-
Gold nanorods (GNRs) have aroused the extensive interest of many researchers in recent years due to their unique physicochemical properties. However, the toxic cetyltrimethylammonium bromide (CTAB) is often introduced into the process of synthesizing GNRs, which hinders the wide-range applications of GNRs in clinical practice. To reduce the toxicity, the CTAB molecules coated on the surface of GNRs should be replaced by nontoxic and biocompatible agents such as phospholipid. Thus the component and morphology of the mixed coating agents on the surface of GNRs affect the physicochemical properties of GNRs. To study the morphology and properties of the coated GNRs at a molecular level, we investigate the self-assembly of GNRs, CTAB, and dimyristoyl phosphatidylcholine (DMPC) by using solvent-free dissipative particle dynamics simulations. Our results show that the morphology of the assembled complex mainly depends on the CTAB/DMPC molar ratio, while neither of the interaction strength between GNRs and the coating agents nor the diameter of GNRs has significant effect on the morphology. At a certain combination of GNRs-coating agent interaction strength with GNRs diameter, the mixture of CTAB and DMPC on the surface of GNRs undergoes a gradual change in morphology as the CTAB/DMPC molar ratio increases, including the forming of intact bilayer membrane, cracked bilayer membrane, long patches of micelles, and short wormlike micelles winding GNRs in spiral shape. The morphology of intact bilayer membrane verifies the experimental guess, while the other three morphologies are brand-new discoveries. We also find that when the GNR’s diameter becomes smaller, or the CTAB/DMPC molar ratio is larger, or the interaction strength is greater, the agents cap the ends of GNRs, meanwhile the membrane thickness becomes thinner. The multiple morphologies of the assembled complexes can be qualitatively explained by the shape energy of a membrane adsorbed on a solid surface. When the surface tension of the membrane (which is proportional to the spontaneous curvature of the membrane) exceeds a critical value (which is equal to the adhesion energy density of the membrane), the membrane dissociates from the solid surface and its shape changes. The change trend is related to the spontaneous curvature of the free membrane. As a result of the synergy and competition among the inherent curvatures of GNRs, the spontaneous curvature of CTAB/DMPC membrane or micelle, as well as the adhesion energy, various interesting morphologies are produced. Our simulations and analyses directly characterize the morphological structures of CTAB and lipid coated GNRs, which allow us to in depth understand the self-assembling behaviors of GNRs at a molecular level. This is also conductive to achieving the controlled assemblies of GNRs.
-
Keywords:
- dissipative particle dynamics /
- gold nanorods /
- lipid /
- surfactant
[1] Cioffi N, Torsi L, Ditaranto N, Tantillo G, Ghibelli L, Sabbatini L, Bleve-Zacheo T, D'Alessio M, Zambonin P G, Traversa E 2005 Chem. Mater. 17 5255
 Google Scholar
Google Scholar
[2] Jin R C, Cao Y W, Mirkin C A, Kelly K L, Schatz G C, Zheng J G 2001 Science 294 1901
 Google Scholar
Google Scholar
[3] Levard C, Hotze E M, Lowry G V, Jr Brown G E 2012 Environ. Sci. Technol. 46 6900
 Google Scholar
Google Scholar
[4] Elahi N, Kamali M, Baghersad M H 2018 Talanta 184 537
 Google Scholar
Google Scholar
[5] 黄运欢, 李璞 2015 64 207301
 Google Scholar
Google Scholar
Huang Y H, Li P 2015 Acta Phys. Sin. 64 207301
 Google Scholar
Google Scholar
[6] 柯善林 阚彩侠 莫博 从博 朱杰君 2012 物理化学学报 28 1275
 Google Scholar
Google Scholar
Ke S L, Kan C X, Mo B, Cong B, Zhu J J 2012 Acta Phys.-Chim. Sin. 28 1275
 Google Scholar
Google Scholar
[7] Singh N, Charan S, Sanjiv K, Huang S H, Hsiao Y C, Kuo C W, Chien F C, Lee T C, Chen P 2012 Bioconjugate Chem. 23 421
 Google Scholar
Google Scholar
[8] von Maltzahn G, Centrone A, Park J H, Ramanathan R, Sailor M J, Hatton T A, Bhatia S N 2009 Adv. Mater. 21 3175
 Google Scholar
Google Scholar
[9] Zhang Y, Qian J, Wang D, Wang Y, He S 2013 Angew. Chem. Int. Ed. 52 1148
 Google Scholar
Google Scholar
[10] Cao J, Galbraith E K, Sun T, Grattan K T V 2012 Sens. Actuators, B 169 360
 Google Scholar
Google Scholar
[11] Li X, Qian J, He S 2008 Nanotechnology 19 355501
 Google Scholar
Google Scholar
[12] Mayer K M, Hafner J H 2011 Chem. Rev. 111 3828
 Google Scholar
Google Scholar
[13] Joshi P P, Yoon S J, Hardin W G, Emelianov S, Sokolov K V 2013 Bioconjugate Chem. 24 878
 Google Scholar
Google Scholar
[14] Luo T, Huang P, Gao G, Shen G X, Fu S, Cui D X, Zhou C Q, Ren Q S 2011 Opt. Express 19 17030
 Google Scholar
Google Scholar
[15] Zhang J B, Balla N K, Gao C, Sheppard C J R, Yung L Y L, Rehman S, Teo J Y, Kulkarni S R, Fu Y H, Yin S J 2012 Aust. J. Chem. 65 290
 Google Scholar
Google Scholar
[16] Troiber C, Kasper J C, Milani S, Scheible M, Martin I, Schaubhut F, Kuchler S, Radler J, Simmel F C, Friess W, Wagner E 2013 Eur. J. Pharmacol. 84 255
 Google Scholar
Google Scholar
[17] Xue H Y, Wong H L 2011 ACS Nano 5 7034
 Google Scholar
Google Scholar
[18] Martin C R 1994 Science 266 1961
 Google Scholar
Google Scholar
[19] Raj M A, John S A 2014 Electrochem. Commun. 45 27
 Google Scholar
Google Scholar
[20] Abdelrasoul G N, Cingolani R, Diaspro A, Athanassiou A, Pignatelli F 2014 J. Photochem. Photobiol., A 275 7
 Google Scholar
Google Scholar
[21] Johnson C J, Dujardin E, Davis S A, Murphy C J, Mann S 2002 J. Mater. Chem. 12 1765
 Google Scholar
Google Scholar
[22] Takahashi H, Niidome Y, Niidome T, Kaneko K, Kawasaki H, Yamada S 2006 Langmuir 22 2
 Google Scholar
Google Scholar
[23] Lyubartsev A P, Rabinovich A L 2011 Soft Matter 7 25
 Google Scholar
Google Scholar
[24] Orendorff C J, Alam T M, Sasaki D Y, Bunker B C, Voigt J A 2009 ACS Nano 3 971
 Google Scholar
Google Scholar
[25] Galati E, Tebbe M, Querejeta-Fernandez A, Xin H L, Gang O, Zhulina E B, Kumacheva E 2017 ACS Nano 11 4995
 Google Scholar
Google Scholar
[26] Galati E, Tao H, Tebbe M, Ansari R, Rubinstein M, Zhulina E B, Kumacheva E 2019 Angew. Chem. Int. Ed. 58 3123
 Google Scholar
Google Scholar
[27] Tao H, Chen L, Galati E, Manion J G, Seferos D S, Zhulina E B, Kumacheva E 2019 Langmuir 35 15872
 Google Scholar
Google Scholar
[28] Wang R Y, Wang H, Wu X, Ji Y, Wang P, Qu Y, Chung T S 2011 Soft Matter 7 8370
 Google Scholar
Google Scholar
[29] Matthews J R, Payne C M, Hafner J H 2015 Langmuir 31 9893
 Google Scholar
Google Scholar
[30] Nakashima H, Furukawa K, Kashimura Y, Torimitsu K 2008 Langmuir 24 5654
 Google Scholar
Google Scholar
[31] Yang J A, Murphy C J 2012 Langmuir 28 5404
 Google Scholar
Google Scholar
[32] Sreeprasad T S, Pradeep T 2011 Langmuir 27 3381
 Google Scholar
Google Scholar
[33] 闫昭, 赵文静, 王荣瑶 2016 65 126101
 Google Scholar
Google Scholar
Yan Z, ZhaoW J, Wang R Y 2016 Acta Phys. Sin. 65 126101
 Google Scholar
Google Scholar
[34] Zhu Y, Qu C, Kuang H, Xu L, Liu L, Hua Y, Wang L, Xu C 2011 Biosens. Bioelectron. 26 4387
 Google Scholar
Google Scholar
[35] Wang Y T, Dellago C 2003 J. Phys. Chem. B 107 9214
 Google Scholar
Google Scholar
[36] da Silva J, Dias R, da Hora G, Soares T, Meneghetti M 2018 J. Braz. Chem. Soc. 29 191
 Google Scholar
Google Scholar
[37] Schmid N, Eichenberger A P, Choutko A, Riniker S, Winger M, Mark A E, van Gunsteren W F 2011 Eur. Biophys. J. 40 843
 Google Scholar
Google Scholar
[38] da Silva J A, Meneghetti M R 2018 Langmuir 34 366
 Google Scholar
Google Scholar
[39] da Silva J A, Netz P A, Meneghetti M R 2020 Langmuir 36 257
 Google Scholar
Google Scholar
[40] Oroskar P A, Jameson C J, Murad S 2016 Mol. Phys. 115 1122
 Google Scholar
Google Scholar
[41] Horsch M A, Zhang Z, Glotzer S C 2005 Phys. Rev. Lett. 95 056105
 Google Scholar
Google Scholar
[42] Wan M W, Li X X, Gao L H, Fang W H 2016 Nanotechnology 27 465704
 Google Scholar
Google Scholar
[43] Li X X, Gao L H, Fang W H 2016 PLoS One 11 e0154568
 Google Scholar
Google Scholar
[44] Sevink G J A, Charlaganov M, Fraaije J G E M 2013 Soft Matter 9 2816
 Google Scholar
Google Scholar
[45] Sevink G J A, Fraaije J 2014 Soft Matter 10 5129
 Google Scholar
Google Scholar
[46] Wan M W, Gao L H, Fang W H 2018 PLoS One 13 e0198049
 Google Scholar
Google Scholar
[47] Groot R D 2003 J. Chem. Phys. 118 11265
 Google Scholar
Google Scholar
[48] Marrink S J, Risselada H J, Yefimov S, Tieleman D P, de Vries A H 2007 J. Phys. Chem. B 111 7812
 Google Scholar
Google Scholar
[49] Humphrey W, Dalke A, Schulten K 1996 J. Mol. Graphics 14 33
 Google Scholar
Google Scholar
[50] Illa-Tuset S, Malaspina D C, Faraudo J 2018 Phys. Chem. Chem. Phys. 20 26422
 Google Scholar
Google Scholar
[51] Lipowsky R 2013 Faraday Discuss. 161 305
 Google Scholar
Google Scholar
[52] Arnarez C, Uusitalo J J, Masman M F, Ingolfsson H I, de Jong D H, Melo M N, Periole X, de Vries A H, Marrink S J 2015 J. Chem. Theory. Comput. 11 260
 Google Scholar
Google Scholar
-
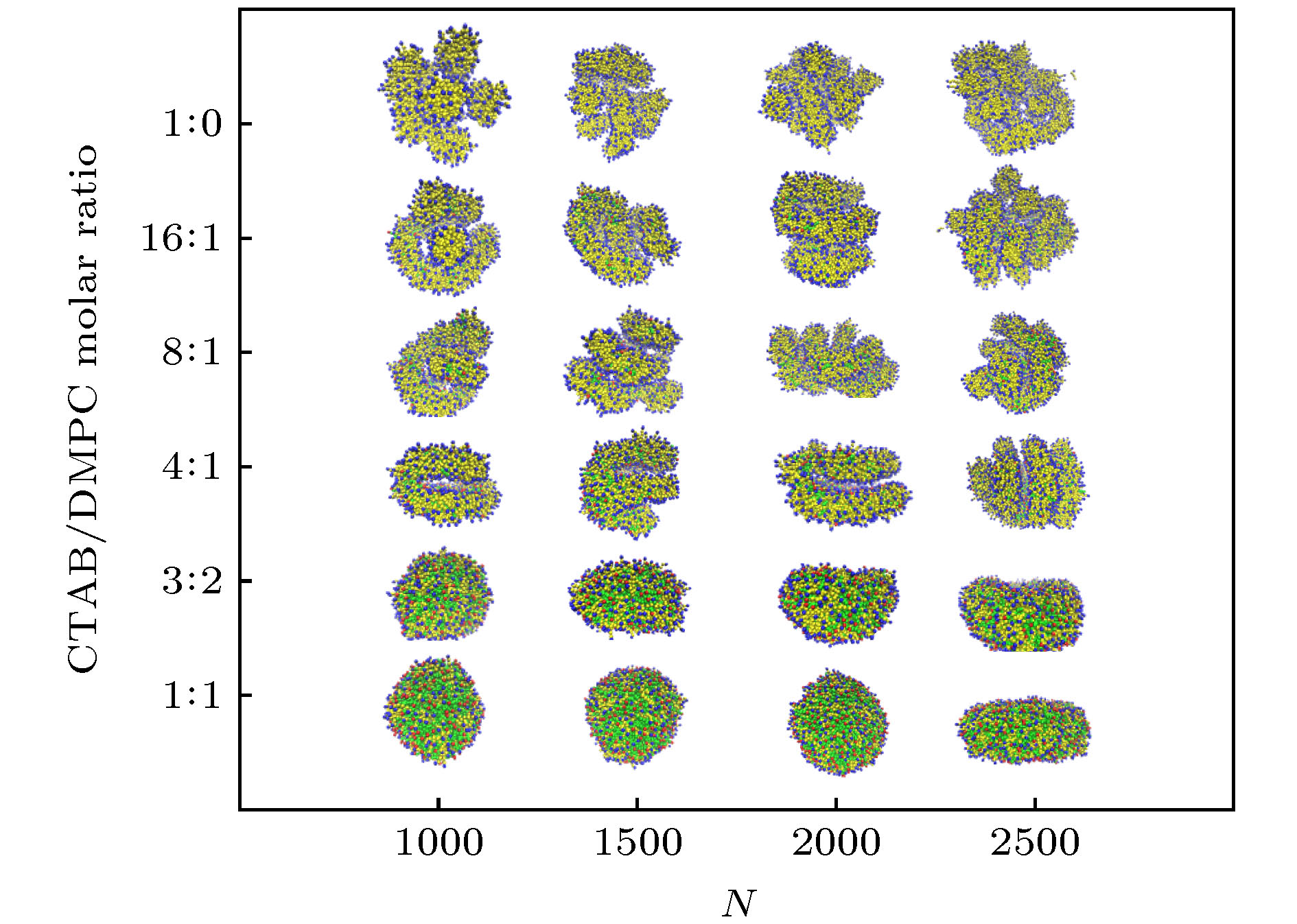
图 3 GNRs-CTAB-DMPC复合体在d = 4 nm, D = 4kBT时的组装形貌, 其中CTAB/DMPC摩尔比分别为(a) 1∶1, (b) 3∶2, (c) 4∶1, (d) 8∶1, (e) 16∶1, (f) 1∶0
Figure 3. Morphology of assembled GNRs-CTAB-DMPC complex with d = 4 nm and D = 4kBT. The CTAB/DMPC molar ratio is (a) 1∶1, (b) 3∶2, (c) 4∶1, (d) 8∶1, (e) 16∶1, (f) 1∶0, respectively.
图 D1 d = 4 nm和D = 4 kBT时GNRs表面修饰剂的投影展开分布图, 其中摩尔比分别为(a) 1∶1, (b) 3∶2, (c) 4∶1, (d) 8∶1, (e) 16∶1, (f) 1∶0
Figure D1. Projected expansion map of coating agents distributing on the surface of GNRs. Data is obtained at d = 4 nm and D = 4 kBT. The CTAB/DMPC molar ratio is (a) 1∶1, (b) 3∶2, (c) 4∶1, (d) 8∶1, (e) 16∶1, (f) 1∶0.
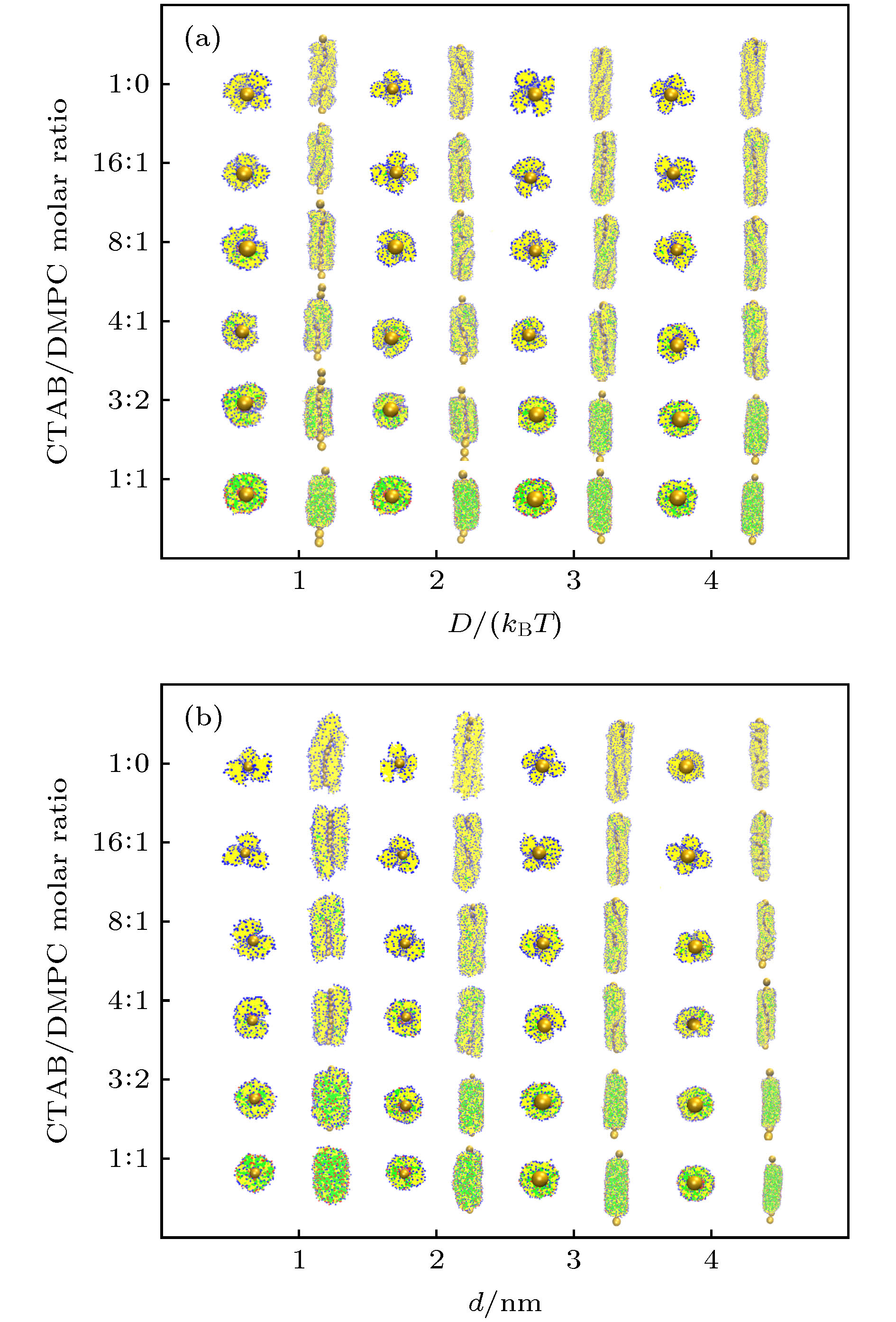
图 4 (a) 当金纳米棒直径d = 4 nm时, GNRs-CTAB-DMPC复合体在不同CTAB/DMPC摩尔比及D下的组装形貌; (b) 当Morse势参数D = 4kBT时, GNRs-CTAB-DMPC复合体在不同CTAB/DMPC摩尔比及d下的组装形貌
Figure 4. (a) Morphology of assembled GNRs-CTAB-DMPC complex at various CTAB/DMPC molar ratio and D with given GNR diameter d = 4 nm; (b) morphology of assembled GNRs-CTAB-DMPC complex at various CTAB/DMPC molar ratio and d with given Morse potential parameter D = 4kBT.
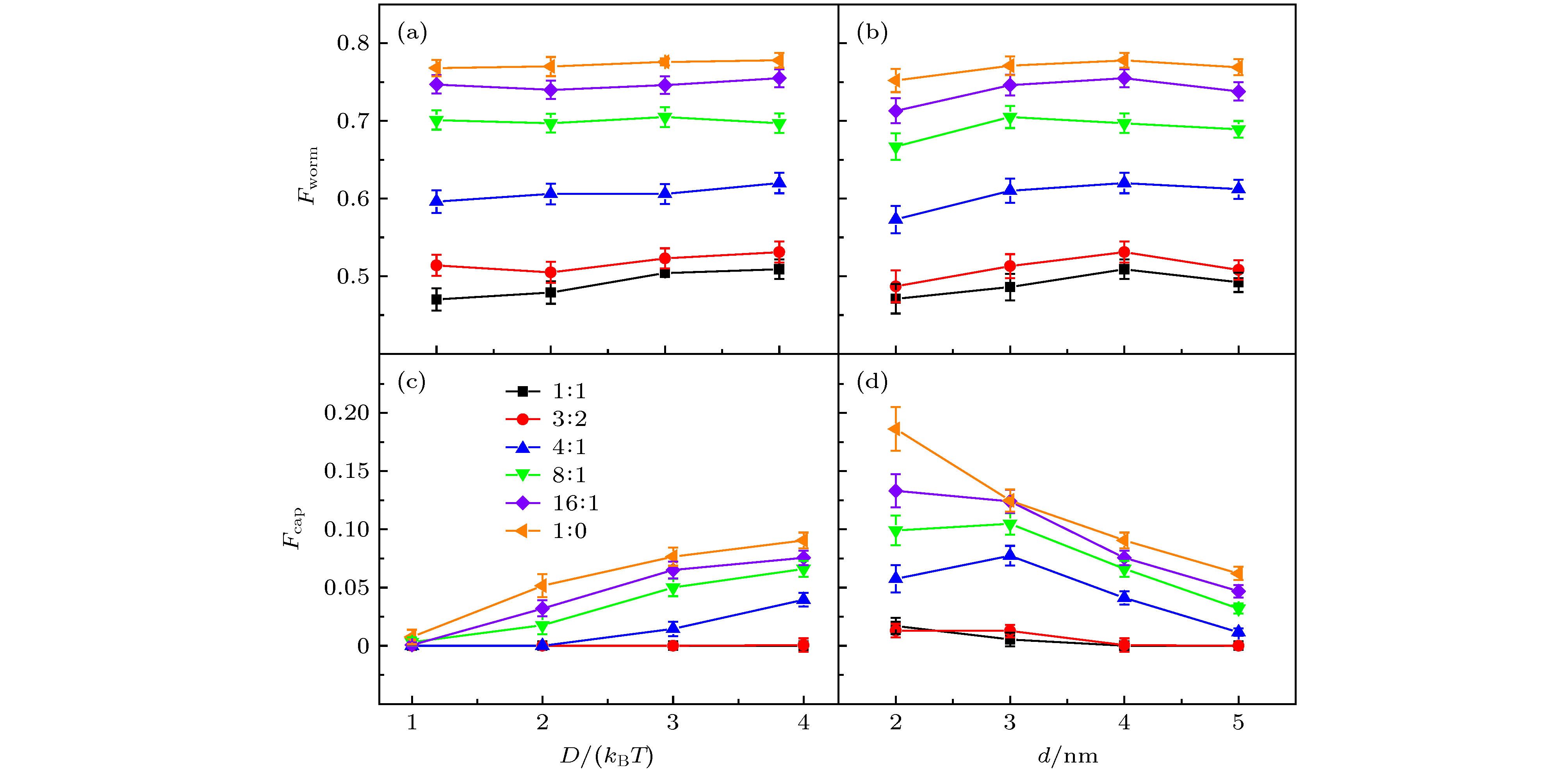
图 6 (a) 修饰剂的蠕虫系数Fworm与D的关系图; (b) Fworm与d的关系图; (c) GNRs的覆盖率Fcap与D的关系图; (d) Fcap与d的关系图; 不同颜色的直线代表不同的摩尔比
Figure 6. (a) Worming coefficient Fworm of coating agents as a function of D; (b) Fworm as a function of d; (c) capping rate Fcap of GNRs as a function of D; (d) Fcap as a function of d. Results are obtained at various CTAB/DMPC molar ratios, shown in different colors.
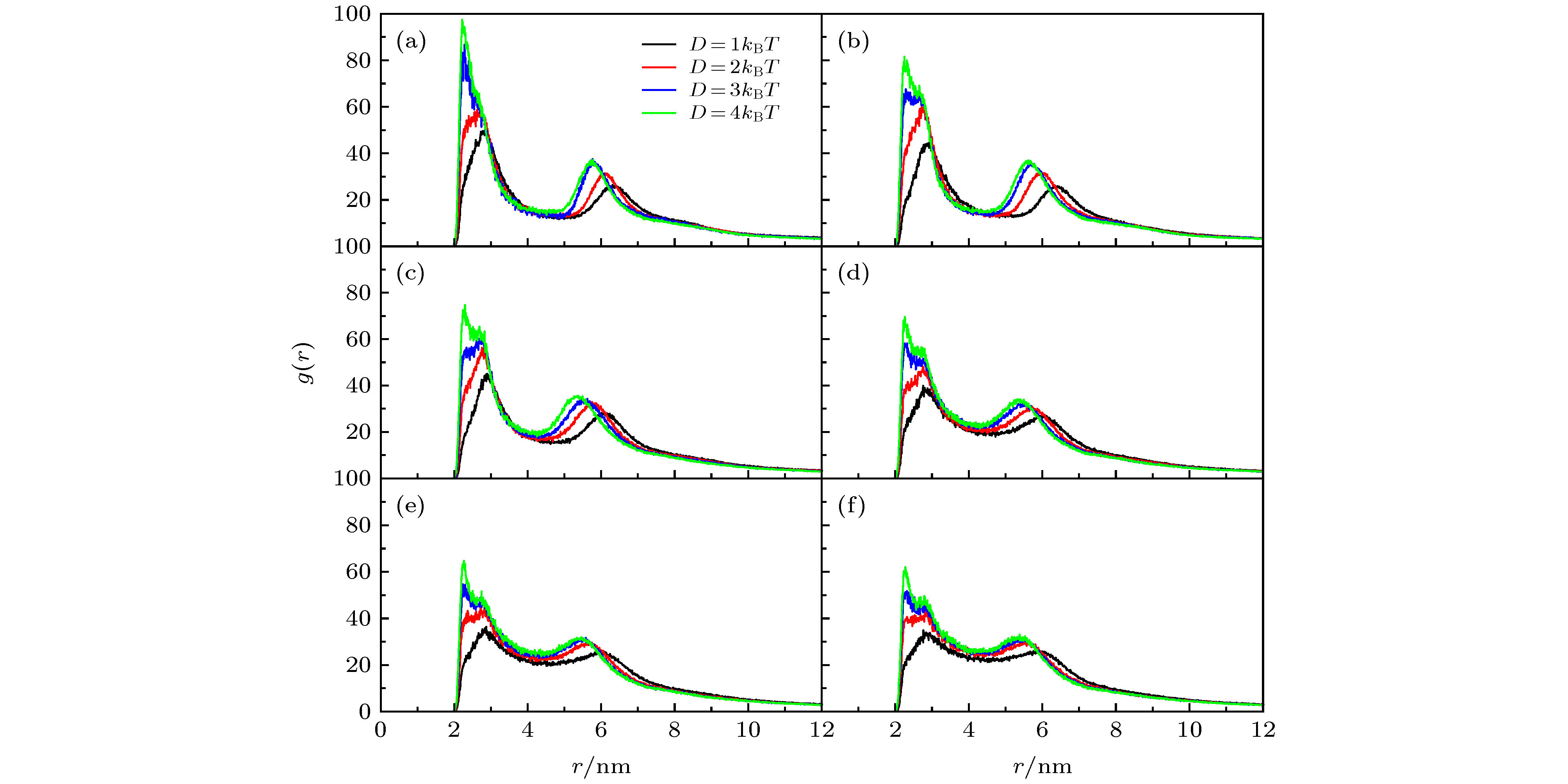
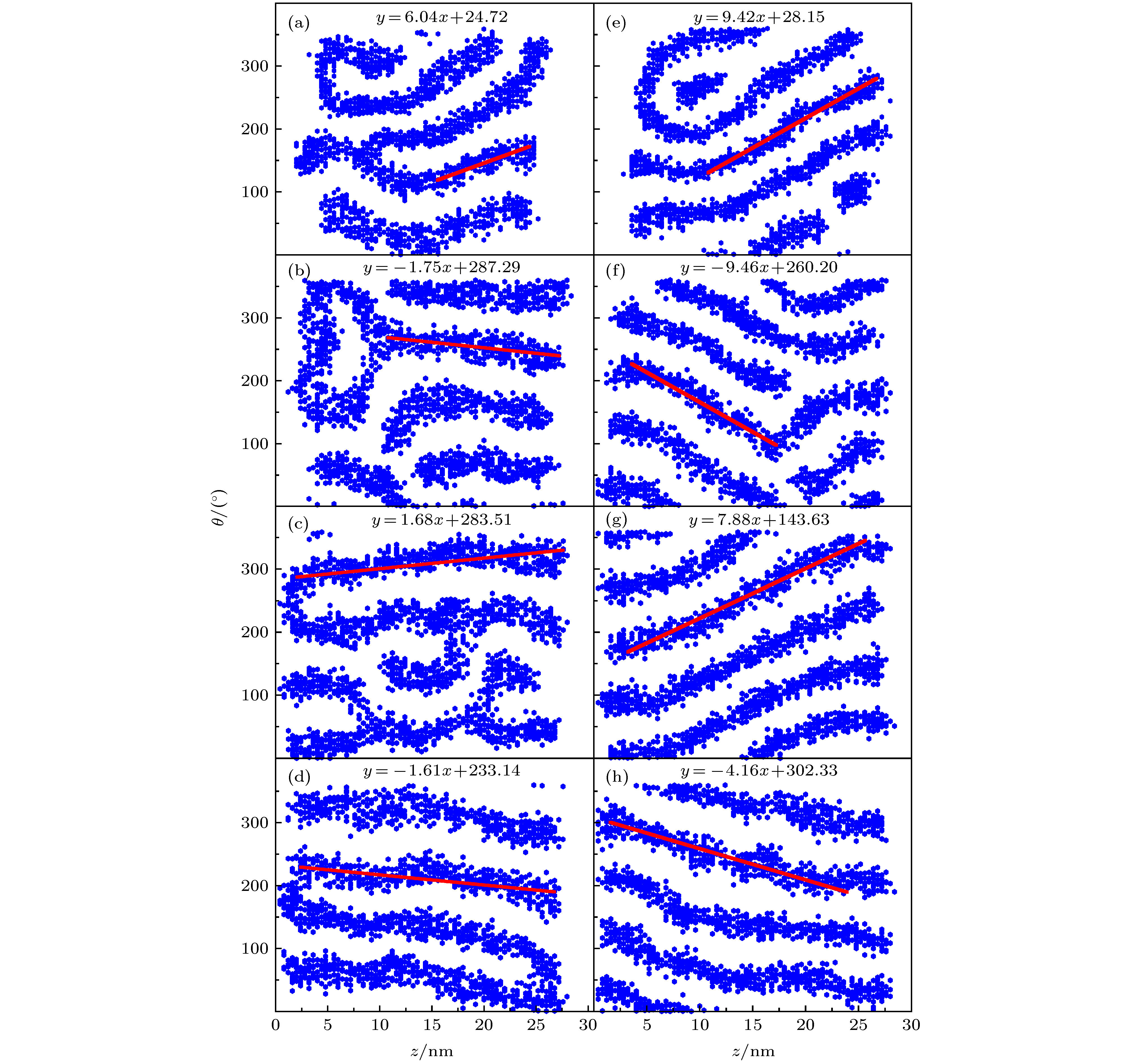
图 9 不同D下得到的GNRs表面修饰剂的投影展开分布图(GNRs直径d = 4 nm), 其中, 线性拟合斜率代表螺旋程度; 左图是CTAB/DMPC摩尔比为16∶1的结果, (a) D = 1kBT, (b) D = 2kBT, (c) D = 3kBT, (d) D = 4kBT; 右图是CTAB/DMPC摩尔比为1∶0的结果, (e) D = 1kBT, (f) D = 2kBT, (g) D = 3kBT, (h) D = 4kBT
Figure 9. Projected expansion map of coating agent distributing on the surface of GNRs with d = 4 nm. The slope of the linear fitting represents the spiral degree. The left panels are results at CTAB/DMPC molar ratio of 16∶1 with (a) D = 1kBT, (b) D = 2kBT, (c) D = 3kBT, (d) D = 4kBT. The right panels are results at CTAB/DMPC molar ratio of 1∶0 with (e) D = 1kBT, (f) D = 2kBT, (g) D = 3kBT, (h) D = 4kBT.
图 C1 纯DMPC和d = 4 nm, D = 4kBT时GNRs-DMPC的组装形貌 (a) DMPC的形貌及(b)切面图; (c) GNRs-DMPC的形貌及(d)切面图
Figure C1. Morphology of the pure DMPC lipids and assembled GNRs-DMPC complex with d = 4 nm and D = 4kBT: (a) Morpohology and (b) cross-sectional view of DMPC; (c) morpohology and (d) cross-sectional view of GNRs-DMPC
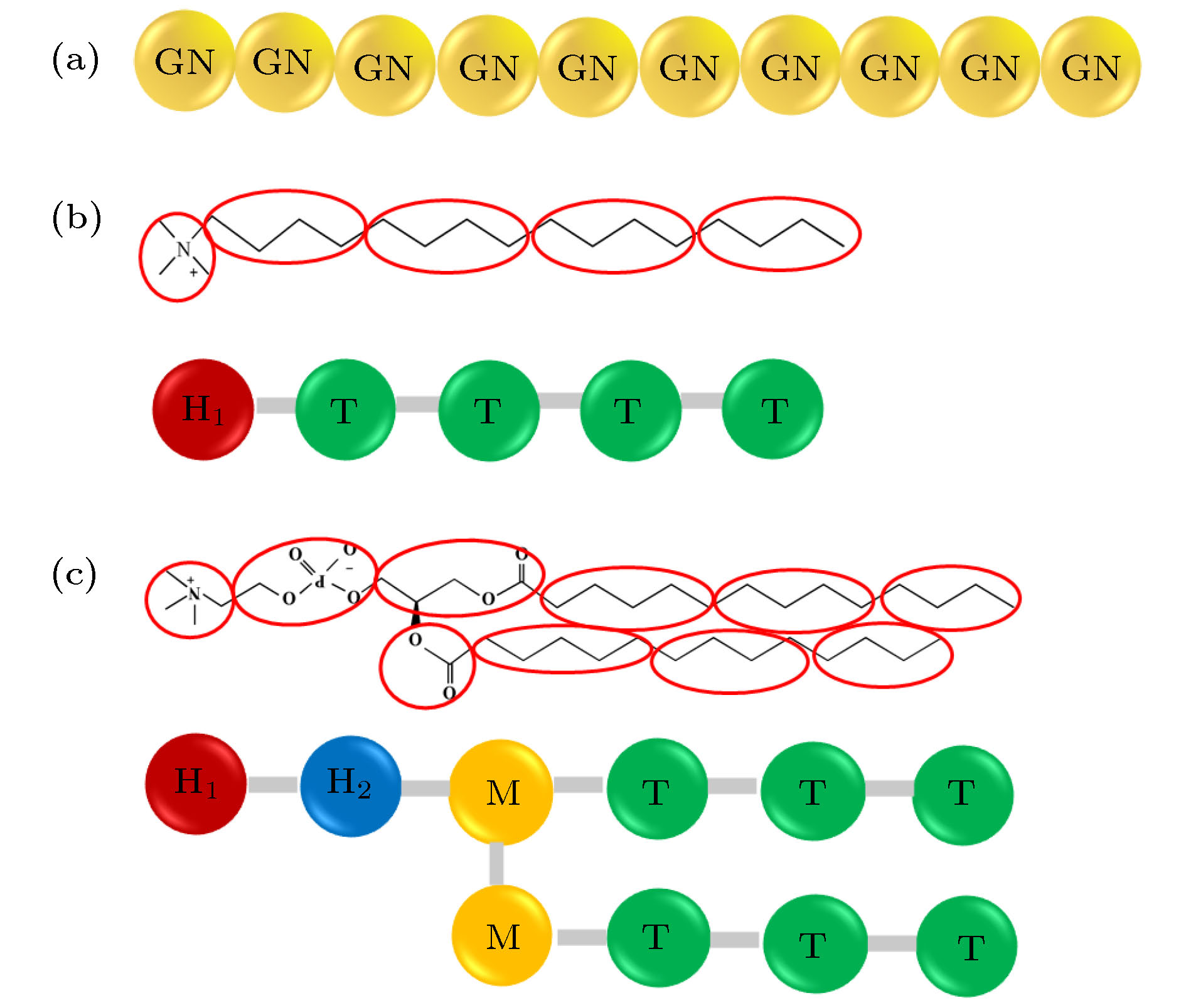
表 A1 DPD力场中aij参数的取值(ION, H1, H2, M, T珠子分别和文献[43]中的W, Q0, Qa, Na, C珠子一一对应)
Table A1. Values of parameters aij in DPD Force field. The ION, H1, H2, M, and T beads correspond to the W, Q0, Qa, Na, and C beads in Ref [43], respectively.
aij ION H1 H2 M T ION 100 98 98 102 130 H1 98 110 100 102 130 H2 98 100 110 102 130 M 102 102 102 100 110 T 130 130 130 110 100 表 A2 Im-DPD力场中c, σ和s参数的值
Table A2. Values of c, σ, s in Im-DPD Force field.
ION H1 H2 M T c 0 19.37 19.37 20.02 24.56 σ 0.5385 0.536 0.536 0.541 0.566 s 0.625 0.625 0.625 0.7135 0.7135 表 A3 Im-DPD力场中的键长、键角和对应的力参数(d是以纳米为单位的金纳米棒的直径)
Table A3. Equilibrium bonds, angles, and corresponding force constants in Im-DPD Force field. d is the diameter of GNRs in unit of nanometer.
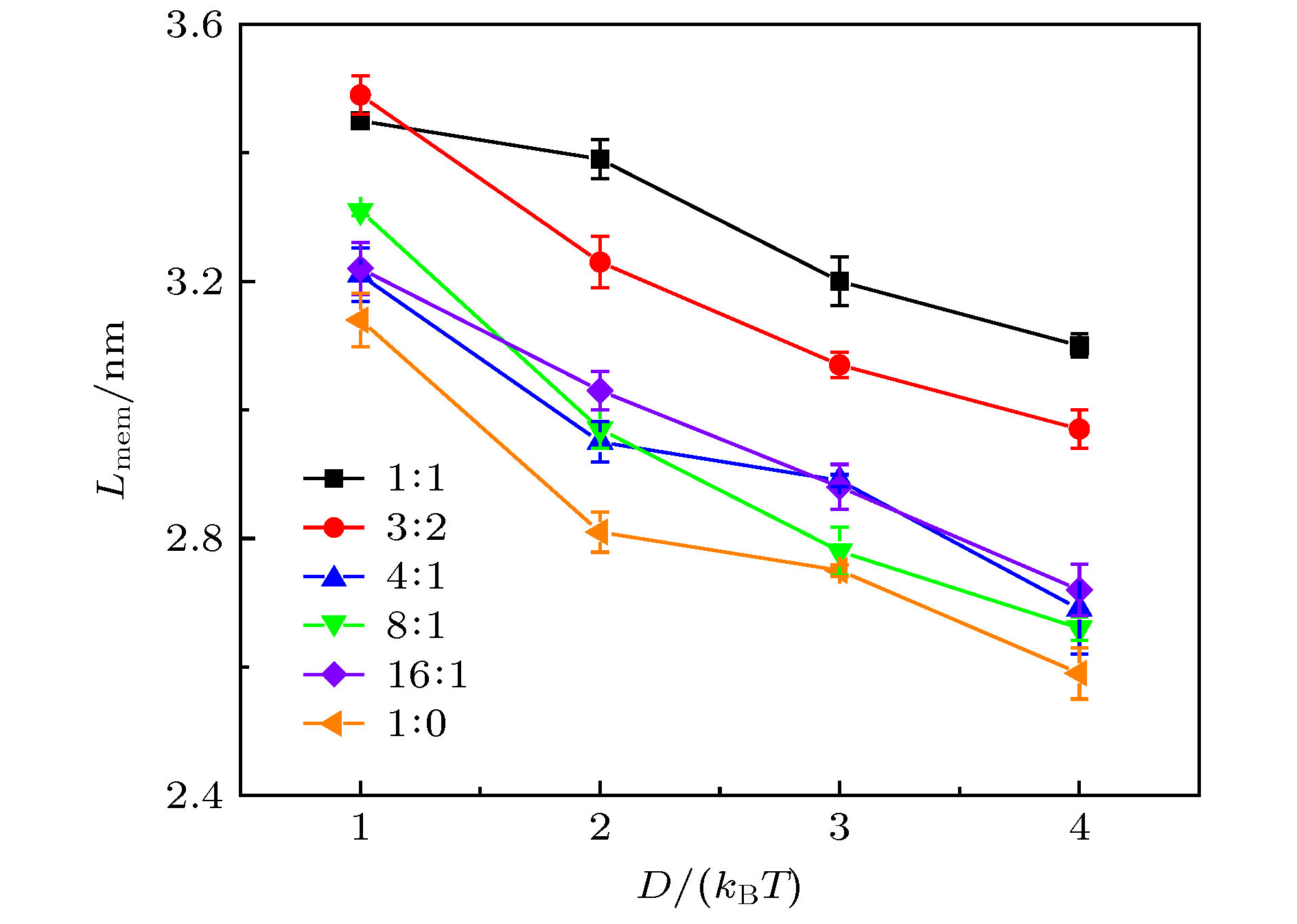
bond L0/r0 K2/kBT·r0–2 angle θ0/(º) K3/kBT GN—GN d/0.71 512 GN—GN—GN 180 600 H1—T 0.47 512 H2—M—M 120 6 H1—H2 0.47 512 H2—M—T 180 6 H2—M 0.47 512 M—T—T 180 6 M—M 0.31 512 T—T—T 180 6 M—T 0.59 512 T—T 0.59 512 表 E1 不同D及CTAB/DMPC摩尔比下的r1, r2, r3和Lmem
Table E1. r1, r2, r3 and Lmem at different combinations of D and CTAB/DMPC molar ratio.
Molar ratio D = 1kBT D = 2kBT D = 3kBT D = 4kBT r2 r3 Lmem r2 r3 Lmem r1 r2 r3 Lmem r1 r2 r3 Lmem 1∶1 2.88 6.33 3.45 2.72 6.11 3.39 2.62 5.82 3.2 2.24 2.68 5.78 3.1 3∶2 2.93 6.42 3.49 2.72 5.95 3.23 2.64 5.71 3.07 2.25 2.66 5.63 2.97 4∶1 2.89 6.1 3.21 2.8 5.75 2.95 2.32 2.73 5.62 2.89 2.29 2.67 5.36 2.69 8∶1 2.75 6.06 3.31 2.74 5.71 2.97 2.3 2.74 5.52 2.78 2.24 2.70 5.36 2.66 16∶1 2.87 6.09 3.22 2.71 5.74 3.03 2.23 2.70 5.58 2.88 2.26 2.74 5.46 2.72 1∶0 2.75 5.89 3.14 2.79 5.60 2.81 2.29 2.67 5.42 2.75 2.29 2.77 5.36 2.59 表 B1 单个CTAB胶束的模拟结果, 其中, D是胶束的直径, e是离心率, α是CTAB分子的电离度(更多细节详见文献[50]), 表格中的前3行数据来自文献[50], 用作对比分析
Table B1. Simulations results of the single CTAB micelle. D is the diameter of the micelle, e is the eccentricity, and α is the degree of ionization of CTAB molecules (see Ref. [50] for details). The data in the first three lines are taken from Ref. [50] for comparison.
Force Field D/nm e α All-atomic 4.79 0.29 0.63 Martini 4.54 0.14 0.78 Dry Martini 4.42 0.12 0.73 Im-DPD 5.13 0.16 0.68 -
[1] Cioffi N, Torsi L, Ditaranto N, Tantillo G, Ghibelli L, Sabbatini L, Bleve-Zacheo T, D'Alessio M, Zambonin P G, Traversa E 2005 Chem. Mater. 17 5255
 Google Scholar
Google Scholar
[2] Jin R C, Cao Y W, Mirkin C A, Kelly K L, Schatz G C, Zheng J G 2001 Science 294 1901
 Google Scholar
Google Scholar
[3] Levard C, Hotze E M, Lowry G V, Jr Brown G E 2012 Environ. Sci. Technol. 46 6900
 Google Scholar
Google Scholar
[4] Elahi N, Kamali M, Baghersad M H 2018 Talanta 184 537
 Google Scholar
Google Scholar
[5] 黄运欢, 李璞 2015 64 207301
 Google Scholar
Google Scholar
Huang Y H, Li P 2015 Acta Phys. Sin. 64 207301
 Google Scholar
Google Scholar
[6] 柯善林 阚彩侠 莫博 从博 朱杰君 2012 物理化学学报 28 1275
 Google Scholar
Google Scholar
Ke S L, Kan C X, Mo B, Cong B, Zhu J J 2012 Acta Phys.-Chim. Sin. 28 1275
 Google Scholar
Google Scholar
[7] Singh N, Charan S, Sanjiv K, Huang S H, Hsiao Y C, Kuo C W, Chien F C, Lee T C, Chen P 2012 Bioconjugate Chem. 23 421
 Google Scholar
Google Scholar
[8] von Maltzahn G, Centrone A, Park J H, Ramanathan R, Sailor M J, Hatton T A, Bhatia S N 2009 Adv. Mater. 21 3175
 Google Scholar
Google Scholar
[9] Zhang Y, Qian J, Wang D, Wang Y, He S 2013 Angew. Chem. Int. Ed. 52 1148
 Google Scholar
Google Scholar
[10] Cao J, Galbraith E K, Sun T, Grattan K T V 2012 Sens. Actuators, B 169 360
 Google Scholar
Google Scholar
[11] Li X, Qian J, He S 2008 Nanotechnology 19 355501
 Google Scholar
Google Scholar
[12] Mayer K M, Hafner J H 2011 Chem. Rev. 111 3828
 Google Scholar
Google Scholar
[13] Joshi P P, Yoon S J, Hardin W G, Emelianov S, Sokolov K V 2013 Bioconjugate Chem. 24 878
 Google Scholar
Google Scholar
[14] Luo T, Huang P, Gao G, Shen G X, Fu S, Cui D X, Zhou C Q, Ren Q S 2011 Opt. Express 19 17030
 Google Scholar
Google Scholar
[15] Zhang J B, Balla N K, Gao C, Sheppard C J R, Yung L Y L, Rehman S, Teo J Y, Kulkarni S R, Fu Y H, Yin S J 2012 Aust. J. Chem. 65 290
 Google Scholar
Google Scholar
[16] Troiber C, Kasper J C, Milani S, Scheible M, Martin I, Schaubhut F, Kuchler S, Radler J, Simmel F C, Friess W, Wagner E 2013 Eur. J. Pharmacol. 84 255
 Google Scholar
Google Scholar
[17] Xue H Y, Wong H L 2011 ACS Nano 5 7034
 Google Scholar
Google Scholar
[18] Martin C R 1994 Science 266 1961
 Google Scholar
Google Scholar
[19] Raj M A, John S A 2014 Electrochem. Commun. 45 27
 Google Scholar
Google Scholar
[20] Abdelrasoul G N, Cingolani R, Diaspro A, Athanassiou A, Pignatelli F 2014 J. Photochem. Photobiol., A 275 7
 Google Scholar
Google Scholar
[21] Johnson C J, Dujardin E, Davis S A, Murphy C J, Mann S 2002 J. Mater. Chem. 12 1765
 Google Scholar
Google Scholar
[22] Takahashi H, Niidome Y, Niidome T, Kaneko K, Kawasaki H, Yamada S 2006 Langmuir 22 2
 Google Scholar
Google Scholar
[23] Lyubartsev A P, Rabinovich A L 2011 Soft Matter 7 25
 Google Scholar
Google Scholar
[24] Orendorff C J, Alam T M, Sasaki D Y, Bunker B C, Voigt J A 2009 ACS Nano 3 971
 Google Scholar
Google Scholar
[25] Galati E, Tebbe M, Querejeta-Fernandez A, Xin H L, Gang O, Zhulina E B, Kumacheva E 2017 ACS Nano 11 4995
 Google Scholar
Google Scholar
[26] Galati E, Tao H, Tebbe M, Ansari R, Rubinstein M, Zhulina E B, Kumacheva E 2019 Angew. Chem. Int. Ed. 58 3123
 Google Scholar
Google Scholar
[27] Tao H, Chen L, Galati E, Manion J G, Seferos D S, Zhulina E B, Kumacheva E 2019 Langmuir 35 15872
 Google Scholar
Google Scholar
[28] Wang R Y, Wang H, Wu X, Ji Y, Wang P, Qu Y, Chung T S 2011 Soft Matter 7 8370
 Google Scholar
Google Scholar
[29] Matthews J R, Payne C M, Hafner J H 2015 Langmuir 31 9893
 Google Scholar
Google Scholar
[30] Nakashima H, Furukawa K, Kashimura Y, Torimitsu K 2008 Langmuir 24 5654
 Google Scholar
Google Scholar
[31] Yang J A, Murphy C J 2012 Langmuir 28 5404
 Google Scholar
Google Scholar
[32] Sreeprasad T S, Pradeep T 2011 Langmuir 27 3381
 Google Scholar
Google Scholar
[33] 闫昭, 赵文静, 王荣瑶 2016 65 126101
 Google Scholar
Google Scholar
Yan Z, ZhaoW J, Wang R Y 2016 Acta Phys. Sin. 65 126101
 Google Scholar
Google Scholar
[34] Zhu Y, Qu C, Kuang H, Xu L, Liu L, Hua Y, Wang L, Xu C 2011 Biosens. Bioelectron. 26 4387
 Google Scholar
Google Scholar
[35] Wang Y T, Dellago C 2003 J. Phys. Chem. B 107 9214
 Google Scholar
Google Scholar
[36] da Silva J, Dias R, da Hora G, Soares T, Meneghetti M 2018 J. Braz. Chem. Soc. 29 191
 Google Scholar
Google Scholar
[37] Schmid N, Eichenberger A P, Choutko A, Riniker S, Winger M, Mark A E, van Gunsteren W F 2011 Eur. Biophys. J. 40 843
 Google Scholar
Google Scholar
[38] da Silva J A, Meneghetti M R 2018 Langmuir 34 366
 Google Scholar
Google Scholar
[39] da Silva J A, Netz P A, Meneghetti M R 2020 Langmuir 36 257
 Google Scholar
Google Scholar
[40] Oroskar P A, Jameson C J, Murad S 2016 Mol. Phys. 115 1122
 Google Scholar
Google Scholar
[41] Horsch M A, Zhang Z, Glotzer S C 2005 Phys. Rev. Lett. 95 056105
 Google Scholar
Google Scholar
[42] Wan M W, Li X X, Gao L H, Fang W H 2016 Nanotechnology 27 465704
 Google Scholar
Google Scholar
[43] Li X X, Gao L H, Fang W H 2016 PLoS One 11 e0154568
 Google Scholar
Google Scholar
[44] Sevink G J A, Charlaganov M, Fraaije J G E M 2013 Soft Matter 9 2816
 Google Scholar
Google Scholar
[45] Sevink G J A, Fraaije J 2014 Soft Matter 10 5129
 Google Scholar
Google Scholar
[46] Wan M W, Gao L H, Fang W H 2018 PLoS One 13 e0198049
 Google Scholar
Google Scholar
[47] Groot R D 2003 J. Chem. Phys. 118 11265
 Google Scholar
Google Scholar
[48] Marrink S J, Risselada H J, Yefimov S, Tieleman D P, de Vries A H 2007 J. Phys. Chem. B 111 7812
 Google Scholar
Google Scholar
[49] Humphrey W, Dalke A, Schulten K 1996 J. Mol. Graphics 14 33
 Google Scholar
Google Scholar
[50] Illa-Tuset S, Malaspina D C, Faraudo J 2018 Phys. Chem. Chem. Phys. 20 26422
 Google Scholar
Google Scholar
[51] Lipowsky R 2013 Faraday Discuss. 161 305
 Google Scholar
Google Scholar
[52] Arnarez C, Uusitalo J J, Masman M F, Ingolfsson H I, de Jong D H, Melo M N, Periole X, de Vries A H, Marrink S J 2015 J. Chem. Theory. Comput. 11 260
 Google Scholar
Google Scholar
Catalog
Metrics
- Abstract views: 19344
- PDF Downloads: 140
- Cited By: 0














 DownLoad:
DownLoad: