of 15.625 MHz. The sample acquisition frequency is set to be 62.5 MHz. In rat experiments in vivo, 14040 angle-stilting images per second are compounded into 520 images, each of which is compounded from 27 tilting images (the tilting angles ranging from –10° to 10°). The experiments are conducted on the anesthetized rats with part of the vertebral plate removed. The ultrafast B-mode images are acquired from a 14.0 mm ×12.7 mm region-of-interest. The 520 frames acquired in one second are processed by the clutter filter based on eigenvalue decomposition and Doppler shift analysis. The eigenvectors and eigenvalues corresponding to the soft tissue are discarded. The power Doppler images of the spinal cord micro-vessels are obtained. The experimental results indicate that the ultrafast ultrasound Doppler imaging method is effective for monitoring the hemodynamic variation in spinal cord. The micro-hemorrhage can be identified from the power Doppler images. The quantitative results indicate that the SNR increases with the steering angles increasing. Compared with the results obtained by using 9 steering angles, 5 dB SNR enhancement can be obtained by using the 27 steering angles. In conclusion, the ultrafast ultrasound Doppler technology has the potential applications in spinal cord microvascular imaging and hemodynamic evaluation of neurovascular function of spinal cord.
-
Keywords:
- ultrafast ultrasound Doppler /
- clutter filtering /
- eigenvalue decomposition /
- spinal cord micro-vessels
[1] Ducker T B, Assenmacher D R 1951 Surg. Forum 3 428
 Google Scholar
Google Scholar
[2] Guha A, Tator C H, Rochon J 1989 Stroke 20 372
 Google Scholar
Google Scholar
[3] Mautes A E M, Weinzierl M R, Donovan F, Noble L J 2000 Phys. Ther. 80 673
 Google Scholar
Google Scholar
[4] Pickett G E, Campos-Benitez M, Keller J L, Duggal N 2006 Spine (Phila Pa 1976) 31 799
 Google Scholar
Google Scholar
[5] Song P, Cuellar C A, Tang S, Islam R, Wen H, Huang C, Manduca A, Trzasko J D, Knudsen B E, Lee K H, Chen S, Lavrov I A 2019 Front. Neurol. 10 279
 Google Scholar
Google Scholar
[6] Bruce M, Hannah A, Hammond R, Khaing Z Z, Tremblay-Darveau C, Burns P N, Hofstetter C P 2020 IEEE Trans. Ultrason. Ferroelectr. Freq. 67 1776
 Google Scholar
Google Scholar
[7] Khaing Z Z, Cates L N, DeWees D M, Hannah A, Mourad P, Bruce M, Hofstetter C P 2018 J. Neurosurg. Spine 29 306
 Google Scholar
Google Scholar
[8] Kornblum H I, Araujo D M, Annala A J, Tatsukawa K J, Phelps M E, Cherry S R 2000 Nat. Biotechnol. 18 655
 Google Scholar
Google Scholar
[9] Phelps M E 1981 Semin. Nucl. Med. 11 32
 Google Scholar
Google Scholar
[10] Ogawa S, Lee T M, Kay A R, Tank D W 1990 Proc. Natl. Acad. Sci. U. S. A. 87 9868
 Google Scholar
Google Scholar
[11] Voorneveld J, Muralidharan A, Hope T, Vos H J, Kruizinga P, van der Steen A F W, Gijsen F J H, Kenjeres S, de Jong N, Bosch J G 2018 IEEE Trans. Ultrason. Ferroelectr. Freq. 65 2222
 Google Scholar
Google Scholar
[12] Tremblay-Darveau C, Sheeran P S, Vu C K, Williams R, Bruce M, Burns P N 2018 IEEE Trans. Ultrason. Ferroelectr. Freq. 65 2286
 Google Scholar
Google Scholar
[13] Starosolski Z, Villamizar C A, Rendon D, Paldino M J, Milewicz D M, Ghaghada K B, Annapragada A V 2015 Sci. Rep. 5 10178
 Google Scholar
Google Scholar
[14] Osmanski B F, Pezet S, Ricobaraza A, Lenkei Z, Tanter M 2014 Nat. Commun. 5 5023
 Google Scholar
Google Scholar
[15] Xia J, Yang Y, Hu C, Meng R, Jiang Q, Liu R, Yu Y, Sheng Z, Yan F, Zhang L, Shi Z, Zheng H, Qiu W 2019 Ultrasound Med. Biol. 45 811
 Google Scholar
Google Scholar
[16] Deffieux T, Demene C, Pernot M, Tanter M 2018 Curr. Opin. Neurobiol. 50 128
 Google Scholar
Google Scholar
[17] Correia M, Provost J, Tanter M, Pernot M 2016 Phys. Med. Biol. 61 48
 Google Scholar
Google Scholar
[18] Provost J, Papadacci C, Arango J E, Imbault M, Fink M, Gennisson J L, Tanter M, Pernot M 2014 Phys. Med. Biol. 59 1
 Google Scholar
Google Scholar
[19] Tanter M, Bercoff J, Sandrin L, Fink M 2002 IEEE Trans. Ultrason. Ferroelectr. Freq. 49 1363
 Google Scholar
Google Scholar
[20] Montaldo G, Tanter M, Bercoff J, Benech N, Fink M 2009 IEEE Trans. Ultrason. Ferroelectr. Freq. 56 489
 Google Scholar
Google Scholar
[21] Mace E, Montaldo G, Cohen I, Baulac M, Fink M, Tanter M 2011 Nat. Methods 8 662
 Google Scholar
Google Scholar
[22] Mace E, Montaldo G, Osmanski B F, Cohen I, Fink M, Tanter M 2013 IEEE Trans. Ultrason. Ferroelectr. Freq. 60 492
 Google Scholar
Google Scholar
[23] Demene C, Baranger J, Bernal M, Delanoe C, Auvin S, Biran V, Alison M, Mairesse J, Harribaud E, Pernot M, Tanter M, Baud O 2017 Sci. Transl. Med. 9 6756
 Google Scholar
Google Scholar
[24] Soloukey S, Harhangi B S, Generowicz B S, Slenter J P H, De Zeeuw C I, Kruizinga P, Koekkoek S K E 2019 IEEE International Ultrasonics Symposium Glasgow England Oct. 06–09, 2019 2259
[25] Garcia D, Le Tarnec L, Muth S, Montagnon E, Poree J, Cloutier G 2013 IEEE Trans. Ultrason. Ferroelectr. Freq. 60 1853
 Google Scholar
Google Scholar
[26] Gazdag J, Sguazzero P 1984 Proc. IEEE 72 1302
 Google Scholar
Google Scholar
[27] Demene C, Deffieux T, Pernot M, Osmanski B F, Biran V, Gennisson J L, Sieu L A, Bergel A, Franqui S, Correas J M 2015 IEEE Trans. Med. Imaging 34 2271
 Google Scholar
Google Scholar
[28] Yu A C H, Lovstakken L 2010 IEEE Trans. Ultrason. Ferroelectr. Freq. 57 1096
 Google Scholar
Google Scholar
[29] Mazensky D, Flesarova S, Sulla I 2017 Anat. Rec. 300 2091
 Google Scholar
Google Scholar
[30] Kang J, Go D, Song I, Yoo Y 2020 IEEE Trans. Ultrason. Ferroelectr. Freq. in press
[31] Jiang C, Li Y, Xu K, Ta D 2020 IEEE Trans. Ultrason. Ferroelectr. Freq. 68 72
 Google Scholar
Google Scholar
[32] Guasch L, Calderon Agudo O, Tang M X, Nachev P, Warner M 2020 NPJ Digital Medicine 3 28
 Google Scholar
Google Scholar
-
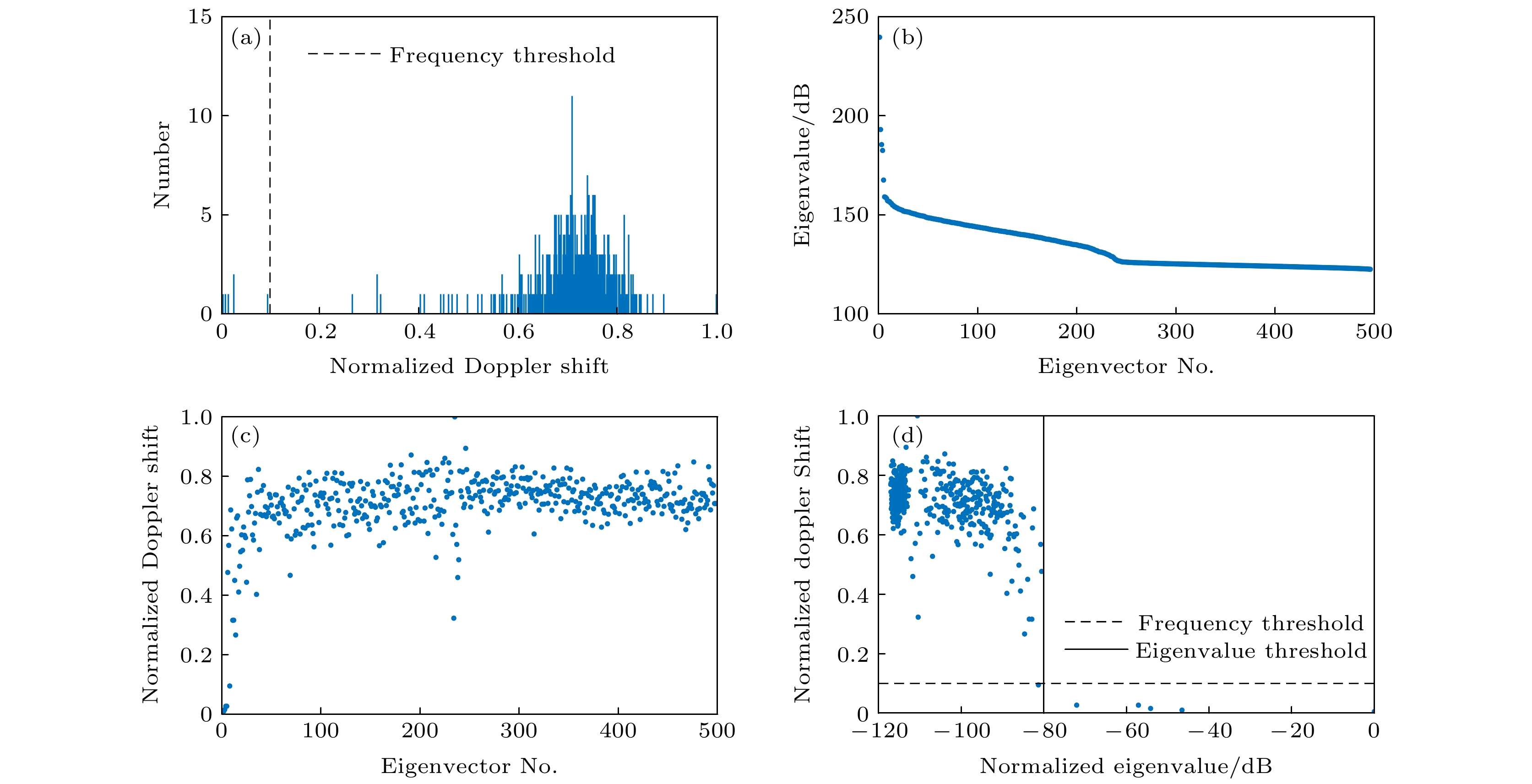
图 5 特征值分解与多普勒频移分析结果 (a) 归一化多普勒频移对应特征向量个数的直方图; (b)特征向量的特征值; (c) 特征向量的归一化多普勒频移; (d) 特征值对应的归一化多普勒频移
Figure 5. Eigenvalue decomposition and Doppler shift analysis results: (a) Histogram of the number of eigenvectors corresponding to normalized Doppler shifts; (b) eigenvalues of eigenvectors; (c) normalized Doppler shifts of eigenvectors; (d) eigenvalues versus normalized Doppler shifts.
图 6 仿体血流成像结果(第400帧) (a) 滤波前的成像结果; (b) 滤波后的血流成像结果; (c) 滤波后的软组织成像结果; (d) 功率多普勒成像结果
Figure 6. Imaging results of the 400th frame of the phantom blood flow: (a) Original image before clutter filtering; (b) blood flow image after clutter filtering; (c) soft tissue image after clutter filtering; (d) power Doppler imaging result.
图 7 特征值分解与多普勒频移分析结果 (a) 归一化多普勒频移对应特征向量个数的直方图; (b)特征向量的特征值; (c) 特征向量的归一化多普勒频移; (d) 特征值对应的归一化多普勒频移
Figure 7. Eigenvalue decomposition and Doppler shift analysis results: (a) Histogram of the number of eigenvectors corresponding to normalized Doppler shifts; (b) eigenvalues of eigenvectors; (c) normalized Doppler shifts of eigenvectors; (d) eigenvalues versus normalized Doppler shifts.
图 8 基于多角度平面波复合成像的大鼠脊髓血流成像结果 (a) 单角度平面波发射成像; (b) 多角度平面波复合成像; (c) 杂波滤除结果. 每一次发射超声平面波的时间间隔为71.225 μs, 27次发射倾斜平面波与接收反射回波的总时长为1.923 ms, 对多角度信号经相干复合可获得单帧超声图像, 其所对应的成像帧率为每秒520帧
Figure 8. Blood flow imaging results of rat spinal cord based on multi-angle compounding method: (a) Beamforming results after a single emission; (b) multi-angle compounding images; (c) images after clutter filtering. The time interval between each emission is 71.225 μs. Each compounded frame is obtained using 27 steering-angle plane-waves within a period of 1.923 ms. Consequently the frame rate is 520 frames per second.
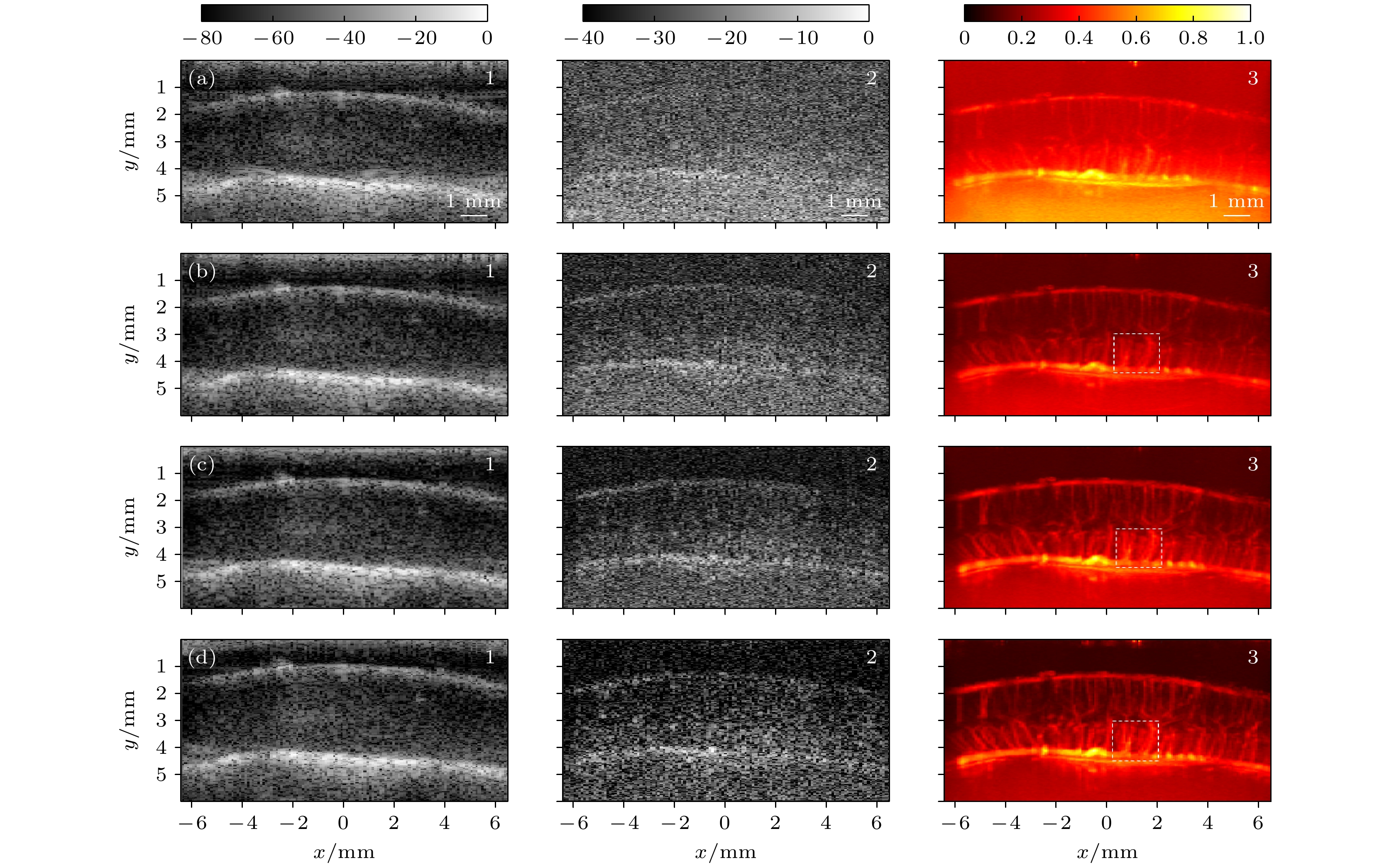
图 9 不同角度复合平面波成像结果对比图(复合帧频均为每秒520帧) (a) 3个角度[–1°—1°]倾斜平面波复合成像结果; (b) 9个角度[–3°—3°]倾斜平面波复合成像结果; (c) 17个角度[–7°—7°]倾斜平面波复合成像结果; (d) 27个角度[–10°—10°]倾斜平面波复合成像结果. 1为单帧原始B模式图像, 2为杂波滤除之后的成像结果, 其中可见微血流变化, 3为1 s内采集数据得到的功率多普勒血流图(1, 2色标单位为dB, 3为归一化数值的多普勒成像结果)
Figure 9. Comparison of compounded images with different numbers of steering angles (composite frame rate is 520 frames per second): (a) Images compounded of data from emitting 3 [–1°—1°] steering plane-waves; (b) images compounded of data from emitting 9 [–3°—3°] steering plane-waves; (c) images compounded of data from emitting 17 [–7°—7°] steering plane-waves; (d) images compounded of data from emitting 27 [–10°—10°] steering plane-waves. Images labeled 1 are original B-mode images; images labeled 2 are imaging results after clutter filtering in which changes of blood flow can be observed; images labeled 3 are power Doppler images of micro-vessels (data was obtained within 1 s).
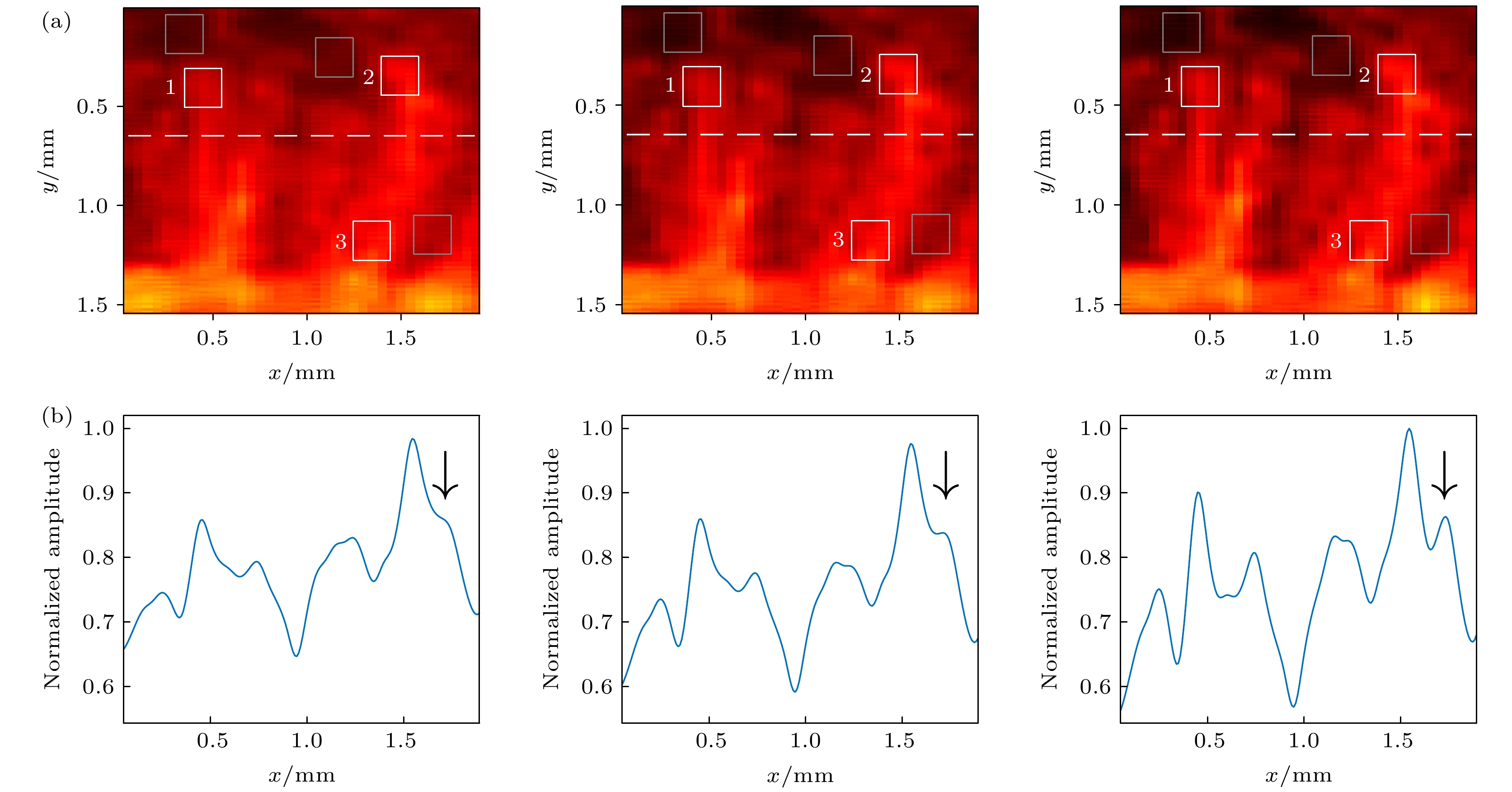
图 10 对比分辨率随复合平面波角度数增加的变化情况 (a) 当角度数N = 9, 17, 27时, 图9(b)-(d)编号3的图中矩形虚线框中图块的放大结果; (b) 当角度数N = 9, 17, 27时, 图10(a)中虚线深度处的幅值曲线图
Figure 10. Change of contrast resolution with the increase of the number of steering angles: (a) Enlarged image blocks in the rectangular dashed box in No.3 figure in Fig.9 (b)-(d) (angle numbers are 9, 17, 27, respectively); (b) amplitude curve of the dotted line position in Fig.10(a) (angle numbers are 9, 17, 27, respectively).
-
[1] Ducker T B, Assenmacher D R 1951 Surg. Forum 3 428
 Google Scholar
Google Scholar
[2] Guha A, Tator C H, Rochon J 1989 Stroke 20 372
 Google Scholar
Google Scholar
[3] Mautes A E M, Weinzierl M R, Donovan F, Noble L J 2000 Phys. Ther. 80 673
 Google Scholar
Google Scholar
[4] Pickett G E, Campos-Benitez M, Keller J L, Duggal N 2006 Spine (Phila Pa 1976) 31 799
 Google Scholar
Google Scholar
[5] Song P, Cuellar C A, Tang S, Islam R, Wen H, Huang C, Manduca A, Trzasko J D, Knudsen B E, Lee K H, Chen S, Lavrov I A 2019 Front. Neurol. 10 279
 Google Scholar
Google Scholar
[6] Bruce M, Hannah A, Hammond R, Khaing Z Z, Tremblay-Darveau C, Burns P N, Hofstetter C P 2020 IEEE Trans. Ultrason. Ferroelectr. Freq. 67 1776
 Google Scholar
Google Scholar
[7] Khaing Z Z, Cates L N, DeWees D M, Hannah A, Mourad P, Bruce M, Hofstetter C P 2018 J. Neurosurg. Spine 29 306
 Google Scholar
Google Scholar
[8] Kornblum H I, Araujo D M, Annala A J, Tatsukawa K J, Phelps M E, Cherry S R 2000 Nat. Biotechnol. 18 655
 Google Scholar
Google Scholar
[9] Phelps M E 1981 Semin. Nucl. Med. 11 32
 Google Scholar
Google Scholar
[10] Ogawa S, Lee T M, Kay A R, Tank D W 1990 Proc. Natl. Acad. Sci. U. S. A. 87 9868
 Google Scholar
Google Scholar
[11] Voorneveld J, Muralidharan A, Hope T, Vos H J, Kruizinga P, van der Steen A F W, Gijsen F J H, Kenjeres S, de Jong N, Bosch J G 2018 IEEE Trans. Ultrason. Ferroelectr. Freq. 65 2222
 Google Scholar
Google Scholar
[12] Tremblay-Darveau C, Sheeran P S, Vu C K, Williams R, Bruce M, Burns P N 2018 IEEE Trans. Ultrason. Ferroelectr. Freq. 65 2286
 Google Scholar
Google Scholar
[13] Starosolski Z, Villamizar C A, Rendon D, Paldino M J, Milewicz D M, Ghaghada K B, Annapragada A V 2015 Sci. Rep. 5 10178
 Google Scholar
Google Scholar
[14] Osmanski B F, Pezet S, Ricobaraza A, Lenkei Z, Tanter M 2014 Nat. Commun. 5 5023
 Google Scholar
Google Scholar
[15] Xia J, Yang Y, Hu C, Meng R, Jiang Q, Liu R, Yu Y, Sheng Z, Yan F, Zhang L, Shi Z, Zheng H, Qiu W 2019 Ultrasound Med. Biol. 45 811
 Google Scholar
Google Scholar
[16] Deffieux T, Demene C, Pernot M, Tanter M 2018 Curr. Opin. Neurobiol. 50 128
 Google Scholar
Google Scholar
[17] Correia M, Provost J, Tanter M, Pernot M 2016 Phys. Med. Biol. 61 48
 Google Scholar
Google Scholar
[18] Provost J, Papadacci C, Arango J E, Imbault M, Fink M, Gennisson J L, Tanter M, Pernot M 2014 Phys. Med. Biol. 59 1
 Google Scholar
Google Scholar
[19] Tanter M, Bercoff J, Sandrin L, Fink M 2002 IEEE Trans. Ultrason. Ferroelectr. Freq. 49 1363
 Google Scholar
Google Scholar
[20] Montaldo G, Tanter M, Bercoff J, Benech N, Fink M 2009 IEEE Trans. Ultrason. Ferroelectr. Freq. 56 489
 Google Scholar
Google Scholar
[21] Mace E, Montaldo G, Cohen I, Baulac M, Fink M, Tanter M 2011 Nat. Methods 8 662
 Google Scholar
Google Scholar
[22] Mace E, Montaldo G, Osmanski B F, Cohen I, Fink M, Tanter M 2013 IEEE Trans. Ultrason. Ferroelectr. Freq. 60 492
 Google Scholar
Google Scholar
[23] Demene C, Baranger J, Bernal M, Delanoe C, Auvin S, Biran V, Alison M, Mairesse J, Harribaud E, Pernot M, Tanter M, Baud O 2017 Sci. Transl. Med. 9 6756
 Google Scholar
Google Scholar
[24] Soloukey S, Harhangi B S, Generowicz B S, Slenter J P H, De Zeeuw C I, Kruizinga P, Koekkoek S K E 2019 IEEE International Ultrasonics Symposium Glasgow England Oct. 06–09, 2019 2259
[25] Garcia D, Le Tarnec L, Muth S, Montagnon E, Poree J, Cloutier G 2013 IEEE Trans. Ultrason. Ferroelectr. Freq. 60 1853
 Google Scholar
Google Scholar
[26] Gazdag J, Sguazzero P 1984 Proc. IEEE 72 1302
 Google Scholar
Google Scholar
[27] Demene C, Deffieux T, Pernot M, Osmanski B F, Biran V, Gennisson J L, Sieu L A, Bergel A, Franqui S, Correas J M 2015 IEEE Trans. Med. Imaging 34 2271
 Google Scholar
Google Scholar
[28] Yu A C H, Lovstakken L 2010 IEEE Trans. Ultrason. Ferroelectr. Freq. 57 1096
 Google Scholar
Google Scholar
[29] Mazensky D, Flesarova S, Sulla I 2017 Anat. Rec. 300 2091
 Google Scholar
Google Scholar
[30] Kang J, Go D, Song I, Yoo Y 2020 IEEE Trans. Ultrason. Ferroelectr. Freq. in press
[31] Jiang C, Li Y, Xu K, Ta D 2020 IEEE Trans. Ultrason. Ferroelectr. Freq. 68 72
 Google Scholar
Google Scholar
[32] Guasch L, Calderon Agudo O, Tang M X, Nachev P, Warner M 2020 NPJ Digital Medicine 3 28
 Google Scholar
Google Scholar
Catalog
Metrics
- Abstract views: 11634
- PDF Downloads: 219
- Cited By: 0















 DownLoad:
DownLoad: