-
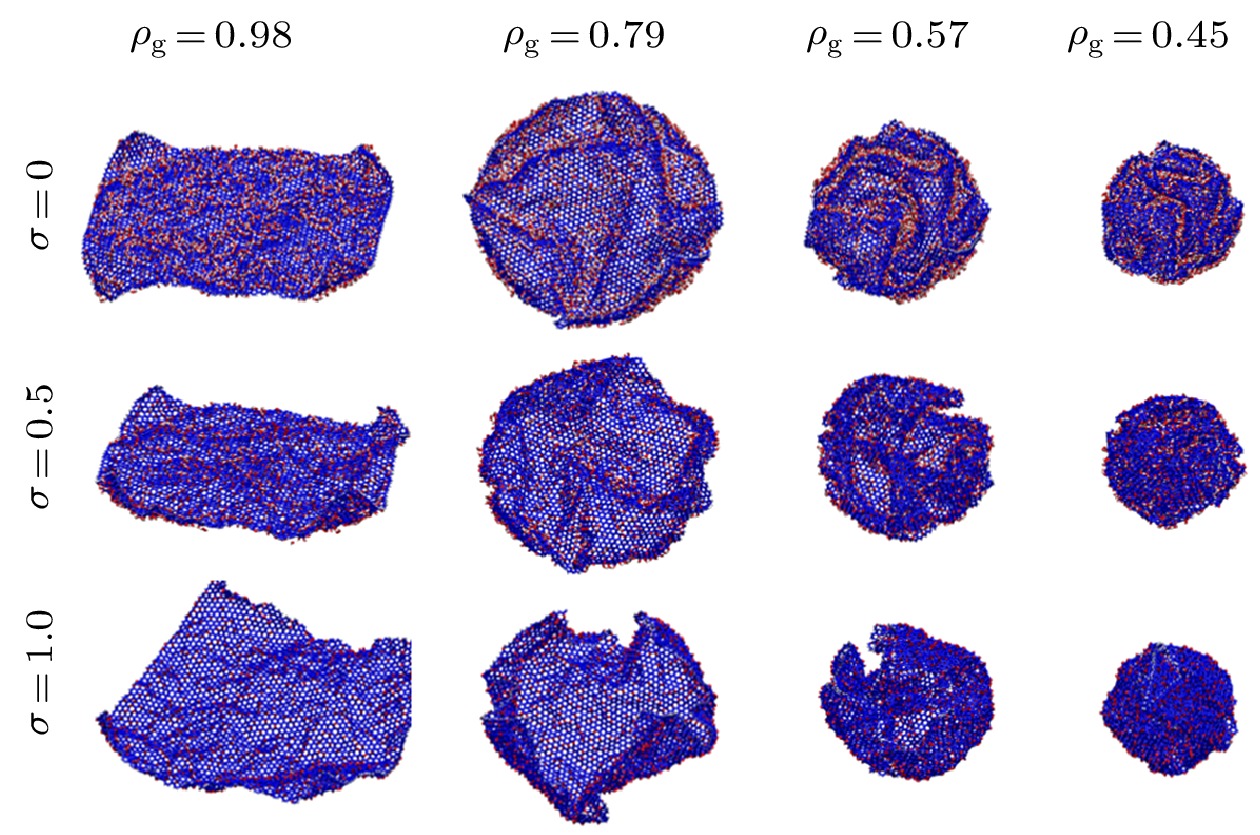
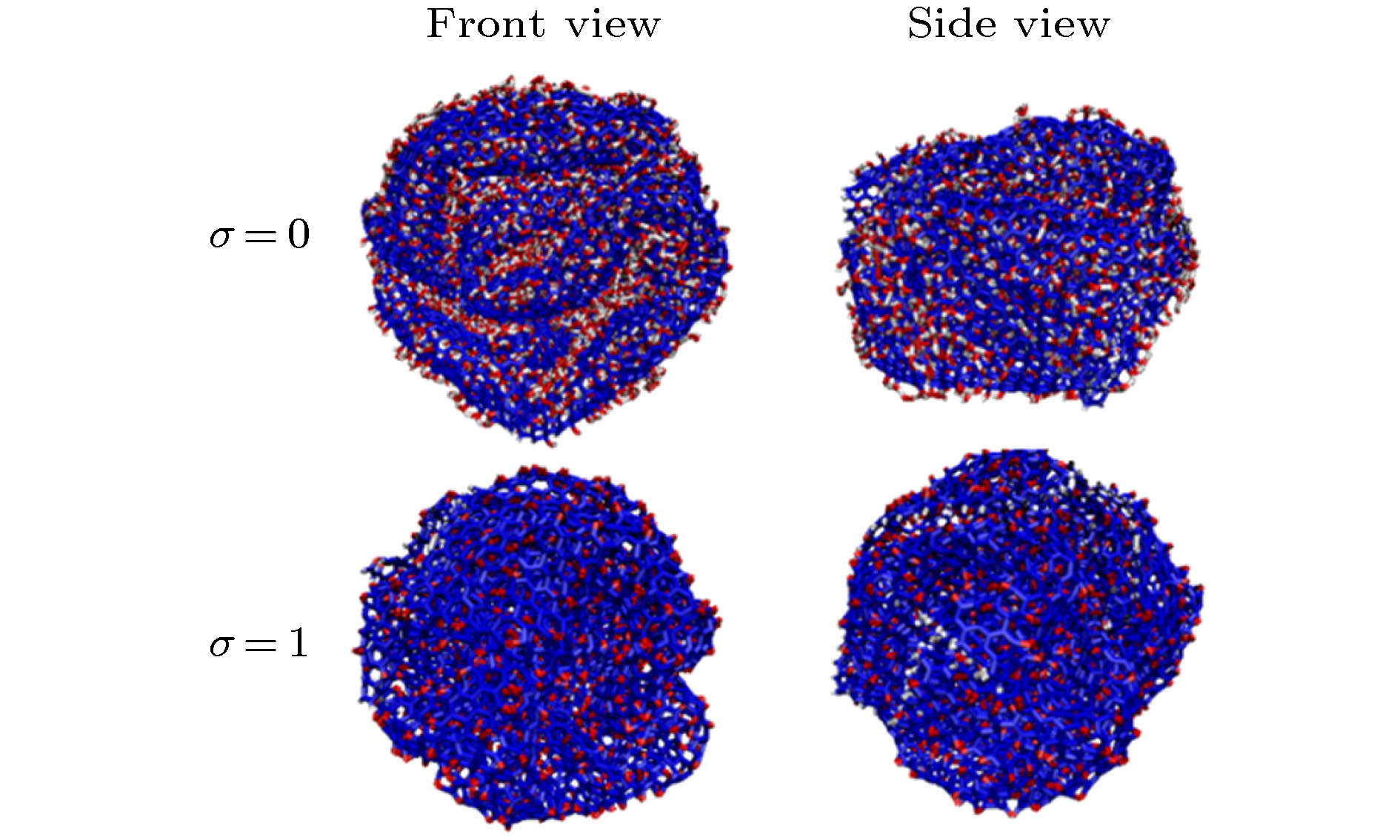
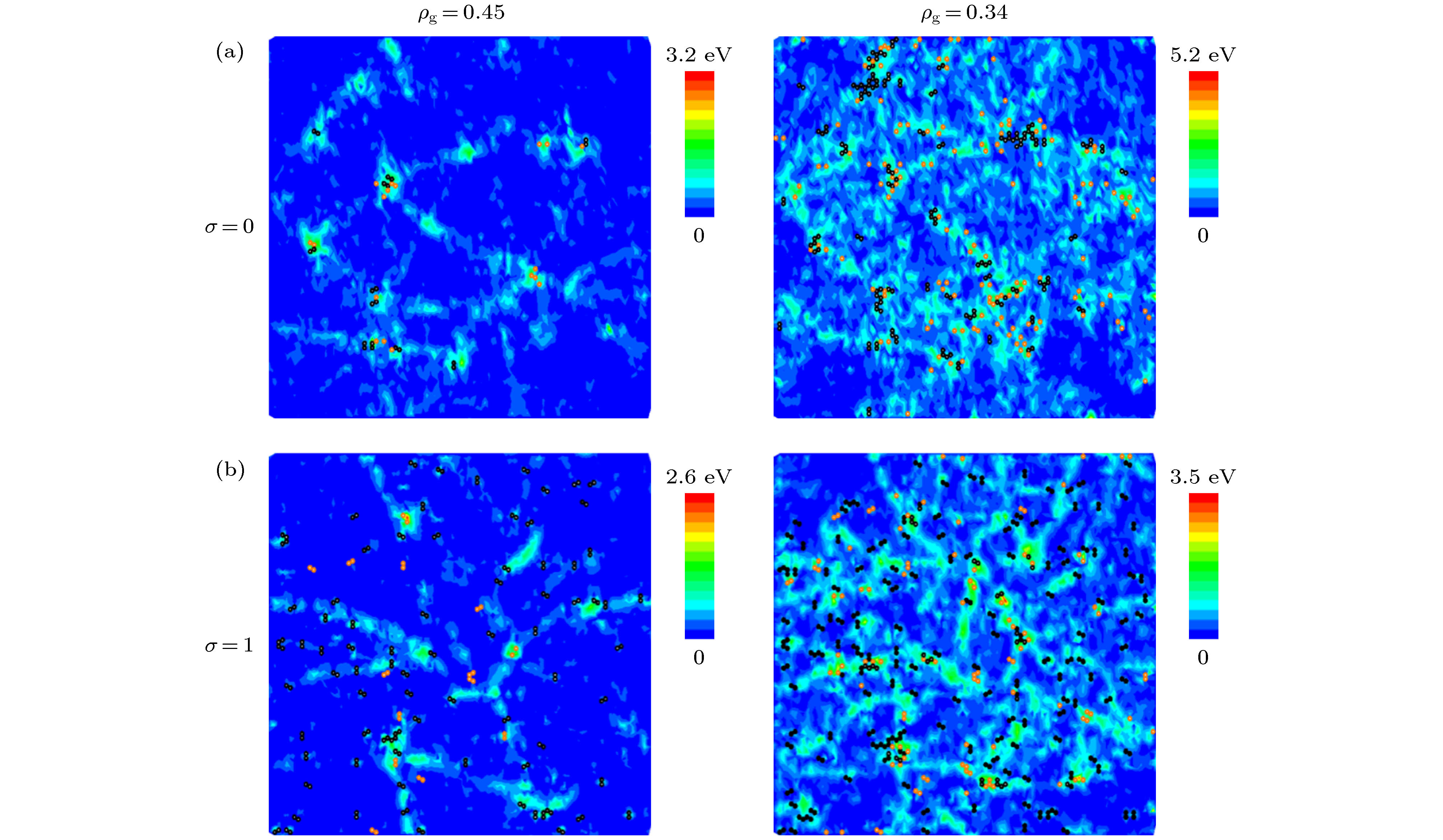
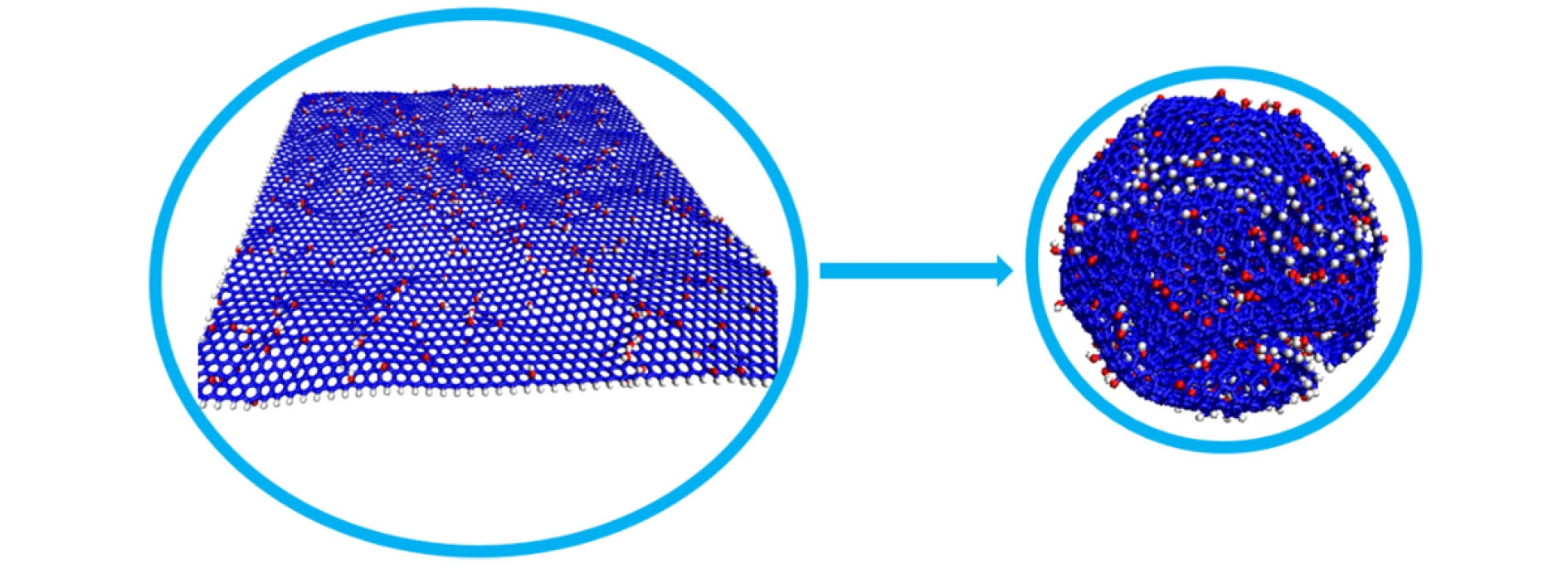
Graphene has a wide range of applications in the fields of electricity, chemistry, biomedicine, and lubrication. But the strong van der Waals interaction between graphene sheets makes it easy to aggregate in preparation process, difficult to produce and put into practical applcation on a large-scale. There are many methods to prevent the graphene sheets from aggregating, such as reducing the size of sheets, adjusting the interaction between solvent and graphene, and using dispersant. Another possible method is to turn the sheet graphene into a three-dimensional structure like the crumpled paper. Compared with sheet graphene, the crumpled graphene ball has excellent aggregation-resistant. The current research on crumpled graphene ball mainly focuses on the effect of the initial structure of graphene sheet on the structure stability of the crumpled ball, but rarely involves the effect of functional groups. In this paper, ReaxFF molecular dynamics is used to simulate the crumpling process of graphene oxide sheet. The effect of functional groups (hydroxyl, epoxy) on the crumpling behavior and the stability of the crumpled ball of graphene oxide are studied. Graphene sheet oxidized by hydroxyl exhibits a push-up crumpling behavior. Graphene sheet oxidized by epoxy exhibits a layer-to-layer fitted crumpling behavior. Different crumpling behavior will lead to the difference in final crumpled ball structure. By analyzing the relationship between the atomic level potential energy incremental distribution and the distribution of broken and formed C—C bonds, we find that the broken and formed C—C bonds mainly occur in areas with a large degree of deformation, and the epoxy group has a stronger weakening effect on the C—C bond connected to it than the hydroxyl group. The release process of graphene oxide crumpled ball is simulated to study its structural stability. The stability of graphene oxide crumpled ball depends on the number of the broken and formed C—C bonds, that is, the more the number of broken and formed C—C bonds, the more stable the structure is, and under the same oxidation rate, the stability of the crumpled ball structure increases with the proportion of epoxy groups increasing. This study shows that the stability of graphene oxide crumpled ball structure can be controlled by changing the relative proportion of functional groups.
-
Keywords:
- graphene oxide /
- crumpling behavior /
- stability /
- molecular dynamics simulation
[1] Varshney V, Patnaik S S, Roy A K, Froudakis G, Farmer B L 2010 ACS Nano 4 1153
 Google Scholar
Google Scholar
[2] Yazyev O V, Louie S G 2010 Nat. Mater. 9 806
 Google Scholar
Google Scholar
[3] Sakhaee-Pour A 2009 Comput. Mater. Sci. 45 266
 Google Scholar
Google Scholar
[4] 尹伟红, 韩勤, 杨晓红 2012 61 248502
 Google Scholar
Google Scholar
Yin W H, Han Q, Yang X H 2012 Acta Phys. Sin. 61 248502
 Google Scholar
Google Scholar
[5] Tozzini V, Pellegrini V 2013 Phys. Chem. Chem. Phys. 15 80
 Google Scholar
Google Scholar
[6] Song Z P, Xu T, Gordin M L, Jiang Y B, Bae I T, Xiao Q F, Zhan H, Liu J, Wang D H 2012 Nano Lett. 12 2205
 Google Scholar
Google Scholar
[7] Yang X W, Zhu J W, Qiu L, Li D 2011 Adv. Mater. 23 2833
 Google Scholar
Google Scholar
[8] Tung V C, Allen M J, Yang Y, Kaner R B 2009 Nat. Nanotechnol. 4 25
 Google Scholar
Google Scholar
[9] Luo J, Cote L J, Tung V C, Tan A T L, Goins P E, Wu J S, Huang J X 2010 J. Am. Chem. Soc. 132 17667
 Google Scholar
Google Scholar
[10] Li D, Mueller M B, Gilje S, Kaner R B, Wallace G G 2008 Nat. Nanotechnol. 3 101
 Google Scholar
Google Scholar
[11] Korkut S, Roy-Mayhew J D, Dabbs D M, Milius D L, Aksay I A 2011 ACS Nano 5 5214
 Google Scholar
Google Scholar
[12] Hamilton C E, Lomeda J R, Sun Z Z, Tour J M, Barron A R 2009 Nano Lett. 9 3460
 Google Scholar
Google Scholar
[13] Tallinen T, Astrom J A, Timonen J 2009 Nat. Mater. 8 25
 Google Scholar
Google Scholar
[14] Matan K, Williams R B, Witten T A, Nagel S R 2002 Phys. Rev. Lett. 88 076101
 Google Scholar
Google Scholar
[15] Lobkovsky A, Gentges S, Li H, Morse D, Witten T A 1995 Science 270 1482
 Google Scholar
Google Scholar
[16] Luo J Y, Jang H D, Sun T, Xiao L, He Z, Katsoulidis A P, Kanatzidis M G, Gibson J M, Huang J X 2011 ACS Nano 5 8943
 Google Scholar
Google Scholar
[17] 邓剑锋, 李慧琴, 于帆, 梁齐 2020 69 076802
 Google Scholar
Google Scholar
Deng J F, Li H Q, Yu F, Liang Q 2020 Acta Phys. Sin. 69 076802
 Google Scholar
Google Scholar
[18] Dou X, Koltonow A R, He X L, Jang H D, Wang Q, Chung Y W, Huang J X 2016 Proc. Natl. Acad. Sci. U. S. A. 113 1528
 Google Scholar
Google Scholar
[19] Cranford S W, Buehler M J 2011 Phys. Rev. B 84 205451
 Google Scholar
Google Scholar
[20] Chang C, Song Z G, Lin J, Xu Z P 2013 RSC Adv. 3 2720
 Google Scholar
Google Scholar
[21] Becton M, Zhang L Y, Wang X Q 2015 Phys. Chem. Chem. Phys. 17 6297
 Google Scholar
Google Scholar
[22] Becton M, Zhang L Y, Wang X Q 2014 Phys. Chem. Chem. Phys. 16 18233
 Google Scholar
Google Scholar
[23] Baimova J A, Rysaeva L K, Liu B, Dmitriev S V, Zhou K 2015 Phys. Status Solidi B 252 1502
 Google Scholar
Google Scholar
[24] Baimova J A, Liu B, Dmitriev S V, Zhou K 2015 J. Phys. D: Appl. Phys. 48 095302
 Google Scholar
Google Scholar
[25] Wan J, Jiang J W, Park H S 2018 J. Phys. D: Appl. Phys. 51 015302
 Google Scholar
Google Scholar
[26] Wang W N, Jiang Y, Biswas P 2012 J. Phys. Chem. Lett. 3 3228
 Google Scholar
Google Scholar
[27] Soler-Crespo R A, Gao W, Xiao P H, Wei X D, Paci J T, Henkelman G, Espinosa H D 2016 J. Phys. Chem. Lett. 7 2702
 Google Scholar
Google Scholar
[28] Chenoweth K, van Duin A C T, Goddard W A, 2008 J. Phys. Chem. A 112 1040
 Google Scholar
Google Scholar
[29] Verma A, Parashar A 2018 Nanotechnology 29 115706
 Google Scholar
Google Scholar
[30] Meng L J, Jiang J, Wang J L, Ding F 2014 J. Phys. Chem. C 118 720
 Google Scholar
Google Scholar
[31] Wang L F, Duan F L 2018 Tribol. Int. 123 266
 Google Scholar
Google Scholar
[32] Li J L, Kudin K N, McAllister M J, Prud'homme R K, Aksay I A, Car R 2006 Phys. Rev. Lett. 96 176101
 Google Scholar
Google Scholar
-
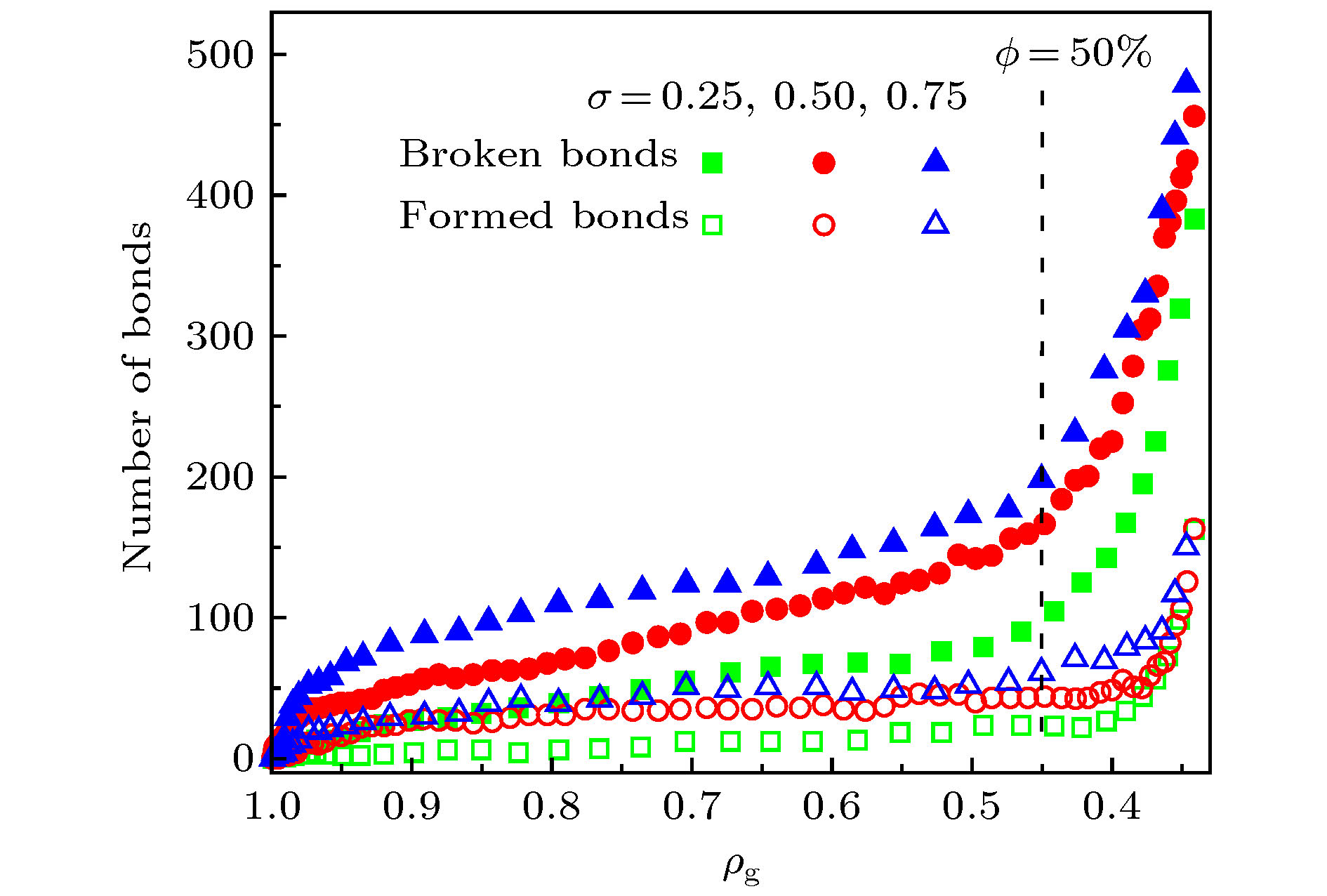
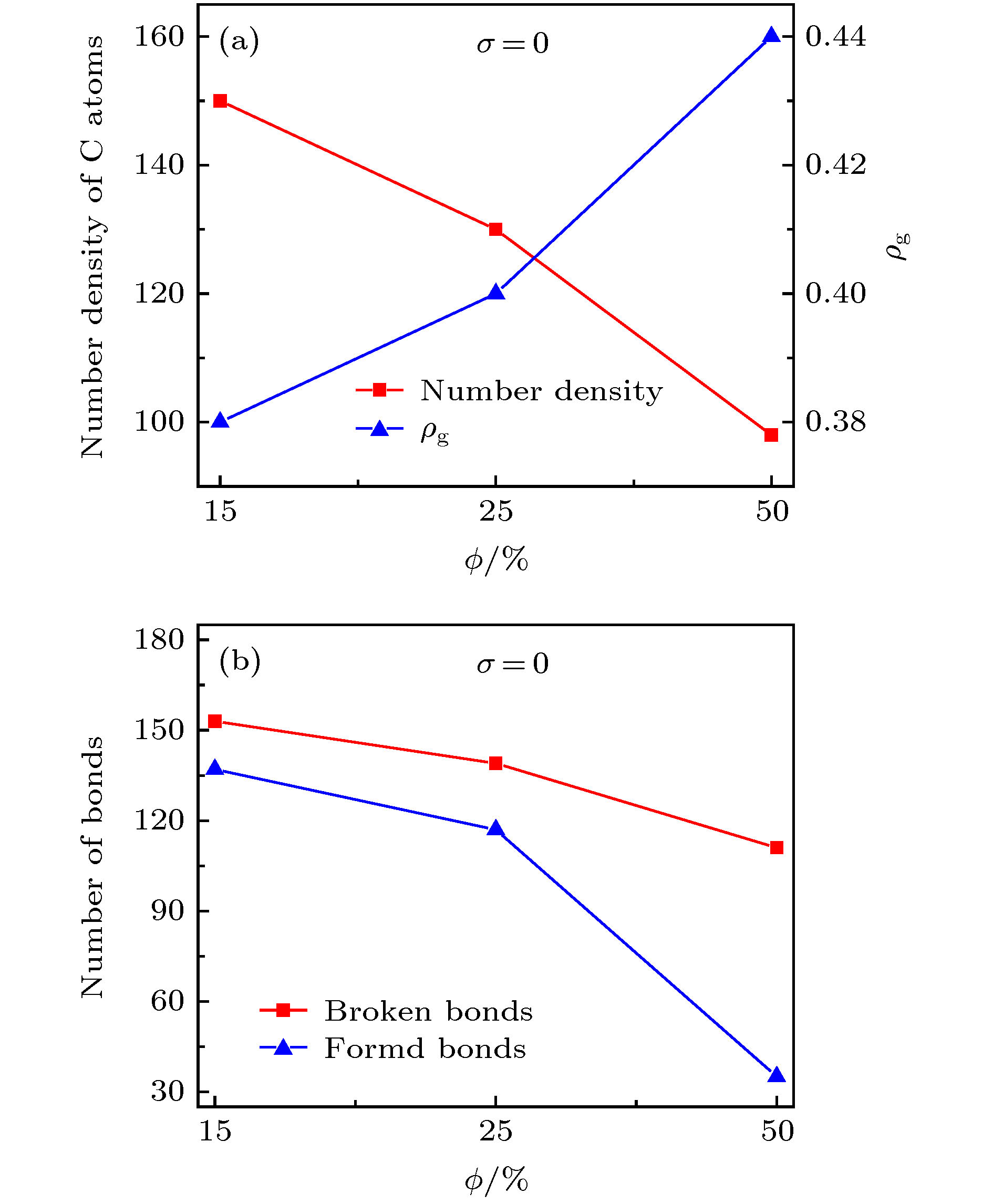
图 7 (a) 释放开始时刻的碳原子数密度与释放稳定后的ρg值; (b) 释放开始时刻的C—C断键、成键数量 (σ = 0)
Figure 7. (a) Number density of C atoms at the beginning of release process and the ρg value at the end of release process; (b) Number of broken and formed C—C bonds at the beginning of release process for the graphene oxide sheets with σ = 0.
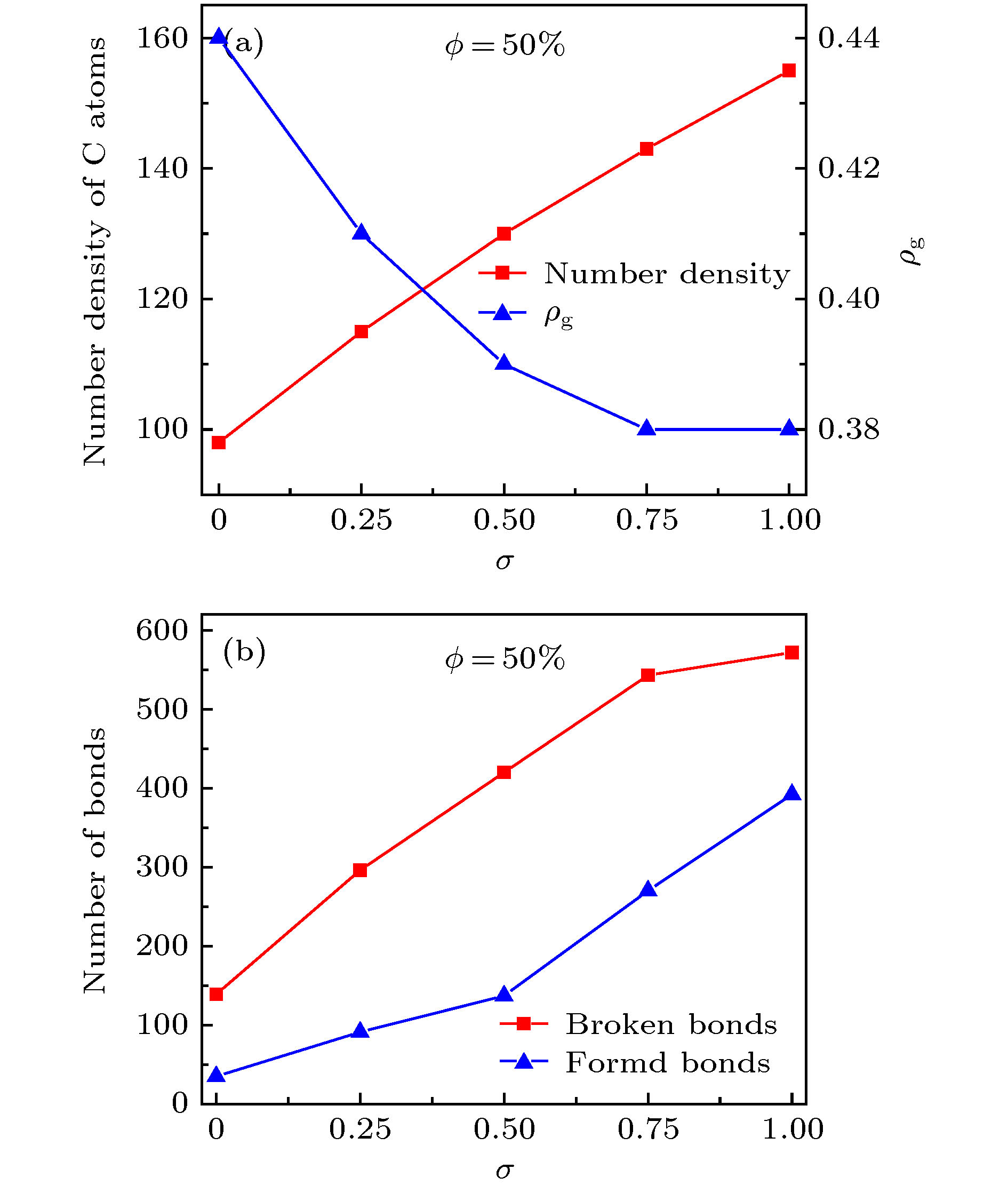
图 8 (a) 释放开始时刻的碳原子数密度与释放稳定后的ρg值; (b) 释放开始时刻的C—C断键、成键数量 (ϕ = 50%)
Figure 8. (a) Number density of C atoms at the beginning of release process and the ρg value at the end of release process; (b) Number of broken and formed C—C bonds at the beginning of release process for the graphene oxide sheets with ϕ = 50%.
-
[1] Varshney V, Patnaik S S, Roy A K, Froudakis G, Farmer B L 2010 ACS Nano 4 1153
 Google Scholar
Google Scholar
[2] Yazyev O V, Louie S G 2010 Nat. Mater. 9 806
 Google Scholar
Google Scholar
[3] Sakhaee-Pour A 2009 Comput. Mater. Sci. 45 266
 Google Scholar
Google Scholar
[4] 尹伟红, 韩勤, 杨晓红 2012 61 248502
 Google Scholar
Google Scholar
Yin W H, Han Q, Yang X H 2012 Acta Phys. Sin. 61 248502
 Google Scholar
Google Scholar
[5] Tozzini V, Pellegrini V 2013 Phys. Chem. Chem. Phys. 15 80
 Google Scholar
Google Scholar
[6] Song Z P, Xu T, Gordin M L, Jiang Y B, Bae I T, Xiao Q F, Zhan H, Liu J, Wang D H 2012 Nano Lett. 12 2205
 Google Scholar
Google Scholar
[7] Yang X W, Zhu J W, Qiu L, Li D 2011 Adv. Mater. 23 2833
 Google Scholar
Google Scholar
[8] Tung V C, Allen M J, Yang Y, Kaner R B 2009 Nat. Nanotechnol. 4 25
 Google Scholar
Google Scholar
[9] Luo J, Cote L J, Tung V C, Tan A T L, Goins P E, Wu J S, Huang J X 2010 J. Am. Chem. Soc. 132 17667
 Google Scholar
Google Scholar
[10] Li D, Mueller M B, Gilje S, Kaner R B, Wallace G G 2008 Nat. Nanotechnol. 3 101
 Google Scholar
Google Scholar
[11] Korkut S, Roy-Mayhew J D, Dabbs D M, Milius D L, Aksay I A 2011 ACS Nano 5 5214
 Google Scholar
Google Scholar
[12] Hamilton C E, Lomeda J R, Sun Z Z, Tour J M, Barron A R 2009 Nano Lett. 9 3460
 Google Scholar
Google Scholar
[13] Tallinen T, Astrom J A, Timonen J 2009 Nat. Mater. 8 25
 Google Scholar
Google Scholar
[14] Matan K, Williams R B, Witten T A, Nagel S R 2002 Phys. Rev. Lett. 88 076101
 Google Scholar
Google Scholar
[15] Lobkovsky A, Gentges S, Li H, Morse D, Witten T A 1995 Science 270 1482
 Google Scholar
Google Scholar
[16] Luo J Y, Jang H D, Sun T, Xiao L, He Z, Katsoulidis A P, Kanatzidis M G, Gibson J M, Huang J X 2011 ACS Nano 5 8943
 Google Scholar
Google Scholar
[17] 邓剑锋, 李慧琴, 于帆, 梁齐 2020 69 076802
 Google Scholar
Google Scholar
Deng J F, Li H Q, Yu F, Liang Q 2020 Acta Phys. Sin. 69 076802
 Google Scholar
Google Scholar
[18] Dou X, Koltonow A R, He X L, Jang H D, Wang Q, Chung Y W, Huang J X 2016 Proc. Natl. Acad. Sci. U. S. A. 113 1528
 Google Scholar
Google Scholar
[19] Cranford S W, Buehler M J 2011 Phys. Rev. B 84 205451
 Google Scholar
Google Scholar
[20] Chang C, Song Z G, Lin J, Xu Z P 2013 RSC Adv. 3 2720
 Google Scholar
Google Scholar
[21] Becton M, Zhang L Y, Wang X Q 2015 Phys. Chem. Chem. Phys. 17 6297
 Google Scholar
Google Scholar
[22] Becton M, Zhang L Y, Wang X Q 2014 Phys. Chem. Chem. Phys. 16 18233
 Google Scholar
Google Scholar
[23] Baimova J A, Rysaeva L K, Liu B, Dmitriev S V, Zhou K 2015 Phys. Status Solidi B 252 1502
 Google Scholar
Google Scholar
[24] Baimova J A, Liu B, Dmitriev S V, Zhou K 2015 J. Phys. D: Appl. Phys. 48 095302
 Google Scholar
Google Scholar
[25] Wan J, Jiang J W, Park H S 2018 J. Phys. D: Appl. Phys. 51 015302
 Google Scholar
Google Scholar
[26] Wang W N, Jiang Y, Biswas P 2012 J. Phys. Chem. Lett. 3 3228
 Google Scholar
Google Scholar
[27] Soler-Crespo R A, Gao W, Xiao P H, Wei X D, Paci J T, Henkelman G, Espinosa H D 2016 J. Phys. Chem. Lett. 7 2702
 Google Scholar
Google Scholar
[28] Chenoweth K, van Duin A C T, Goddard W A, 2008 J. Phys. Chem. A 112 1040
 Google Scholar
Google Scholar
[29] Verma A, Parashar A 2018 Nanotechnology 29 115706
 Google Scholar
Google Scholar
[30] Meng L J, Jiang J, Wang J L, Ding F 2014 J. Phys. Chem. C 118 720
 Google Scholar
Google Scholar
[31] Wang L F, Duan F L 2018 Tribol. Int. 123 266
 Google Scholar
Google Scholar
[32] Li J L, Kudin K N, McAllister M J, Prud'homme R K, Aksay I A, Car R 2006 Phys. Rev. Lett. 96 176101
 Google Scholar
Google Scholar
Catalog
Metrics
- Abstract views: 10669
- PDF Downloads: 266
- Cited By: 0














 DownLoad:
DownLoad: