-
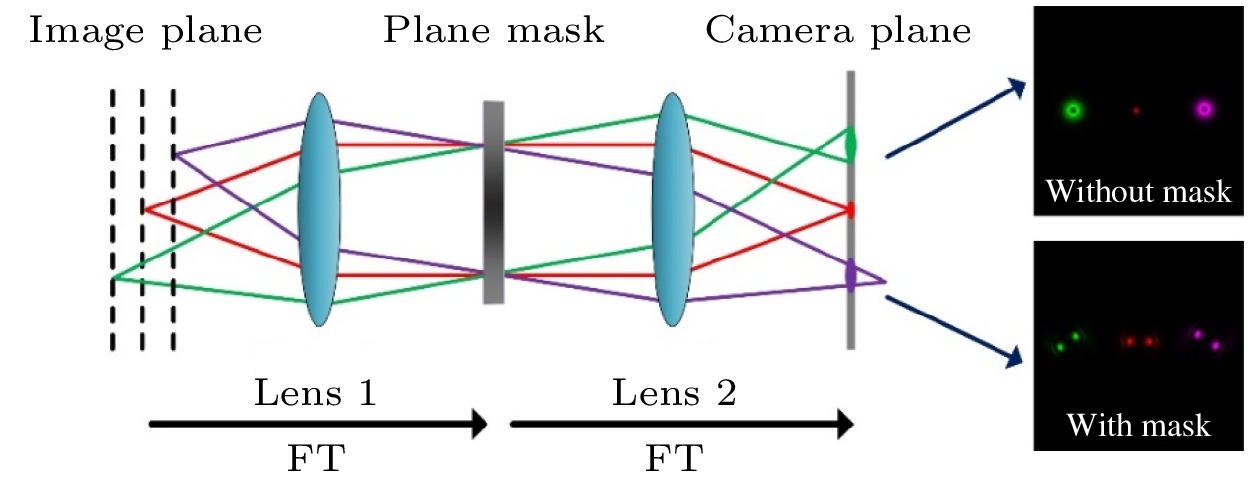
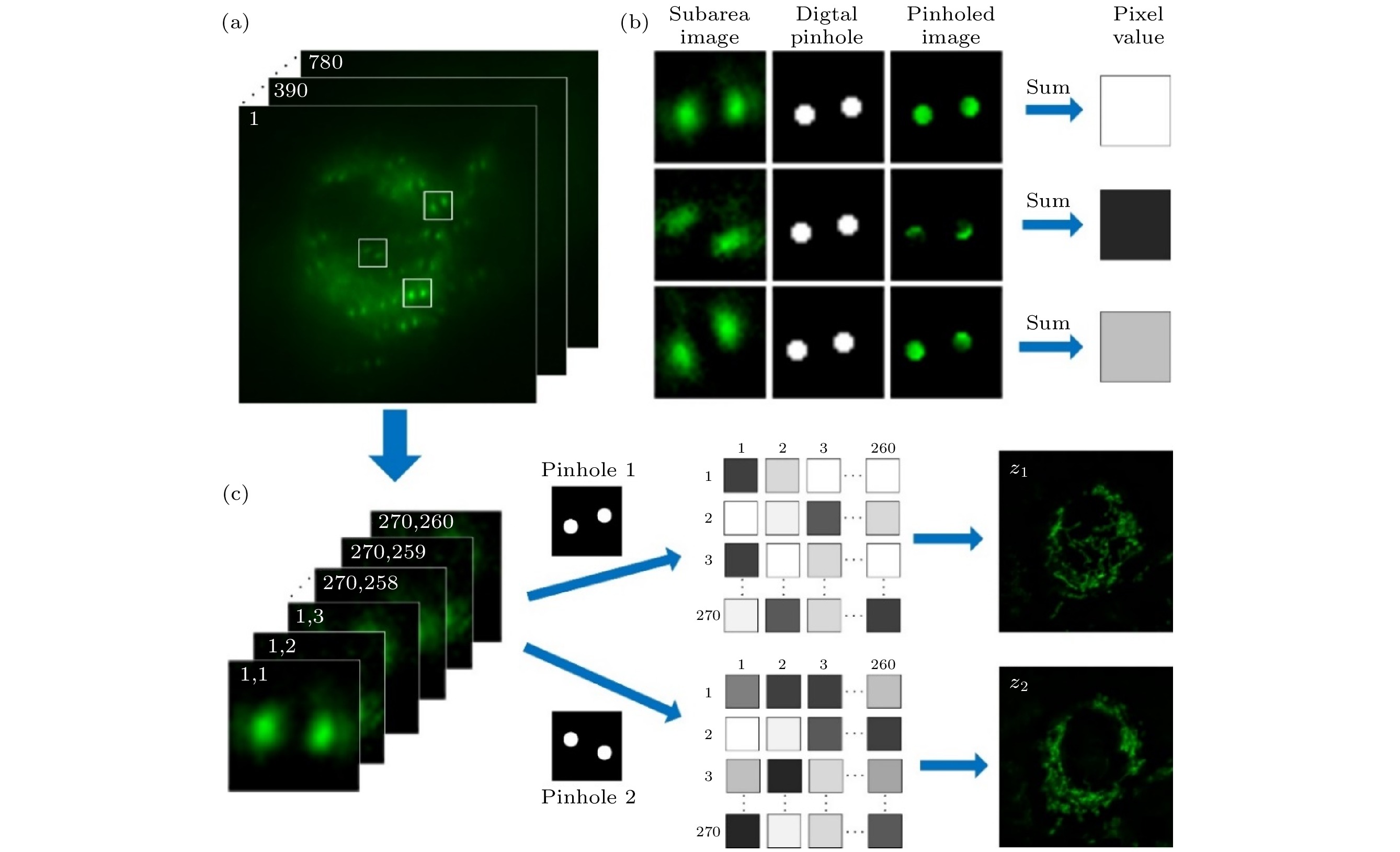
Confocal laser scanning microscopy (CLSM) is a powerful imaging tool providing high resolution and optical sectioning. In its standard optical configuration, a pair of confocal pinholes is used to reject out-of-focus light. The diffraction limited resolution can be broken by reducing the confocal pinhole size. But this comes at the cost of extremely low signal-to-noise ratio (SNR). The limited SNR problem can be solved by image scanning microscopy (ISM), in which the single-point detector of a regular point-scanning confocal microscopy is substituted with an array detector such as CCD or CMOS, thus the two-fold super-resolution imaging can be achieved by pixel reassignment and deconvolution. However, the practical application of ISM is challenging due to its limited image acquisition speed. Here, we present a hybrid microscopy technique, named multifocal refocusing after scanning using helical phase engineering microscopy (MRESCH), which combines the double-helix point spread function (DH-PSF) engineering with multifocal structured illumination to dramatically improve the image acquisition speed. In the illumination path, sparse multifocal illumination patterns are generated by a digital micromirror device for parallel imaging information acquisition. In the detection path, a phase mask is introduced to modulate the conventional PSF to the DH-PSF, which provides volumetric information, and meanwhile, we also present a digital refocusing strategy for processing the collected raw data to recover the wild-filed image from different sample layers. To demonstrate imaging capabilities of MRESCH, we acquire the images of mitochondria in live HeLa cells and make a detailed comparison with those from the wide-field microscopy. In contrast to the conventional wide-field approach, the MRESCH can expand the imaging depth in a range from –1 μm to 1 μm. Next, we sample the F-actin of bovine pulmonary artery endothelial cells to characterize the lateral resolution of the MRESCH. The results show that the MRESCH has a better resolution capability than the conventional wide-field illumination microscopy. Finally, the proposed image scanning microscopy can record three-dimensional specimen information from a single multi-spot two-dimensional scan, which ensures faster data acquisition and larger field of view than ISM.
[1] Pawley J B 2006 Handbook of Biological Confocal Microscopy (USA: Springer) p16
[2] Denk W, Strickler J H, Webb W W 1990 Science 248 73
 Google Scholar
Google Scholar
[3] Yan J, Zhang Q L, Lin D Q, Yao S J 2016 Curr. Biochem. Eng. 3 56
 Google Scholar
Google Scholar
[4] Sheppard C J R 1988 Optik 80 53
[5] Müller C B, Enderlein J 2010 Phys. Rev. Lett. 104 198101
 Google Scholar
Google Scholar
[6] Ward E N, Pal R 2017 J. Microsc. 266 221
 Google Scholar
Google Scholar
[7] Sheppard C J R, Mehta S B, Heintzmann R 2013 Opt. Lett. 38 2889
 Google Scholar
Google Scholar
[8] Castello M, Sheppard C J R, Diaspro A, Vicidomini G 2015 Opt. Lett. 40 5355
 Google Scholar
Google Scholar
[9] Jesacher A, Ritschmarte M, Piestun R 2015 Optica 2 210
 Google Scholar
Google Scholar
[10] Roider C, Heintzmann R, Piestun R 2016 Opt. Express 24 15456
 Google Scholar
Google Scholar
[11] Roider C, Piestun R, Jesacher A 2017 Optica 4 1373
 Google Scholar
Google Scholar
[12] Wang Z J, Cai Y N, Liang Y S, Zhou X, Yan S H, Dan D, Bianco P R, Lei M, Yao B L 2017 Biomed. Opt. Express 8 5493
 Google Scholar
Google Scholar
[13] Li S W, Wu J J, Li H, Lin D Y, Yu B, Qu J L 2018 Opt. Express 26 23585
 Google Scholar
Google Scholar
[14] York A G, Parekh S H, Nogare D D, Fischer R S, Temprine K, Mione M, Chitnis A B, Combs C A, Shroff H 2012 Nat. Methods 9 749
 Google Scholar
Google Scholar
[15] Pavani S R P, Greengard A, Piestun R 2009 Appl. Phys. Lett. 95 021103
 Google Scholar
Google Scholar
[16] Grover G, Pavani S R P, Piestun R 2010 Opt. Lett. 35 3306
 Google Scholar
Google Scholar
[17] Grover G, Quirin S, Fiedler C, Piestun R 2011 Biomed. Opt. Express 2 3010
 Google Scholar
Google Scholar
[18] 于斌, 李恒, 陈丹妮, 牛憨笨 2013 62 154206
 Google Scholar
Google Scholar
Yu B, Li H, Chen D N, Niu H B 2013 Acta Phys. Sin. 62 154206
 Google Scholar
Google Scholar
[19] Pavani S R P, Piestun R 2008 Opt. Express 16 3484
 Google Scholar
Google Scholar
[20] Grover G, DeLuca K, Quirin S 2012 Opt. Express 20 26681
 Google Scholar
Google Scholar
[21] Roider C, Jesacher A, Bernet S 2014 Opt. Express 22 4029
 Google Scholar
Google Scholar
[22] 李恒, 于斌, 陈丹妮, 牛憨笨 2013 62 144201
 Google Scholar
Google Scholar
Li H, Yu B, Chen D N, Niu H B 2013 Acta Phys. Sin. 62 144201
 Google Scholar
Google Scholar
-
图 2 (a) DMD上载入的投影模式; (b) 激发罗丹明染料样品探测到的荧光点阵分布; (c) 存在相位片的条件下, 激发罗丹明染料样品探测到的双螺旋荧光点阵分布
Figure 2. (a) Project pattern of DMD; (b) the fluorescence image of the excitation foci in a uniform solution of Rhodamine 6G at the sample plane; (c) the fluorescence image of the excitation foci in a uniform solution of Rhodamine 6G at the sample plane with DH phase mask.
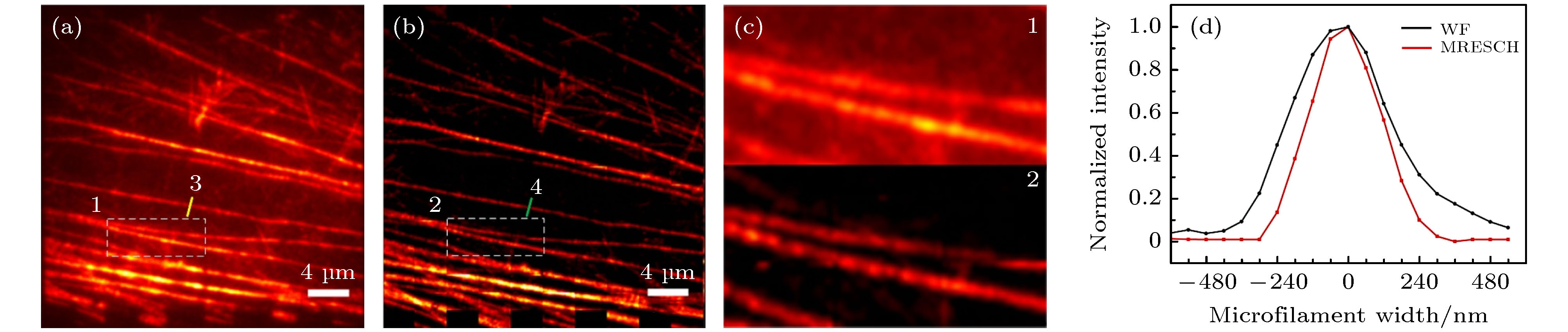
图 5 宽场照明和MRESCH对纤维状肌动蛋白的成像结果比较 (a) 纤维状肌动蛋白的宽场照明成像结果; (b) MRESCH的成像结果; (c) 图(a)和图(b)中白色方块区域的放大; (d)图(a)和图(b)中划线位置的横切面强度图(半高宽分别为: 宽场(WF)照明图像374 nm、MRESCH图像 277 nm)
Figure 5. Comparison of F-actin imaging results with wide-field illumination and MRESCH: (a) Wide-field image of F-actin; (b) MRESCH image of F-actin; (c) magnification of white box region in panels (a) and (b); (d) plots of intensity along the colored lines in panels (a) and (b); the FWHM values are 374 nm and 277 nm for wide-field (WF) and MRESCH, respectively.
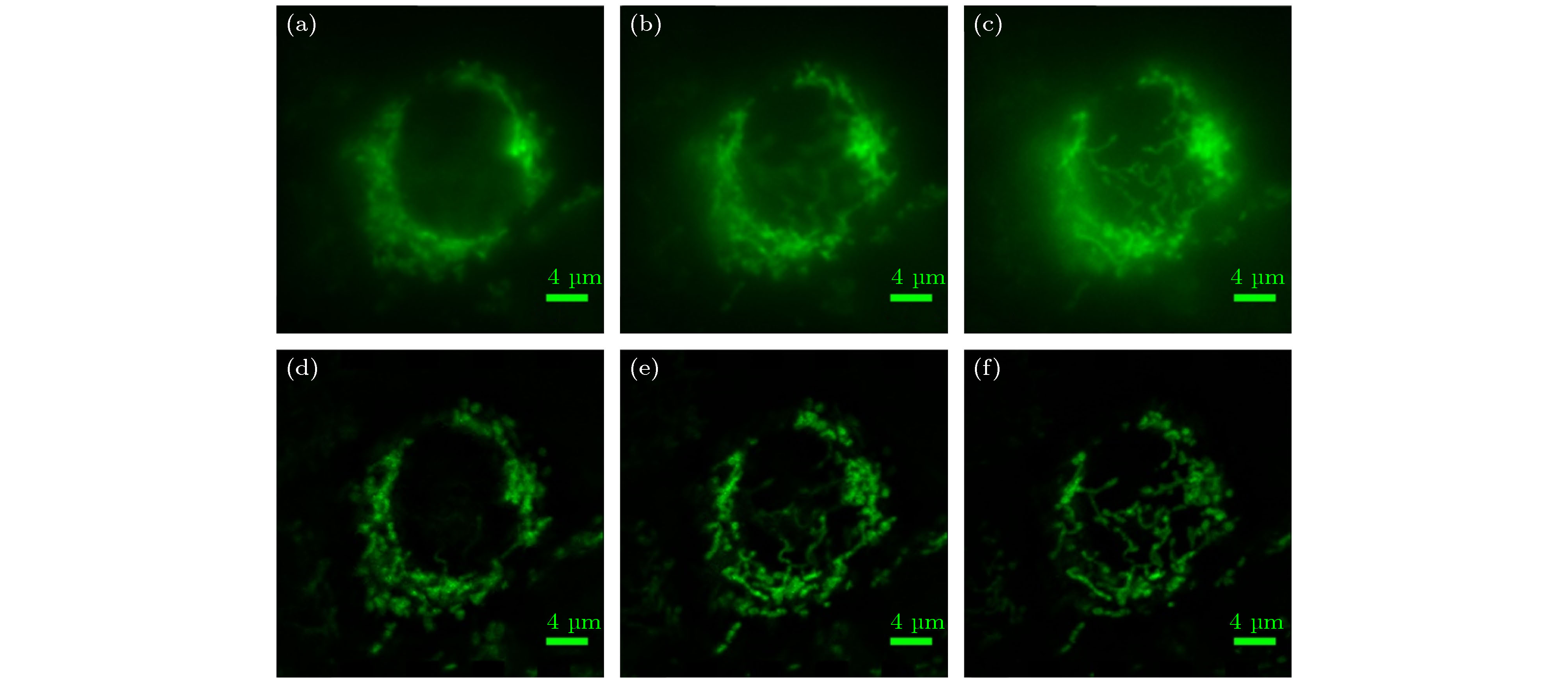
图 6 宽场照明和MRESCH对海拉细胞线粒体成像结果比较 (a) 线粒体在z = –1000 nm位置的宽场成像结果; (b) 线粒体在z = 0 nm位置的宽场成像结果; (c) 线粒体在z = 1000 nm位置的宽场成像结果; (d) 线粒体在z = –1000 nm位置的MRESCH成像结果; (e) 线粒体在z = 0 nm位置的MRESCH成像结果; (f) 线粒体在z = 1000 nm位置的MRESCH成像结果
Figure 6. Comparison of mitochondrial imaging results of HeLa cells with wide-field illumination and MRESCH: (a) Wide-field image of mitochondria at z = –1000 nm; (b) wide-field image of mitochondrion at z = 0 nm; (c) wide-field image of mitochondria at z = 1000 nm; (d) image obtained via MRESCH at z = –1000 nm; (e) image obtained via MRESCH at z = 0 nm; (f) image obtained via MRESCH at z = 1000 nm.
-
[1] Pawley J B 2006 Handbook of Biological Confocal Microscopy (USA: Springer) p16
[2] Denk W, Strickler J H, Webb W W 1990 Science 248 73
 Google Scholar
Google Scholar
[3] Yan J, Zhang Q L, Lin D Q, Yao S J 2016 Curr. Biochem. Eng. 3 56
 Google Scholar
Google Scholar
[4] Sheppard C J R 1988 Optik 80 53
[5] Müller C B, Enderlein J 2010 Phys. Rev. Lett. 104 198101
 Google Scholar
Google Scholar
[6] Ward E N, Pal R 2017 J. Microsc. 266 221
 Google Scholar
Google Scholar
[7] Sheppard C J R, Mehta S B, Heintzmann R 2013 Opt. Lett. 38 2889
 Google Scholar
Google Scholar
[8] Castello M, Sheppard C J R, Diaspro A, Vicidomini G 2015 Opt. Lett. 40 5355
 Google Scholar
Google Scholar
[9] Jesacher A, Ritschmarte M, Piestun R 2015 Optica 2 210
 Google Scholar
Google Scholar
[10] Roider C, Heintzmann R, Piestun R 2016 Opt. Express 24 15456
 Google Scholar
Google Scholar
[11] Roider C, Piestun R, Jesacher A 2017 Optica 4 1373
 Google Scholar
Google Scholar
[12] Wang Z J, Cai Y N, Liang Y S, Zhou X, Yan S H, Dan D, Bianco P R, Lei M, Yao B L 2017 Biomed. Opt. Express 8 5493
 Google Scholar
Google Scholar
[13] Li S W, Wu J J, Li H, Lin D Y, Yu B, Qu J L 2018 Opt. Express 26 23585
 Google Scholar
Google Scholar
[14] York A G, Parekh S H, Nogare D D, Fischer R S, Temprine K, Mione M, Chitnis A B, Combs C A, Shroff H 2012 Nat. Methods 9 749
 Google Scholar
Google Scholar
[15] Pavani S R P, Greengard A, Piestun R 2009 Appl. Phys. Lett. 95 021103
 Google Scholar
Google Scholar
[16] Grover G, Pavani S R P, Piestun R 2010 Opt. Lett. 35 3306
 Google Scholar
Google Scholar
[17] Grover G, Quirin S, Fiedler C, Piestun R 2011 Biomed. Opt. Express 2 3010
 Google Scholar
Google Scholar
[18] 于斌, 李恒, 陈丹妮, 牛憨笨 2013 62 154206
 Google Scholar
Google Scholar
Yu B, Li H, Chen D N, Niu H B 2013 Acta Phys. Sin. 62 154206
 Google Scholar
Google Scholar
[19] Pavani S R P, Piestun R 2008 Opt. Express 16 3484
 Google Scholar
Google Scholar
[20] Grover G, DeLuca K, Quirin S 2012 Opt. Express 20 26681
 Google Scholar
Google Scholar
[21] Roider C, Jesacher A, Bernet S 2014 Opt. Express 22 4029
 Google Scholar
Google Scholar
[22] 李恒, 于斌, 陈丹妮, 牛憨笨 2013 62 144201
 Google Scholar
Google Scholar
Li H, Yu B, Chen D N, Niu H B 2013 Acta Phys. Sin. 62 144201
 Google Scholar
Google Scholar
Catalog
Metrics
- Abstract views: 9147
- PDF Downloads: 185
- Cited By: 0















 DownLoad:
DownLoad: