-
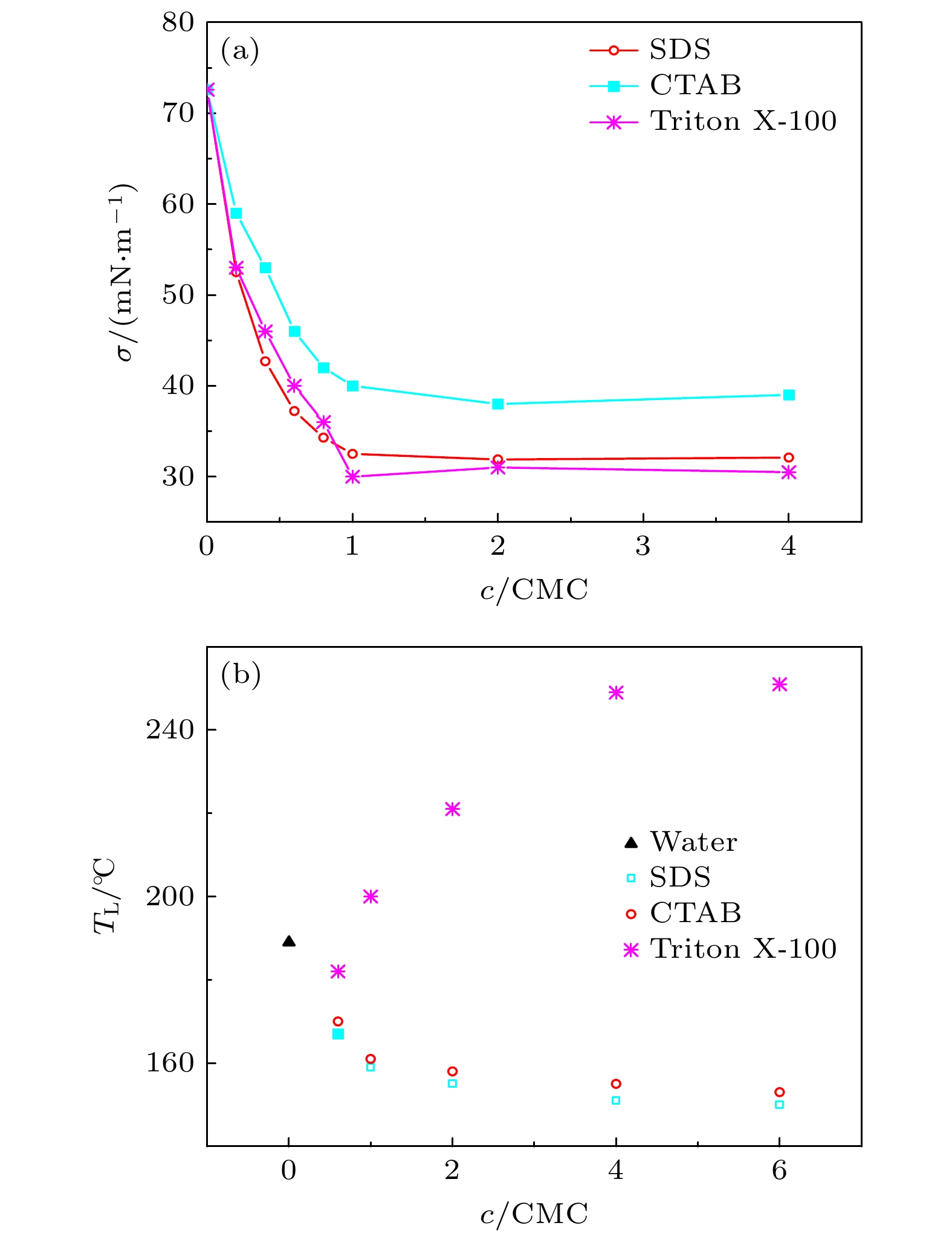
表面活性剂液滴撞击不同温度基底的动力学过程, 在传热、冷却和打印等领域均有广泛涉及. 本文利用高速摄影技术对表面活性剂 SDS, CTAB和Triton X-100的水溶液液滴撞击热铝板的过程进行观测, 研究不同表面活性剂液滴撞击热铝板动力学过程. 实验发现, 处于过渡沸腾的表面活性剂液滴, 在蒸发的最后阶段会形成一个处于非浸润状态的二次液滴. 分析表明, 液滴撞击基底后, 液滴的三相接触线和液滴顶部产生温度梯度, 三相接触线附近的表面活性剂分子浓度显著大于液滴顶部. 由浓度梯度驱动的Marangoni效应使上层液体得以保持, 并在蒸发的最终阶段, 逐渐收缩为球形, 形成二次液滴, 在底部气泡爆炸产生的冲击下脱离基底并起跳. 二次液滴的半径随初始液滴浓度升高而增大, 最终达到饱和半径. 该项工作阐明了二次液滴形成过程中表面活性剂的作用, 为理解Leidenfrost效应的物理机制以及调控沸腾传热提供了参考.
-
关键词:
- 热基底 /
- 表面活性剂 /
- Leidenfrost效应 /
- 二次液滴
The dynamic processes of surfactant droplets impacting onto substrates of varied temperatures have been widely studied in heat transfer, cooling and printing. In this work, we observe the impacting process of aqueous droplets of surfactants SDS, CTAB, and Triton X-100 on a hot aluminum plate via a high-speed camera, in order to study the dynamics of different surfactant droplets impacting on a hot aluminum substrate. Experimentally, it is discovered that the surfactant droplets in transition boiling produce a secondary droplet of non-wetting state in the final stage of evaporation. The analysis demonstrates that after the droplet impacts the substrate, a temperature gradient is created between the top of the droplet and the triple-phase contact line, increasing the surfactant concentration near the triple-phase contact line as compared with that of the top. The top liquid is maintained by the Marangoni effect, which is caused by the concentration gradient. In the final stage of the evaporation process, the residual droplet gradually shrinks into a sphere. It is detached from the substrate and taken off under the impulse force of the bubble explosion at the bottom, generating the secondary droplet. The radius of the secondary drop increases with the raising of initial concentration of the drop, but ultimately reaches the saturation size. This work explains the role of surfactants in forming secondary droplets. Additionally, this work provides a reference for understanding the physical mechanism of Leidenfrost effect and the controlling of boiling heat transmission.-
Keywords:
- hot substrate /
- surfactant /
- Leidenfrost effect /
- secondary droplet
[1] Zhong L S, Guo Z G 2017 Nanoscale 9 6219
 Google Scholar
Google Scholar
[2] Zang D Y, Tarafdar S, Tarasevich Y Y, Choudhury M D, Dutta T 2019 Phys. Rep. 804 1
 Google Scholar
Google Scholar
[3] 马理强, 常建忠, 刘汉涛, 刘谋斌 2012 61 054701
 Google Scholar
Google Scholar
Ma L Q, Chang J Z, Liu H T, Liu M B 2012 Acta Phys. Sin. 61 054701
 Google Scholar
Google Scholar
[4] Girard F, Antoni M, Sefiane K 2010 Langmuir 26 4576
 Google Scholar
Google Scholar
[5] Quéré D 2013 Annu. Rev. Fluid Mech. 45 197
 Google Scholar
Google Scholar
[6] 梁刚涛, 郭亚丽, 沈胜强 2013 62 024705
 Google Scholar
Google Scholar
Liang G T, Guo Y L, Shen S Q 2013 Acta Phys. Sin. 62 024705
 Google Scholar
Google Scholar
[7] Lü S, Tan H S, Wakata Y, Yang X J, Law C K, Lohse D, Sun C 2021 PNAS 118 e2016107118
 Google Scholar
Google Scholar
[8] Cossali G E, Marengo M, Santini M 2008 Int. J. Heat Fluid Flow 29 167
 Google Scholar
Google Scholar
[9] Shirota M, van Limbeek M A J, Sun C, Prosperetti A, Lohse D 2016 Phys. Rev. Lett. 116 064501
 Google Scholar
Google Scholar
[10] Tran T, Staat H J, Prosperetti A, Sun C, Lohse D 2012 Phys. Rev. Lett. 108 036101
 Google Scholar
Google Scholar
[11] Celestini F, Frisch T, Pomeau Y 2012 Phys. Rev. Lett. 109 034501
 Google Scholar
Google Scholar
[12] Lü S, Mathai V, Wang Y J, Sobac B, Colinet P, Lohse D, Sun C 2019 Sci. Adv. 5 eaav8081
 Google Scholar
Google Scholar
[13] Wu X W, Ni Y X, Zhu J, Burrows N D, Murphy C J, Dumitrica T, Wang X J 2016 ACS Appl. Mater. Interfaces 8 10581
 Google Scholar
Google Scholar
[14] Zhao L, Seshadri S, Liang X C, Bailey S J, Haggmark M, Gordon M, Helgeson M E, de Alaniz J R, Luzzatto-Fegiz P, Zhu Y Y 2022 ACS Central Sci. 8 235
 Google Scholar
Google Scholar
[15] Prasad G V V, Dhar P, Samanta D 2022 Int. J. Heat Mass Tran. 189 122675
 Google Scholar
Google Scholar
[16] Morgan A I, Bromley L A 1949 Ind. Eng. Chem. 41 2767
 Google Scholar
Google Scholar
[17] Hetsroni G, Zakin J L, Lin Z, Mosyak A, Pancallo E A, Rozenblit R 2001 Int. J. Heat Mass Tran. 44 485
 Google Scholar
Google Scholar
[18] Sham A, Notley S M 2016 J. Colloid Interface Sci. 469 196
 Google Scholar
Google Scholar
[19] Wang J, Li F C, Li X B 2016 Int. J. Heat Mass Tran. 101 800
 Google Scholar
Google Scholar
[20] Kwon H M, Bird J C, Varanasi K 2013 Appl. Phys. Lett. 103 201601
 Google Scholar
Google Scholar
[21] Huang C K, Carey V P 2007 Int. J. Heat Mass Tran. 50 269
 Google Scholar
Google Scholar
[22] Liang G T, Shen S Q, Guo Y L, Zhang J L 2016 Int. J. Heat Mass Tran. 100 48
 Google Scholar
Google Scholar
[23] Denis R, Clanet C, Quéré D 2002 Nature 417 811
 Google Scholar
Google Scholar
[24] Zhang P P, Peng B X, Yang X, Wang J M, Jiang L 2020 Adv. Mater. Interfaces 7 2000501
 Google Scholar
Google Scholar
[25] Chaves H, Kubitzek A M, Obermeier F 1999 Int. J. Heat Fluid Flow 20 470
 Google Scholar
Google Scholar
[26] Vakarelski I U, Patankar N A, Marston J O, Chan D Y C, Thoroddsen S T 2012 Nature 489 274
 Google Scholar
Google Scholar
[27] Zhang B J, Park J, Kim K J 2013 Int. J. Heat Mass Tran. 63 224
 Google Scholar
Google Scholar
[28] Ahn H, Hwan K M 2013 Int. J. Air-Cond. Refrig. 21 1
 Google Scholar
Google Scholar
[29] Bertola V 2009 Int. J. Heat Mass Tran. 52 1786
 Google Scholar
Google Scholar
[30] Cheng L X, Mewes D, Luke A 2007 Int. J. Heat Mass Tran. 50 2744
 Google Scholar
Google Scholar
-
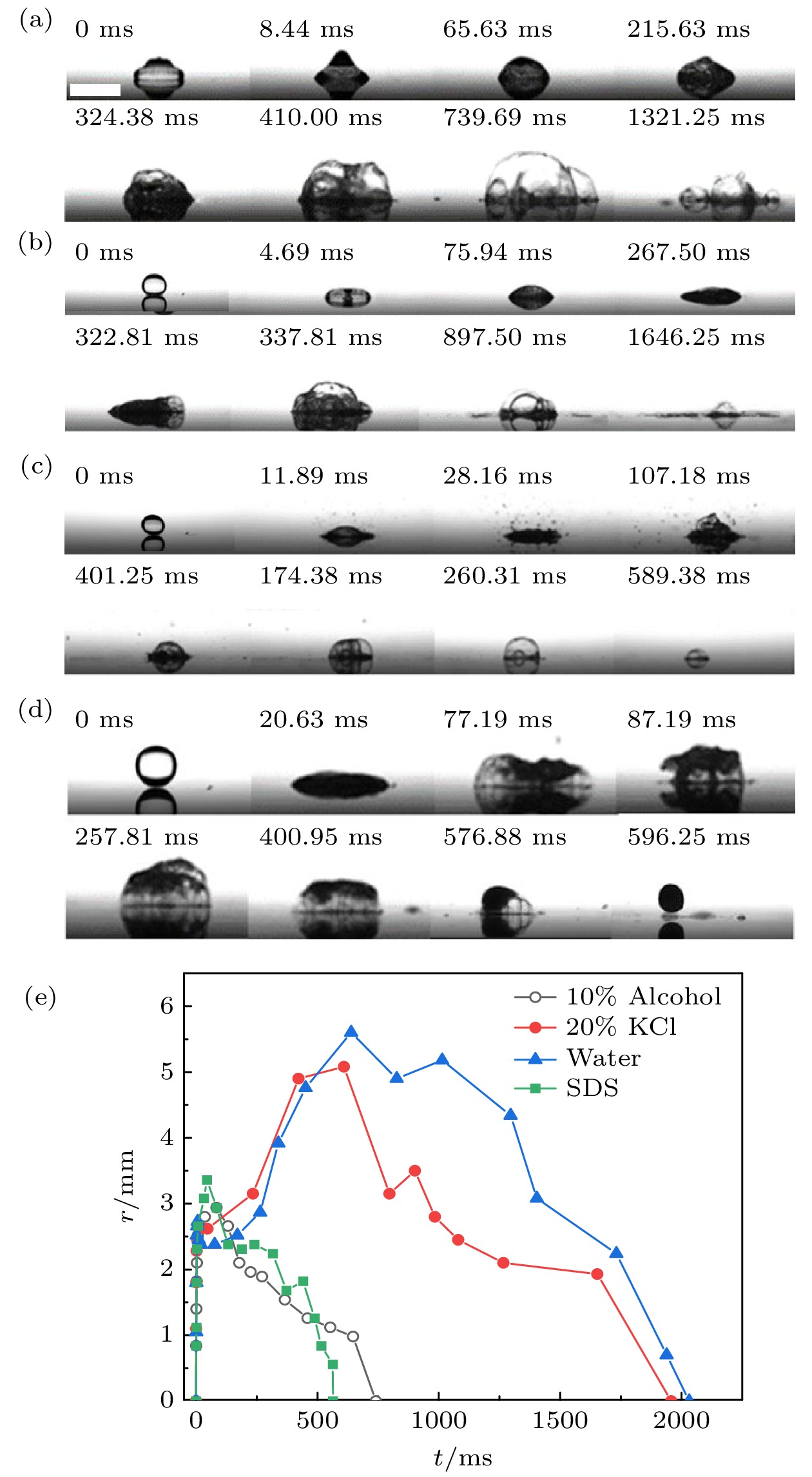
图 2 4种液滴的Leidenfrost现象 (a) 纯水; (b) 10%乙醇水溶液; (c) 20% KCl水溶液; (d) 1 CMC SDS水溶液. (e) 3种液滴第一次撞击基底时接触半径随时间变化. r减小为0时, 液滴反弹脱离基底, 标尺为2 mm
Fig. 2. Leidenfrost phenomenon of four droplets: (a) Pure water; (b) 10% ethanol aqueous solution; (c) 20% KCl aqueous solution; (d) 1 CMC SDS aqueous solution. (e) Contact radius varies with time when droplets hit the substrate for the first time. When r decreased to 0, the droplets bounced off the base, and the scale bar represents 2 mm.
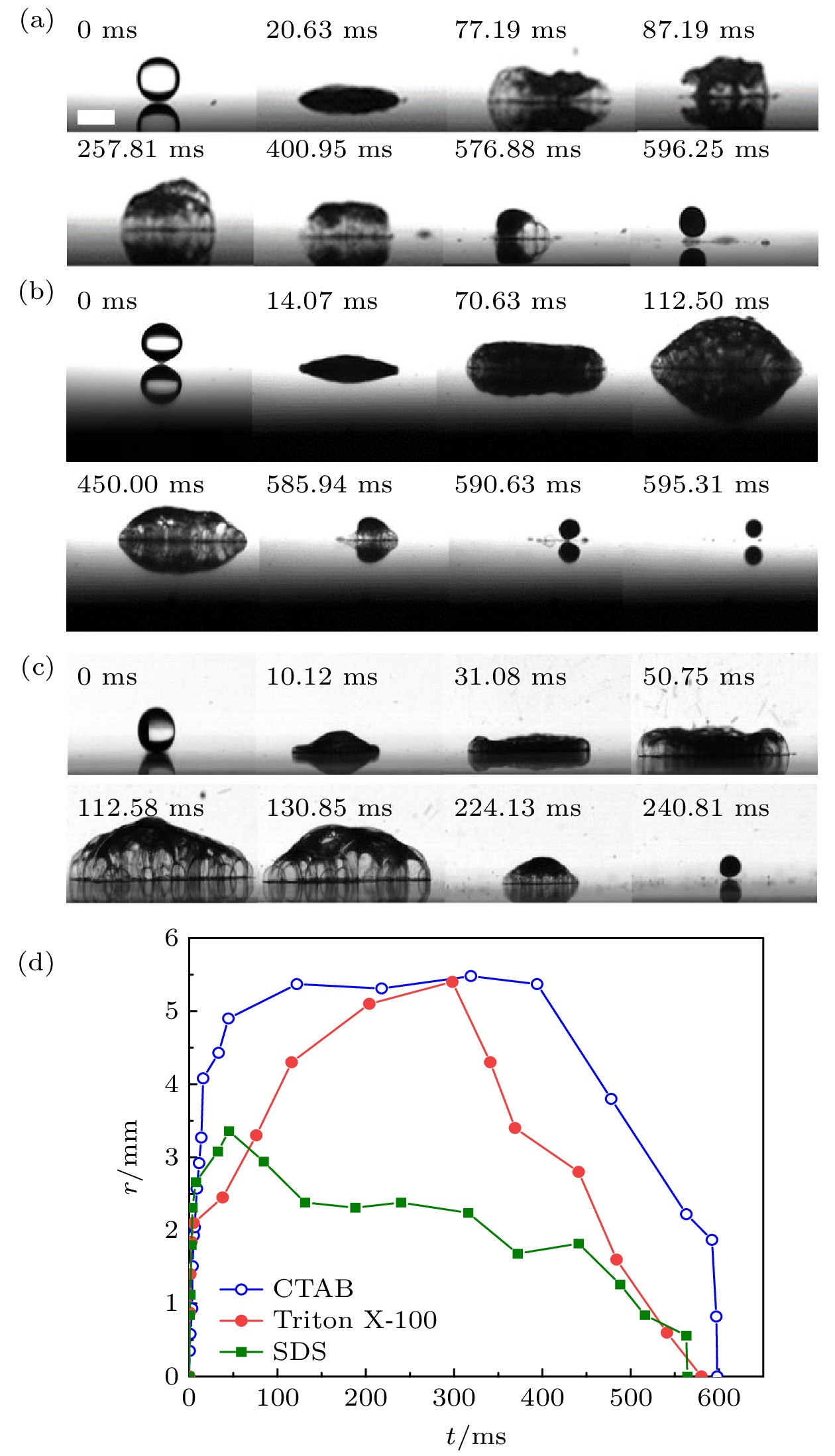
图 4 4种液滴过渡沸腾的过程 (a) 纯水液滴; (b) 20% KCl水液滴; (c) 10%乙醇水液滴; (d) 4 CMC SDS液滴. (e) 4种液滴接触半径随时间变化. SDS液滴的r降为0指二次液滴起飞, 其他液滴降为0代表液滴完全蒸发. 标尺为5 mm
Fig. 4. Transition boiling process of four droplets: (a) Pure water; (b) 20% KCl aqueous solution; (c) 10% ethanol aqueous solution; (d) 4 CMC SDS aqueous solution. (e) Contact radius of the four droplets varies with time. The r of SDS droplet decreased to 0 indicated that the secondary droplet took off, the other droplets indicated that the droplets completely evaporated. The scale bar represents 5 mm.
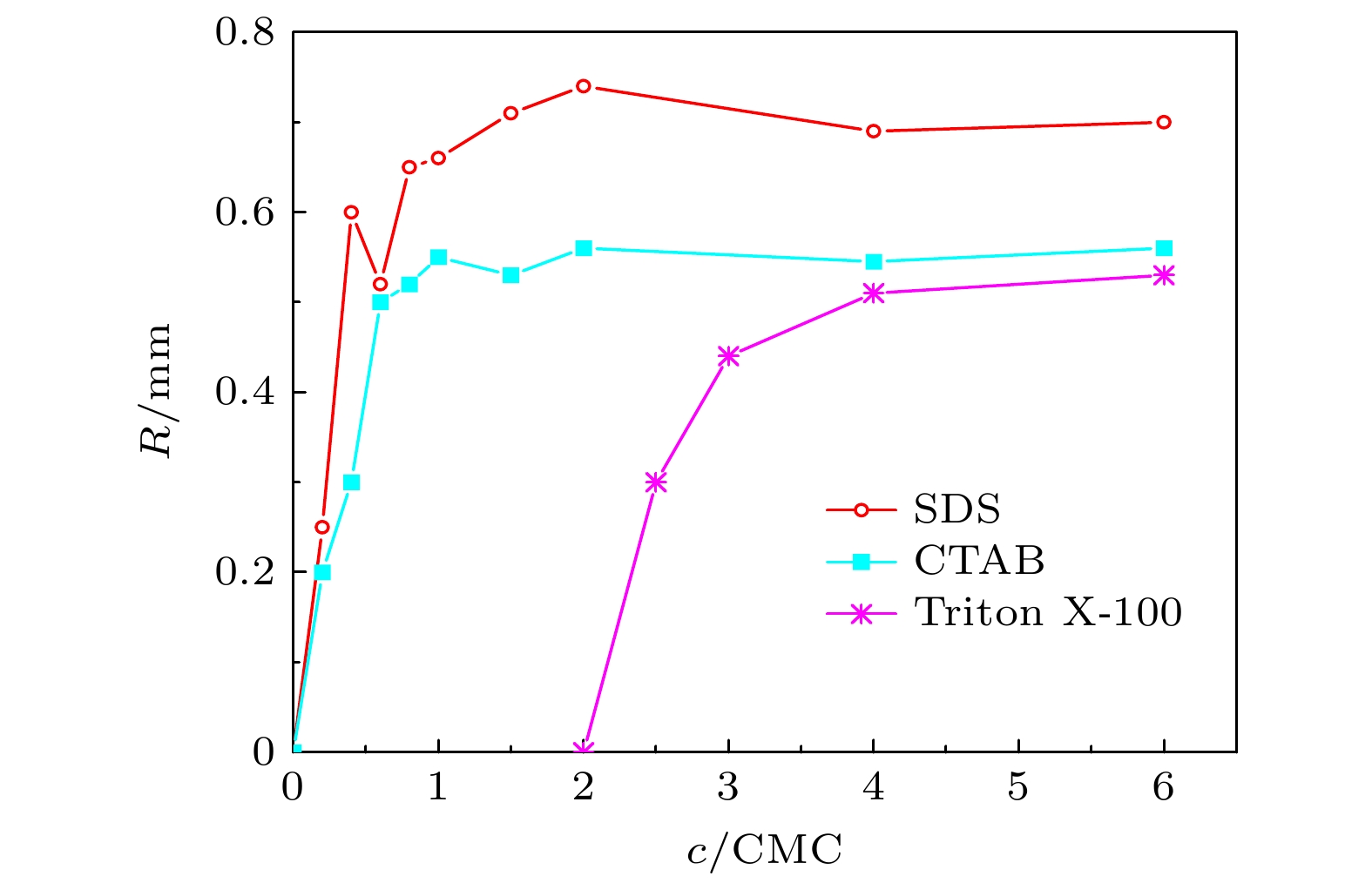
图 5 二次液滴形成 (a) 1 CMC的SDS液滴, 140 ℃; (b) 1 CMC的CTAB液滴, 140 ℃; (c) 4 CMC的Triton X-100液滴, 140 ℃. (d) 3种液滴接触半径随时间变化. r第2次为0代表二次液滴起跳. 标尺为2 mm
Fig. 5. Formation of secondary droplets: (a) 1 CMC SDS droplets at 140 ℃; (b) 1 CMC CTAB droplets at 140 ℃; (c) 4 CMC Triton X-100 droplets at 140 ℃. (d) Contact radius of the three droplets varies with time, the r of SDS droplets decreased to 0 indicated that the secondary droplets took off. The scale bar represents 2 mm.
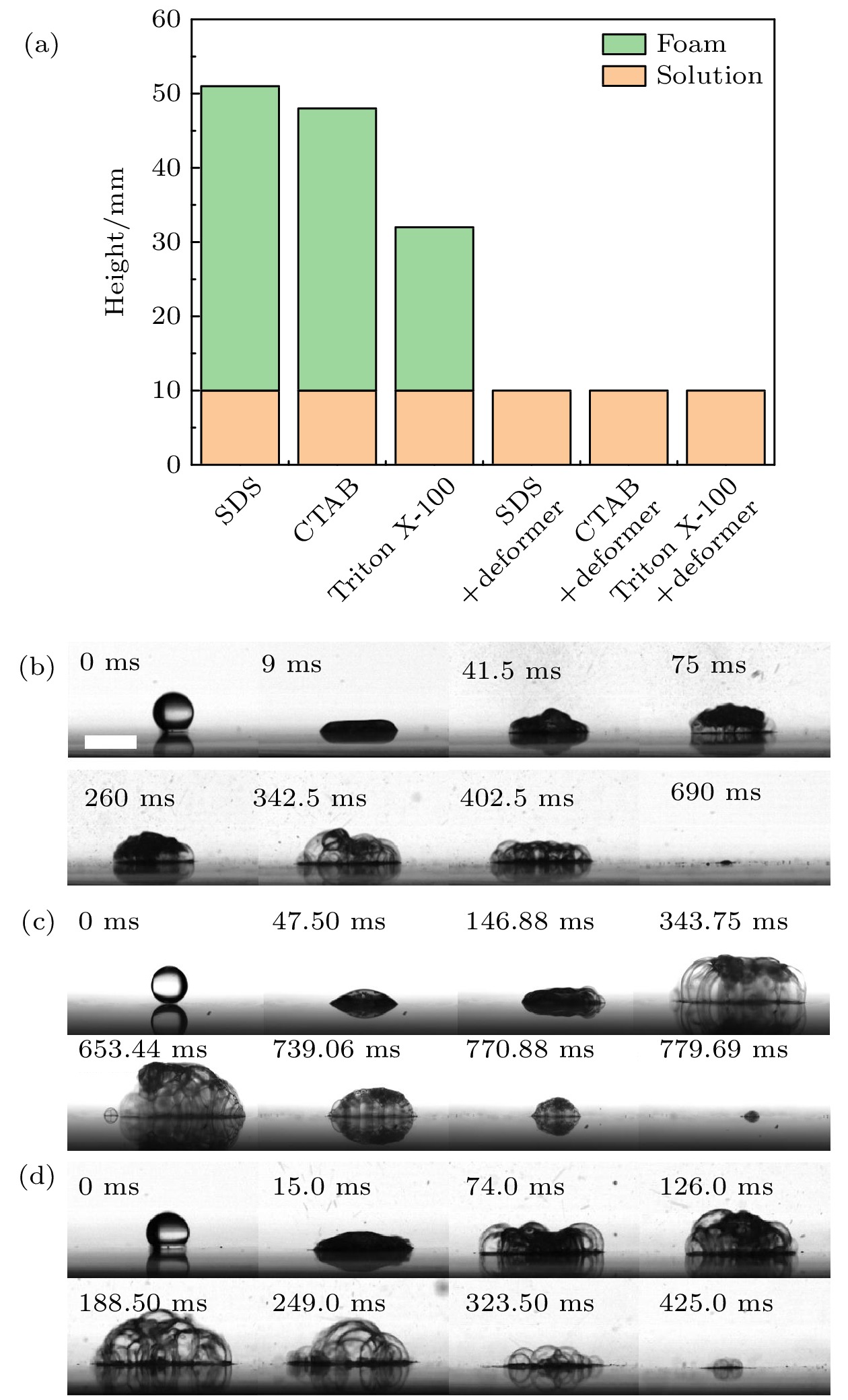
图 7 表面活性剂液滴加入消泡剂 (a) 3种表面活性剂溶液中加入消泡剂剧烈振荡的结果; (b) 加入消泡剂的SDS液滴撞击热基底; (c) 加入消泡剂的CTAB液滴撞击热基底; (d) 加入消泡剂的Triton X-100液滴撞击热基底. 标尺为5 mm
Fig. 7. Addition of defoamer to surfactant solution: (a) Result of three surfactant solutions added defoamer shaken vigorously; (b) SDS droplet with defoamer impacted on the hot substrate; (c) CTAB droplet with defoamer impacted on the hot substrate; (d) Triton X-100 droplet with defoamer impacted on the hot substrate. The scale bar represents 5 mm.
图 8 表面活性剂和消泡剂分子导致的液滴动态行为示意图 (a)表面活性剂液滴撞击热基底的动态过程 (ⅰ) 下落的液滴; (ⅱ) 液滴产生浓度梯度; (ⅲ) 固液界面气泡生长; (ⅳ)气泡支撑上部液体; (ⅴ) 剩余液体收缩为二次液滴; (ⅵ) 二次液滴起跳. (b)添加消泡剂分子后的动态过程 (ⅰ)单个消泡剂液滴吸附表面活性剂分子; (ⅱ) 大液滴中的表面活性剂分子被消泡剂大量消耗, 液滴表面无显著浓度梯度; (ⅲ)顶部液体无法聚集, 箭头方向为Marangoni流动方向
Fig. 8. Schematic of dynamic droplet behavior caused by surfactant and deformer molecules. (a) Dynamic process of surfactant droplet impacting on hot substrate: (ⅰ) Falling droplet; (ⅱ) formation of concentration gradient; (ⅲ) bubbles growth at the solid-liquid interface; (ⅳ) bubbles held up the top liquid; (ⅴ) the remained liquid shrunk into secondary droplet; (ⅵ) the secondary droplet detached from the substrate. (b) Dynamic process after adding defoamer molecules: (ⅰ) A single defoamer drop adsorbed surfactant molecules; (ⅱ) the surfactant molecules in the large droplets were consumed in large quantities by deformers, there was no significant concentration gradient on the droplet surface; (ⅲ) the top liquid failed to aggregate. Arrows showed the direction of Marangoni flow.
表 1 实验中的主要仪器
Table 1. Main instruments in the experiment.
名称 型号 功能(误差) 恒温加热台 C-MAG HP 0—(500±1) ℃ 高速摄像机 Photron Fastcam
Mini UX100103—2×103 f/s 光源 KM-FL120120-W — 热电偶温度计 ES1310 0—(500±0.1) ℃ -
[1] Zhong L S, Guo Z G 2017 Nanoscale 9 6219
 Google Scholar
Google Scholar
[2] Zang D Y, Tarafdar S, Tarasevich Y Y, Choudhury M D, Dutta T 2019 Phys. Rep. 804 1
 Google Scholar
Google Scholar
[3] 马理强, 常建忠, 刘汉涛, 刘谋斌 2012 61 054701
 Google Scholar
Google Scholar
Ma L Q, Chang J Z, Liu H T, Liu M B 2012 Acta Phys. Sin. 61 054701
 Google Scholar
Google Scholar
[4] Girard F, Antoni M, Sefiane K 2010 Langmuir 26 4576
 Google Scholar
Google Scholar
[5] Quéré D 2013 Annu. Rev. Fluid Mech. 45 197
 Google Scholar
Google Scholar
[6] 梁刚涛, 郭亚丽, 沈胜强 2013 62 024705
 Google Scholar
Google Scholar
Liang G T, Guo Y L, Shen S Q 2013 Acta Phys. Sin. 62 024705
 Google Scholar
Google Scholar
[7] Lü S, Tan H S, Wakata Y, Yang X J, Law C K, Lohse D, Sun C 2021 PNAS 118 e2016107118
 Google Scholar
Google Scholar
[8] Cossali G E, Marengo M, Santini M 2008 Int. J. Heat Fluid Flow 29 167
 Google Scholar
Google Scholar
[9] Shirota M, van Limbeek M A J, Sun C, Prosperetti A, Lohse D 2016 Phys. Rev. Lett. 116 064501
 Google Scholar
Google Scholar
[10] Tran T, Staat H J, Prosperetti A, Sun C, Lohse D 2012 Phys. Rev. Lett. 108 036101
 Google Scholar
Google Scholar
[11] Celestini F, Frisch T, Pomeau Y 2012 Phys. Rev. Lett. 109 034501
 Google Scholar
Google Scholar
[12] Lü S, Mathai V, Wang Y J, Sobac B, Colinet P, Lohse D, Sun C 2019 Sci. Adv. 5 eaav8081
 Google Scholar
Google Scholar
[13] Wu X W, Ni Y X, Zhu J, Burrows N D, Murphy C J, Dumitrica T, Wang X J 2016 ACS Appl. Mater. Interfaces 8 10581
 Google Scholar
Google Scholar
[14] Zhao L, Seshadri S, Liang X C, Bailey S J, Haggmark M, Gordon M, Helgeson M E, de Alaniz J R, Luzzatto-Fegiz P, Zhu Y Y 2022 ACS Central Sci. 8 235
 Google Scholar
Google Scholar
[15] Prasad G V V, Dhar P, Samanta D 2022 Int. J. Heat Mass Tran. 189 122675
 Google Scholar
Google Scholar
[16] Morgan A I, Bromley L A 1949 Ind. Eng. Chem. 41 2767
 Google Scholar
Google Scholar
[17] Hetsroni G, Zakin J L, Lin Z, Mosyak A, Pancallo E A, Rozenblit R 2001 Int. J. Heat Mass Tran. 44 485
 Google Scholar
Google Scholar
[18] Sham A, Notley S M 2016 J. Colloid Interface Sci. 469 196
 Google Scholar
Google Scholar
[19] Wang J, Li F C, Li X B 2016 Int. J. Heat Mass Tran. 101 800
 Google Scholar
Google Scholar
[20] Kwon H M, Bird J C, Varanasi K 2013 Appl. Phys. Lett. 103 201601
 Google Scholar
Google Scholar
[21] Huang C K, Carey V P 2007 Int. J. Heat Mass Tran. 50 269
 Google Scholar
Google Scholar
[22] Liang G T, Shen S Q, Guo Y L, Zhang J L 2016 Int. J. Heat Mass Tran. 100 48
 Google Scholar
Google Scholar
[23] Denis R, Clanet C, Quéré D 2002 Nature 417 811
 Google Scholar
Google Scholar
[24] Zhang P P, Peng B X, Yang X, Wang J M, Jiang L 2020 Adv. Mater. Interfaces 7 2000501
 Google Scholar
Google Scholar
[25] Chaves H, Kubitzek A M, Obermeier F 1999 Int. J. Heat Fluid Flow 20 470
 Google Scholar
Google Scholar
[26] Vakarelski I U, Patankar N A, Marston J O, Chan D Y C, Thoroddsen S T 2012 Nature 489 274
 Google Scholar
Google Scholar
[27] Zhang B J, Park J, Kim K J 2013 Int. J. Heat Mass Tran. 63 224
 Google Scholar
Google Scholar
[28] Ahn H, Hwan K M 2013 Int. J. Air-Cond. Refrig. 21 1
 Google Scholar
Google Scholar
[29] Bertola V 2009 Int. J. Heat Mass Tran. 52 1786
 Google Scholar
Google Scholar
[30] Cheng L X, Mewes D, Luke A 2007 Int. J. Heat Mass Tran. 50 2744
 Google Scholar
Google Scholar
计量
- 文章访问数: 5764
- PDF下载量: 117
- 被引次数: 0














 下载:
下载: