-
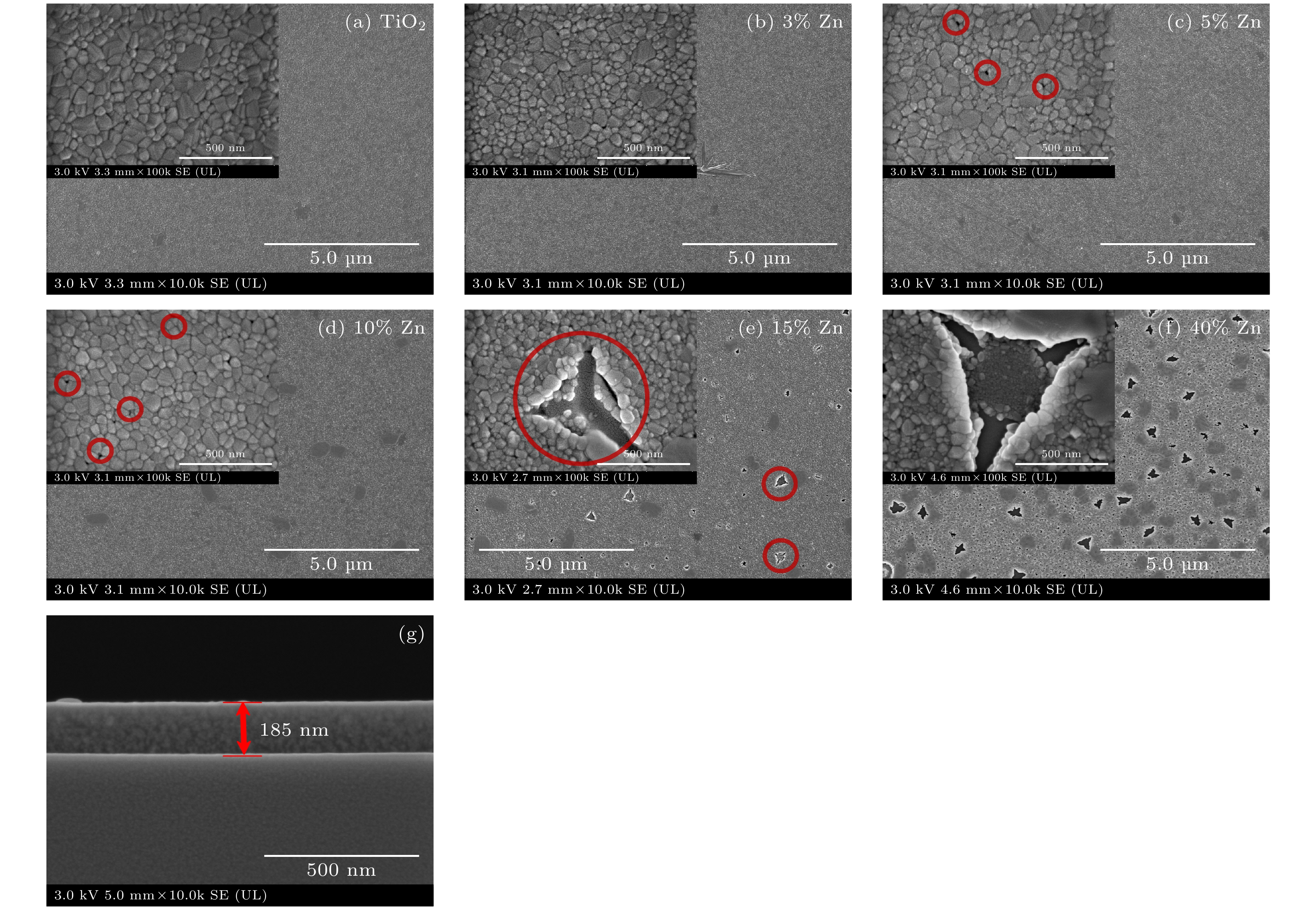
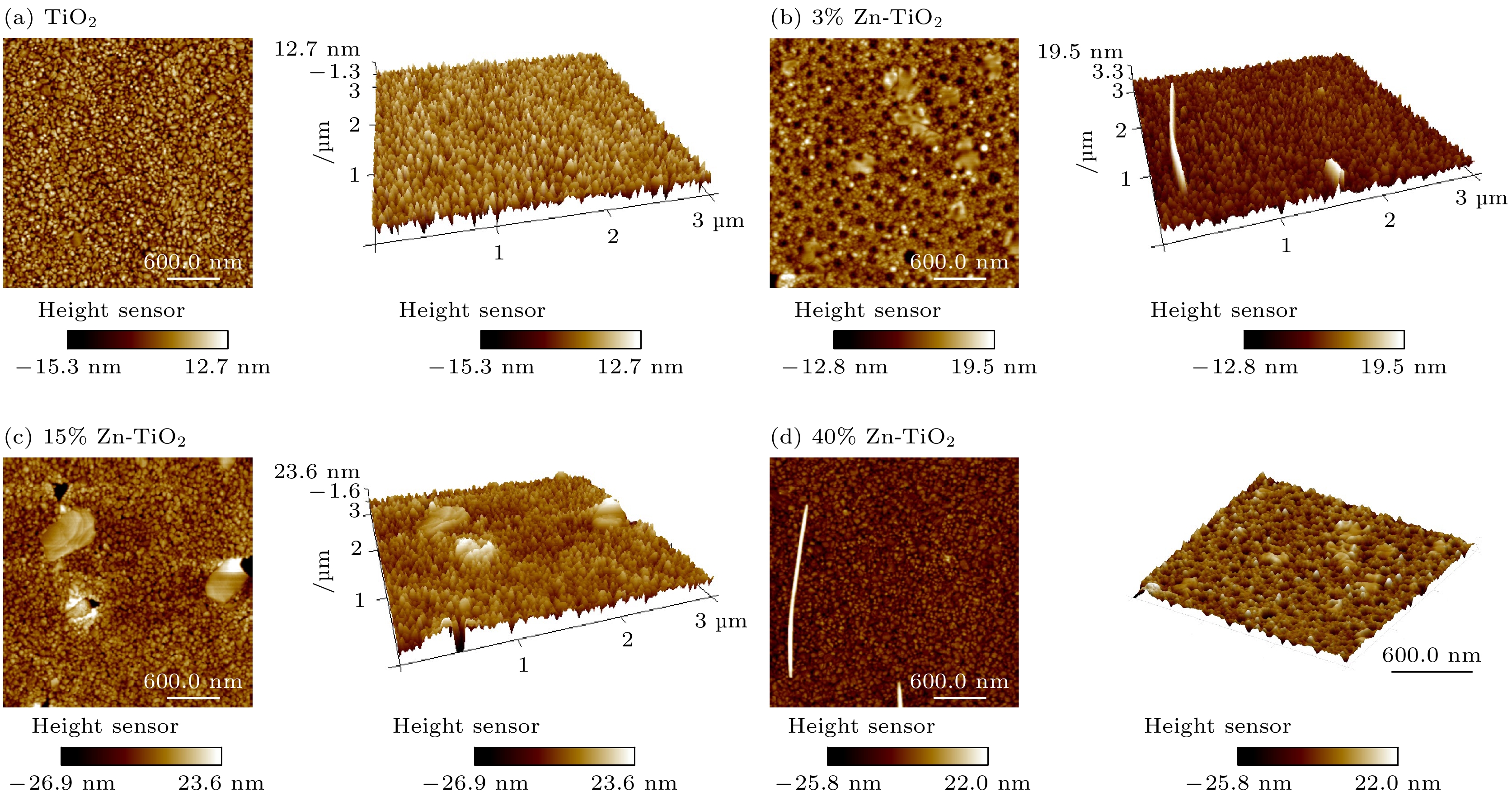
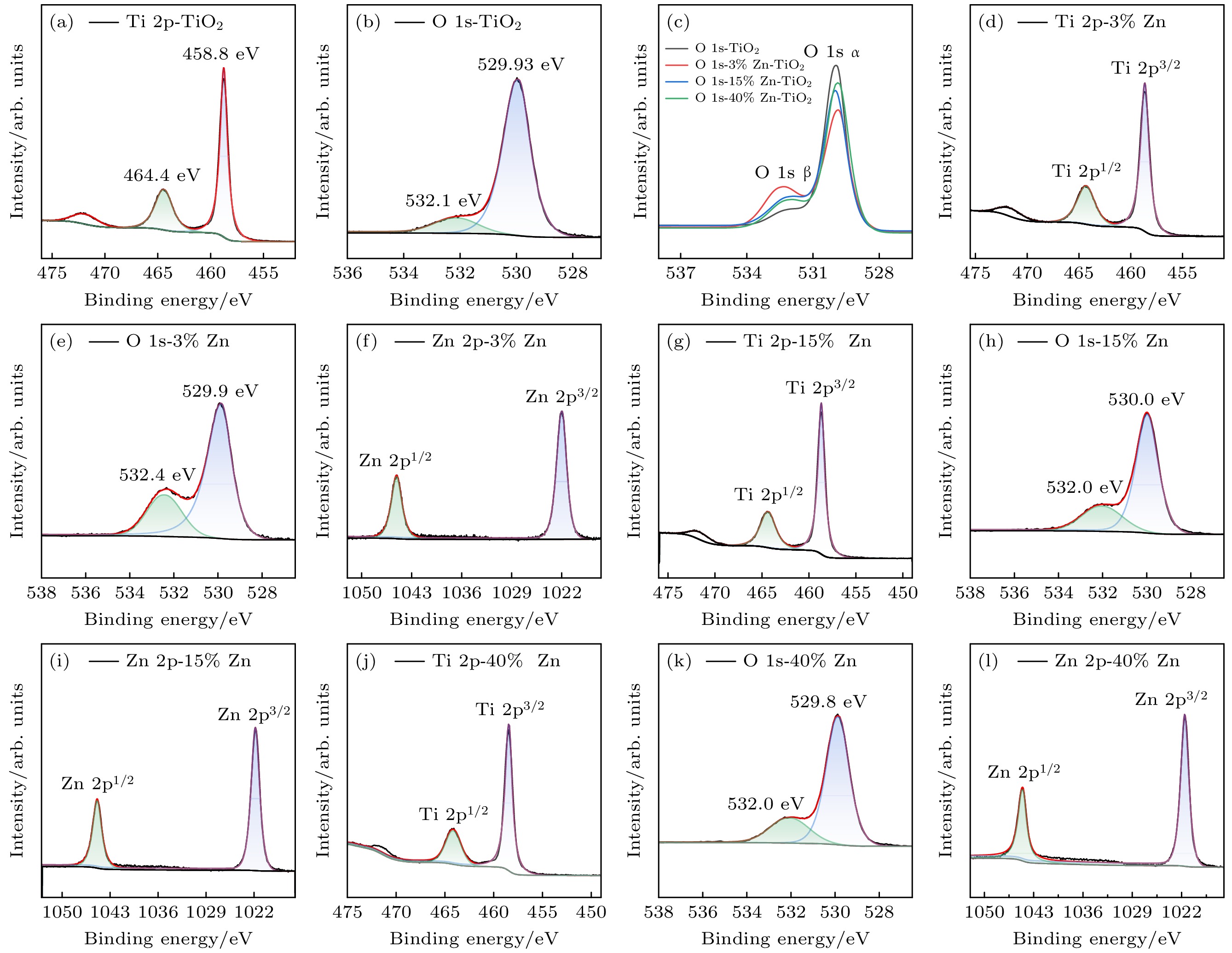
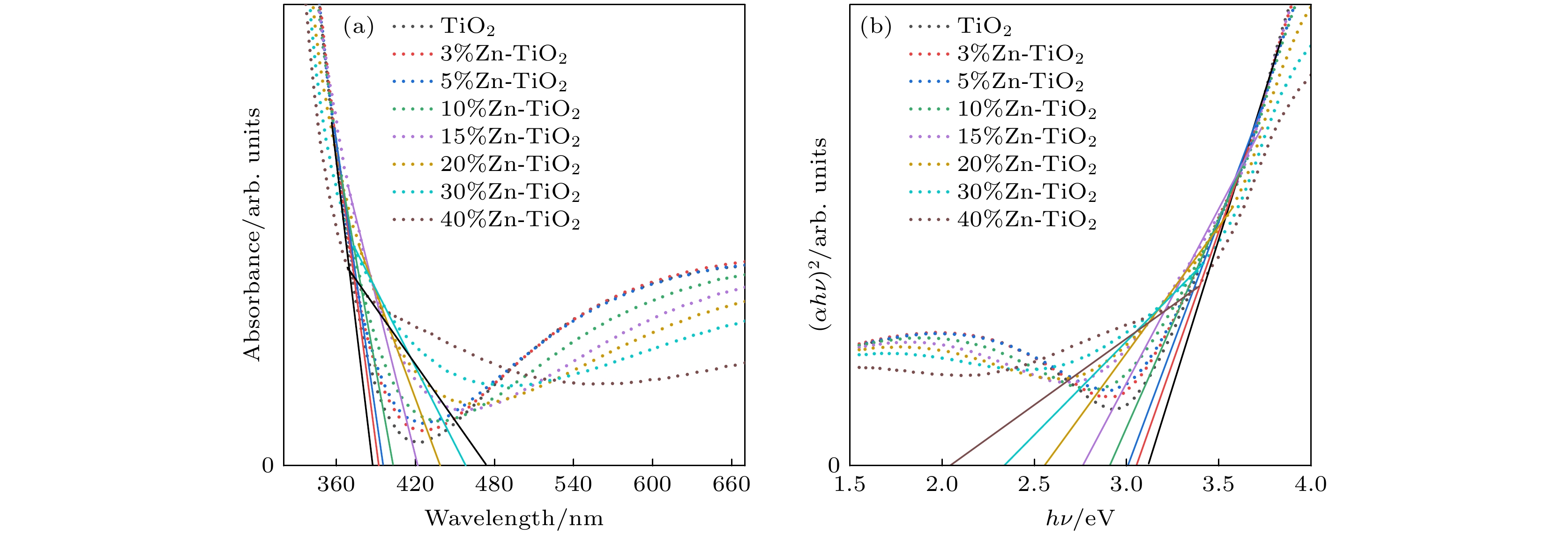
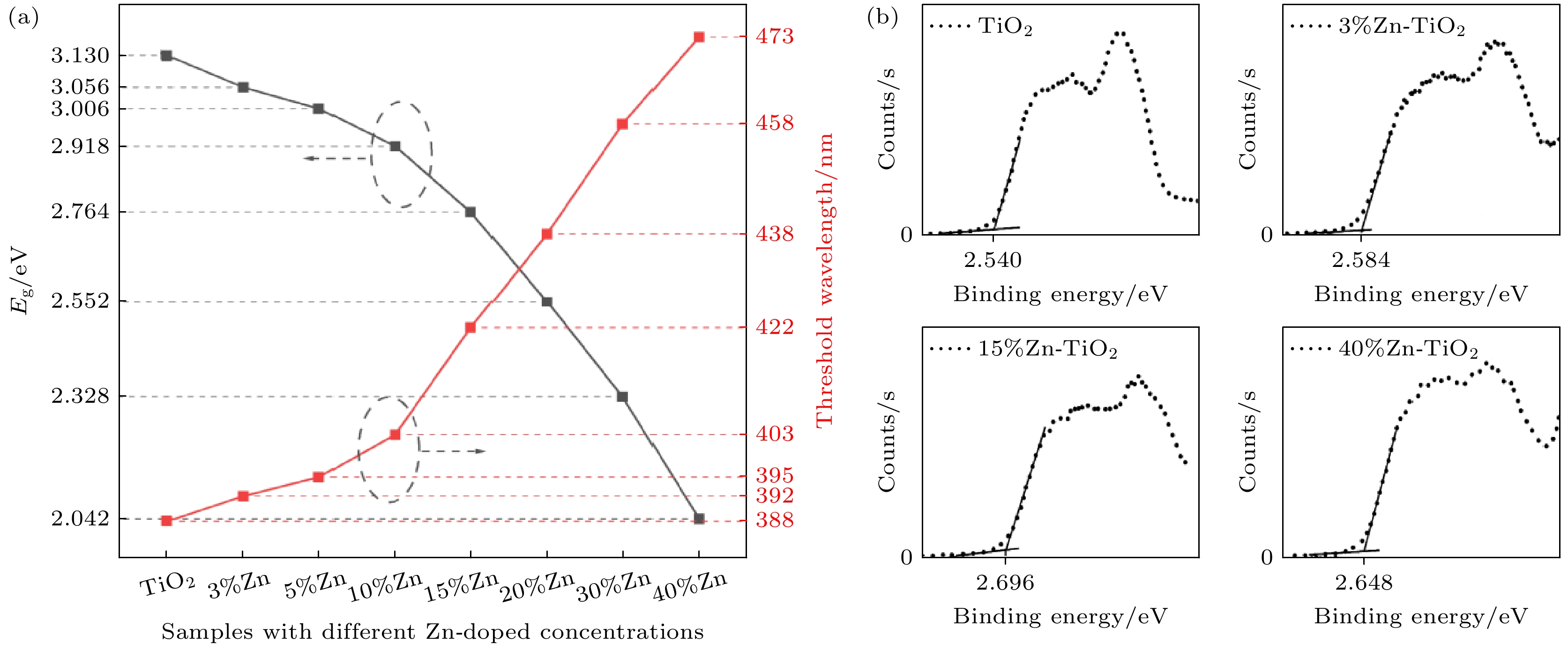
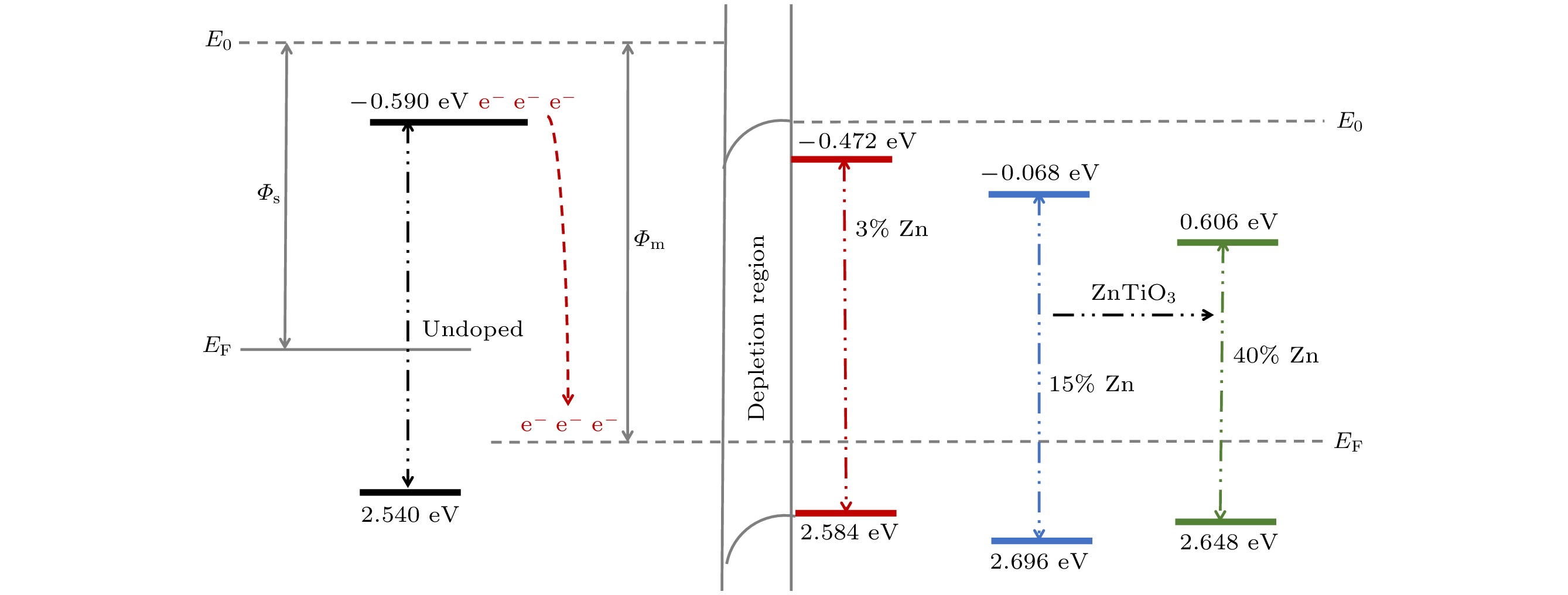
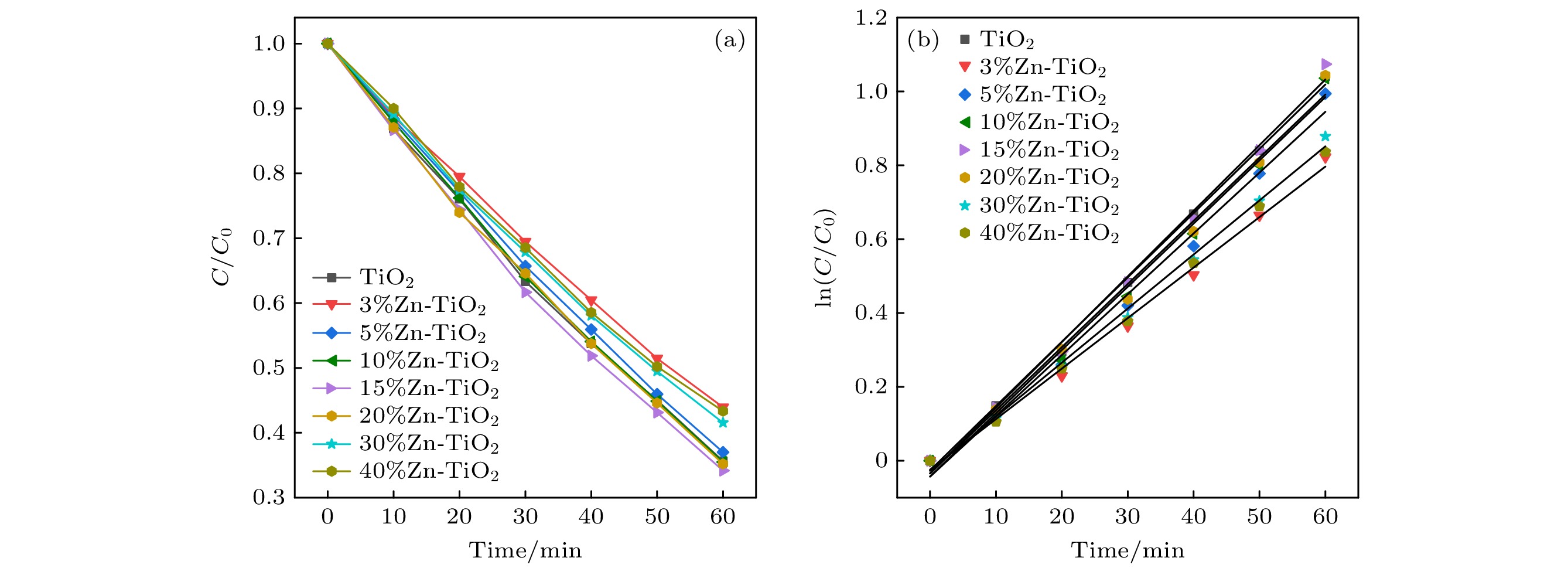
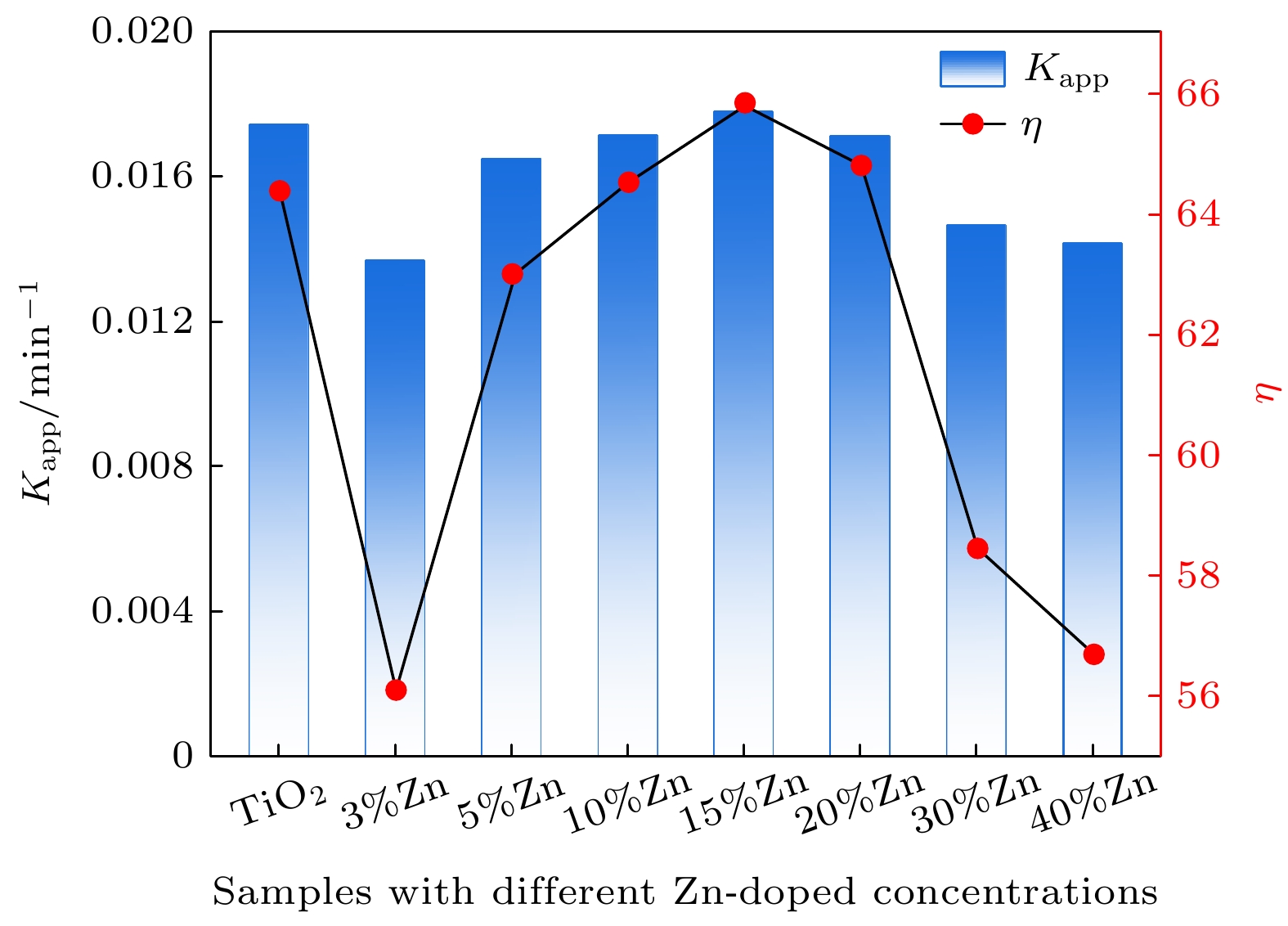
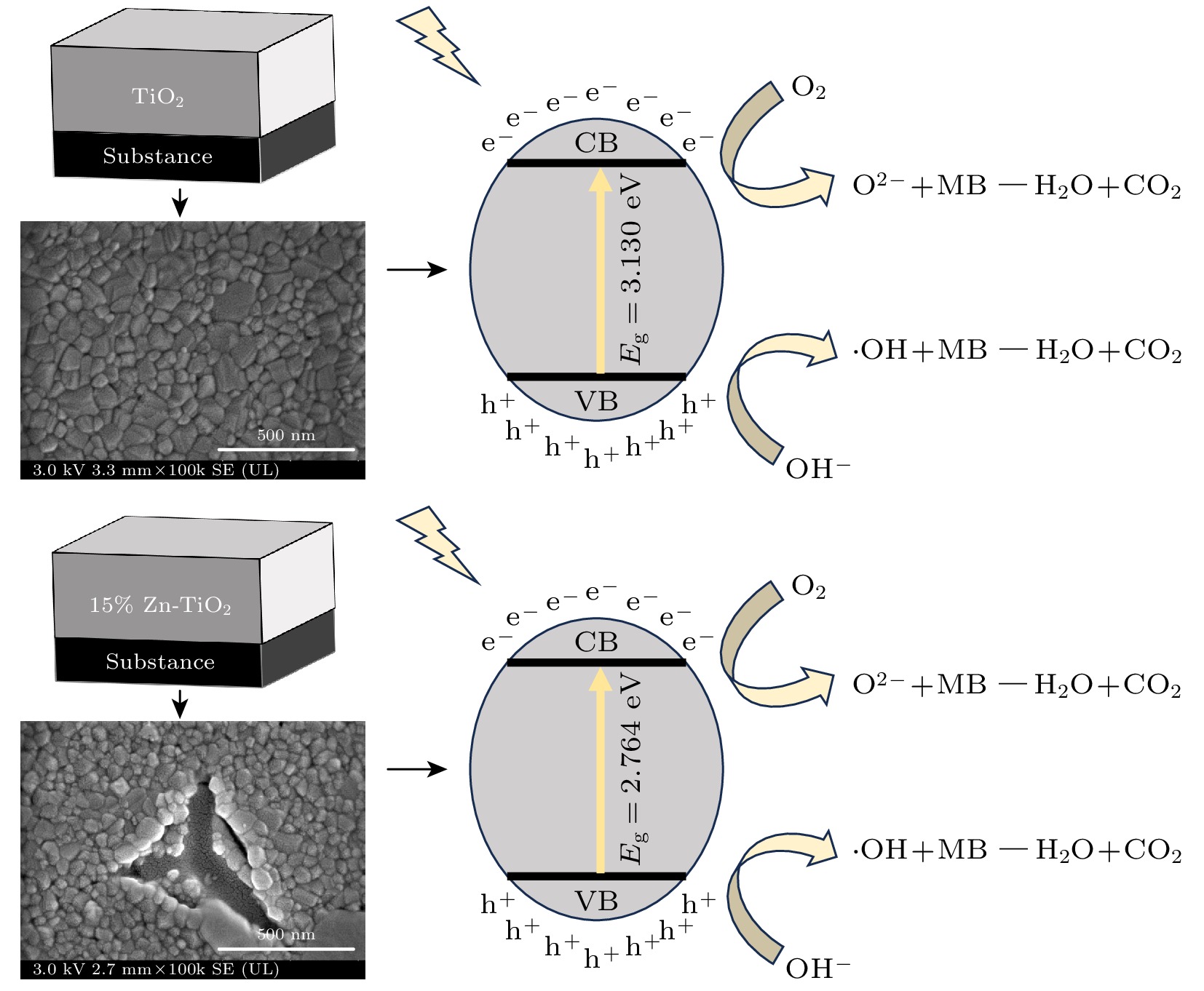
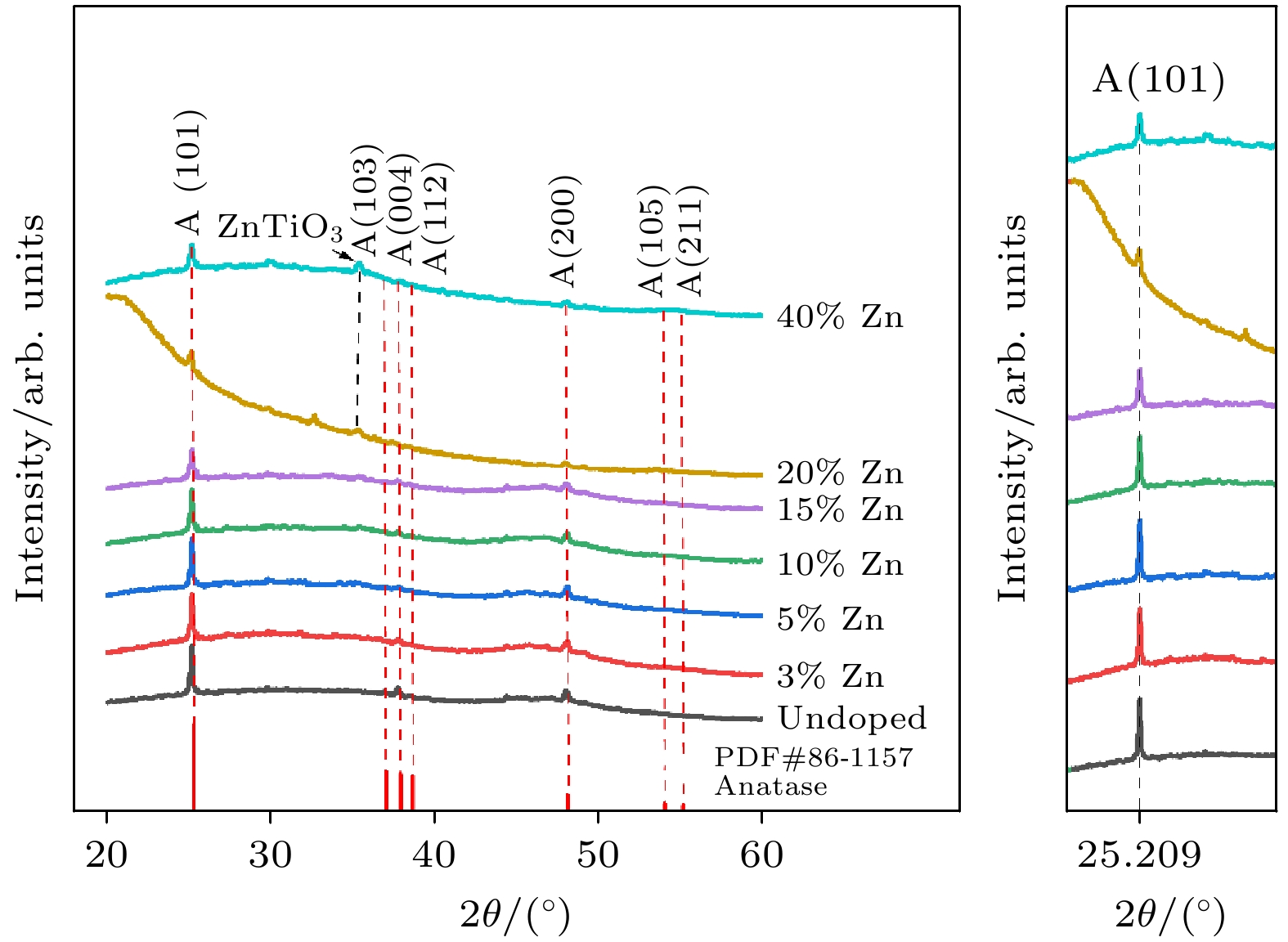
使用溶胶-凝胶法在单晶硅衬底上制备一批不同Zn2+成分调节的TiO2薄膜, 观测Zn2+和TiO2合金化过程中光学和光催化性能的变化. X射线衍射光谱仪用于观测在合金化过程中薄膜的晶体结构变化并追踪ZnTiO3化合物的形成. 扫描电子显微镜、原子力显微镜用于观测合金化过程中因TiO2晶格对Zn2+溶解度有限而导致薄膜表面出现大量孔洞的现象. X射线光电子能谱和光学带隙用于观测Zn2+与TiO2合金化过程中电子结构层面的变化. 最后, 通过降解亚甲基蓝(MB)溶液, 表明少量Zn2+掺杂完全溶解在TiO2中, 并破坏TiO2结晶质量. 在Zn2+的成分占比继续提高至15%的过程中, XPS峰形拟合结果验证了TiO2对Zn2+的溶解度有限, 导致薄膜出现大量孔洞结构, 薄膜的活性比表面积得以提升, 同时Zn2+可以有效地捕获光生e–/h+. 为了继续观察Zn2+浓度对TiO2的影响, 将Zn2+的浓度提升至40%, 观察Zn2+与TiO2合金化过程中的现象. 表明化合物ZnTiO3的出现可以充当e–/h+的复合中心以及TiO2占比的大幅下降导致合金化之后的薄膜光催化效率逐渐下降.A batch of TiO2 films with different Zn2+ compositions are prepared on a single crystal silicon substrate by using sol-gel method to observe the changes in optical and photocatalytic properties in the alloying process of Zn2+ and TiO2. X-ray diffractometer (XRD) is used to observe the changes in the crystal structures of the films in the alloying process and to track the formation of ZnTiO3 compounds. Scanning electron microscope (SEM) and atomic force microscope (AFM) are used to observe the phenomena of a large number of holes on the surfaces of the films due to the limited solubility of the crystal lattice for Zn2+ in the alloying process. X-ray photoelectron spectroscopy (XPS) and optical bandgap are used to observe the changes at a level of the electronic structure of the films in the alloying process of Zn2+ with TiO2. Finally, by degrading the methylene blue solution, it is shown that a small amount of Zn2+ doping is completely dissolved in TiO2, destroying the TiO2 crystalline quality. As the compositional share of Zn2+ continues to increase to 15%, the limited solubility of TiO2 for Zn2+ is verified in the XPS peak fitting, resulting in a large number of hole structures in the film, and the active specific surface area of the film is enhanced, while Zn2+ effectively traps the photogenerated e–/h+. In order to continue to observe the effect of Zn2+ concentration on TiO2, we increase the concentration of Zn2+ to 40% and observe the phenomenon in the alloying process of Zn2+ with TiO2. It is shown that the appearance of the compound ZnTiO3 can act as a complex center for e–/h+ and a significant decrease in the percentage of TiO2 leads to a gradual decrease in the photocatalytic efficiency of the films after alloying.
-
Keywords:
- TiO2 /
- Zn2+ /
- photocatalysis /
- bandgap /
- surface
[1] Lukong V T, Ukoba K, Jen T C 2022 Int. J. Adv. Manuf. Technol. 122 3525
 Google Scholar
Google Scholar
[2] 李冬冬, 王丽莉 2012 61 034212
 Google Scholar
Google Scholar
Li D D, Wang L L 2012 Acta Phys. Sin. 61 034212
 Google Scholar
Google Scholar
[3] Chinnarani M, Suresh S, Prabu K M, Kandasamy M, Pugazhenthiran N 2024 Inorg. Chim. Acta 560 121842
 Google Scholar
Google Scholar
[4] Sanattalab E, Gürdağ G, Sığırcı B D 2023 Biomater. Adv. 148 213365
 Google Scholar
Google Scholar
[5] Ziental D, Goslinska B C, Mlynarczyk D T, Sobotta A G, Stanisz B, Goslinski T L 2020 Nanomaterials 10 387
 Google Scholar
Google Scholar
[6] Zhu Z W, Chen S F, Zhang Y, Wang W 2022 Prog. Org. Coat. 173 107226
 Google Scholar
Google Scholar
[7] Alrowaili Z A, Alsohaimi I H, Betiha M A, Essawy A A, Mousa A A, Alruwaili S F, Hassan H M A 2020 Mater. Chem. Phys. 241 122403
 Google Scholar
Google Scholar
[8] Ike P O, Ezugwu S, Chikwenze R, Nwanya A C, Ezugwu A E, Madiba I G, Ezekoye B A, Ahmed M S, Maaza M, Ezema F I 2019 Optik 191 1
 Google Scholar
Google Scholar
[9] Aruna S T, Vismaya A, Balaji N 2021 Mater. Manuf. Processes 36 868
 Google Scholar
Google Scholar
[10] Mittireddi R T, Makani N H, Prajapati D, Raj A, Banerjee R, Panda E 2023 Mater. Charact. 199 112818
 Google Scholar
Google Scholar
[11] Sun N R, Wang J W, Yao J Z, Chen H M, Deng C H 2019 Microchim. Acta 186 159
 Google Scholar
Google Scholar
[12] Zhang Q, Li C Y 2020 Nanomaterials 10 911
 Google Scholar
Google Scholar
[13] Kusior A, Banas J, Zajac A T, Zubrzycka P, Ilnicka A M, Radecka M 2018 J. Mol. Struct. 1157 327
 Google Scholar
Google Scholar
[14] Al-Tayeb M A-A, Khan M Y, Drmosh Q A, Hossain M K, Asim M, Dafalla H, Khan A 2023 Inorg. Chim. Acta 556 121611
 Google Scholar
Google Scholar
[15] Ma Q, Qiu T, Liu S J, Weng L Q, Dong W Y 2010 Appl. Phys. A 104 365
 Google Scholar
Google Scholar
[16] Liu Y, Peng Q, Zhou Z P, Yang G 2018 Chin. Phys. Lett. 35 048101
 Google Scholar
Google Scholar
[17] Resende P D, Maria R, Silva J D, Azevedo I, Santos L A, Tadeu V 2020 J. Mater. Res. Technol. 9 10121
 Google Scholar
Google Scholar
[18] Chen C C, Hu S H, Fu Y P 2015 J. Alloys Compd. 632 326
 Google Scholar
Google Scholar
[19] Ahadi S, Moalej N S, Sheibani S 2019 Solid State Sci. 96 105975
 Google Scholar
Google Scholar
[20] Yoon Y H, Lee S Y, Gwon J, Cho H J, Wu Q, Kim Y H, Lee W H 2018 Ceram. Int. 44 16647
 Google Scholar
Google Scholar
[21] Qu X, Lin J B, Wei Q, Chen C T, Sun D P 2022 Chemosphere 308 136239
 Google Scholar
Google Scholar
[22] Jaimy K B, Safeena V P, Ghosh S, Hebalkar N, Warrier K 2012 Dalton Trans. 41 4824
 Google Scholar
Google Scholar
[23] Chen K T, Hsu C H, Jiang S C, Liang L S, Gao P, Qiu Y, Wu W Y, Zhang S, Zhu W Z, Lien S Y 2022 IEEE Trans. Electron Devices 69 1149
 Google Scholar
Google Scholar
[24] Nair P K, Mizukami F, Nair J, Salou M, Oosawa Y, Izutsu H, Maeda K, Okubo T 1998 Mater. Res. Bull. 33 1495
 Google Scholar
Google Scholar
[25] Chen P C, Chen C C, Chen S H 2017 Curr. Nanosci. 13 373
 Google Scholar
Google Scholar
[26] Ren T Z, Yuan Z Y, Su B L 2004 Colloids Surf., A 241 67
 Google Scholar
Google Scholar
[27] Lang J, Takahashi K, Kubo M, Shimada M 2022 Catalysts 12 365
 Google Scholar
Google Scholar
[28] Mercado C C, Seeley Z M, Bandyopadhyay A, Bose S, McHale J L 2011 ACS Appl. Mater. Interfaces 3 2281
 Google Scholar
Google Scholar
[29] Henderson M 2002 Surf. Sci. Rep. 46 1
 Google Scholar
Google Scholar
[30] Dikici T, Yılmaz O, Akalin A S, Demirci S, Gültekin S, Yıldırım S, Yurddaşkal M 2022 J. Aust. Ceram. Soc. 58 1415
 Google Scholar
Google Scholar
[31] Xu W, Li X J, Zhang S J, Wang J G, Xu Z K 2005 Trans. Nonferrous Met. Soc. China 15 119
 Google Scholar
Google Scholar
[32] Wang C T, Lin H S, Wang W P 2019 Mater. Sci. Semicond. Process. 99 85
 Google Scholar
Google Scholar
[33] Liao C Z, Li Y C, Tjong S C 2020 Nanomaterials 10 124
 Google Scholar
Google Scholar
[34] Ismael M 2020 Sol. Energy 211 522
 Google Scholar
Google Scholar
[35] Matsumoto Y, Katayama M, Abe T, Ohsawa T, Ohkubo I, Kumigashira H, Oshima M, Koinuma H 2010 J. Ceram. Soc. Jpn. 118 993
 Google Scholar
Google Scholar
[36] Ribao P, Rivero M J, Ortiz I 2017 Environ. Sci. Pollut. Res. 24 12628
 Google Scholar
Google Scholar
[37] Tang T, Wang T, Gao Y, Xiao H, Xu J H 2019 J. Mater. Sci. -Mater. Electron. 30 8471
 Google Scholar
Google Scholar
[38] Xavier A M, Jacob I D, Surender S, Saravana kumaar M S S, Elangovan P 2022 Inorg. Chem. Commun. 146 110168
 Google Scholar
Google Scholar
[39] Pecherskaya M D, Butanov K T, Ruzimuradov O N, Mamatkulov S I, Parpiev O 2022 Glass Phys. Chem. 48 327
 Google Scholar
Google Scholar
[40] Heiba Z K, Mohamed M B, Badawi A, Abdellatief M 2022 J. Mater. Sci. -Mater. Electron. 33 10399
 Google Scholar
Google Scholar
[41] Castellón E, Zayat M, Lévy D 2017 Adv. Funct. Mater. 28 04717
 Google Scholar
Google Scholar
[42] Chu J Y, Sun Y C, Han X J, Zhang B, Du Y C, Song B, Xu P 2019 ACS Appl. Mater. Interfaces 11 18475
 Google Scholar
Google Scholar
[43] Maleki K, Abdizadeh H, Golobostanfard M R, Adelfar R 2017 Appl. Surf. Sci. 394 37
 Google Scholar
Google Scholar
-
表 1 从 XRD 图谱获得的结构参数
Table 1. Structural parameters obtained from XRD spectra.
Samples (h k l ) FWHM β/(°) D/nm TiO2 (1 0 1) 0.177 8.384 3% Zn-TiO2 (1 0 1) 0.161 9.217 5% Zn-TiO2 (1 0 1) 0.197 7.533 10% Zn-TiO2 (1 0 1) 0.180 8.244 15% Zn-TiO2 (1 0 1) 0.259 5.730 20% Zn-TiO2 (1 0 1) 0.152 9.762 40% Zn-TiO2 (1 0 1) 0.227 6.537 表 2 从AFM获得的粗糙度参数(Ra为算数平均粗糙度, RMS为均方根粗糙度, RSk为粗糙度斜率, RKu为粗糙度峰度)
Table 2. Roughness parameters obtained from AFM (Ra, RMS, RSk, RKu are the average, root- mean-square, Skewness, and Kurtosis values of surface roughness, respectively).
Samples Roughness
Ra/nmRoughness
RMS/nmSkewness
RSkKurtosis
RKuTiO2 3.515 4.359 –0.492 0.276 3%Zn-TiO2 2.230 2.851 –0.077 0.089 15%Zn-TiO2 5.590 6.990 –0.313 0.819 40%Zn-TiO2 6.115 8.703 –1.079 3.318 -
[1] Lukong V T, Ukoba K, Jen T C 2022 Int. J. Adv. Manuf. Technol. 122 3525
 Google Scholar
Google Scholar
[2] 李冬冬, 王丽莉 2012 61 034212
 Google Scholar
Google Scholar
Li D D, Wang L L 2012 Acta Phys. Sin. 61 034212
 Google Scholar
Google Scholar
[3] Chinnarani M, Suresh S, Prabu K M, Kandasamy M, Pugazhenthiran N 2024 Inorg. Chim. Acta 560 121842
 Google Scholar
Google Scholar
[4] Sanattalab E, Gürdağ G, Sığırcı B D 2023 Biomater. Adv. 148 213365
 Google Scholar
Google Scholar
[5] Ziental D, Goslinska B C, Mlynarczyk D T, Sobotta A G, Stanisz B, Goslinski T L 2020 Nanomaterials 10 387
 Google Scholar
Google Scholar
[6] Zhu Z W, Chen S F, Zhang Y, Wang W 2022 Prog. Org. Coat. 173 107226
 Google Scholar
Google Scholar
[7] Alrowaili Z A, Alsohaimi I H, Betiha M A, Essawy A A, Mousa A A, Alruwaili S F, Hassan H M A 2020 Mater. Chem. Phys. 241 122403
 Google Scholar
Google Scholar
[8] Ike P O, Ezugwu S, Chikwenze R, Nwanya A C, Ezugwu A E, Madiba I G, Ezekoye B A, Ahmed M S, Maaza M, Ezema F I 2019 Optik 191 1
 Google Scholar
Google Scholar
[9] Aruna S T, Vismaya A, Balaji N 2021 Mater. Manuf. Processes 36 868
 Google Scholar
Google Scholar
[10] Mittireddi R T, Makani N H, Prajapati D, Raj A, Banerjee R, Panda E 2023 Mater. Charact. 199 112818
 Google Scholar
Google Scholar
[11] Sun N R, Wang J W, Yao J Z, Chen H M, Deng C H 2019 Microchim. Acta 186 159
 Google Scholar
Google Scholar
[12] Zhang Q, Li C Y 2020 Nanomaterials 10 911
 Google Scholar
Google Scholar
[13] Kusior A, Banas J, Zajac A T, Zubrzycka P, Ilnicka A M, Radecka M 2018 J. Mol. Struct. 1157 327
 Google Scholar
Google Scholar
[14] Al-Tayeb M A-A, Khan M Y, Drmosh Q A, Hossain M K, Asim M, Dafalla H, Khan A 2023 Inorg. Chim. Acta 556 121611
 Google Scholar
Google Scholar
[15] Ma Q, Qiu T, Liu S J, Weng L Q, Dong W Y 2010 Appl. Phys. A 104 365
 Google Scholar
Google Scholar
[16] Liu Y, Peng Q, Zhou Z P, Yang G 2018 Chin. Phys. Lett. 35 048101
 Google Scholar
Google Scholar
[17] Resende P D, Maria R, Silva J D, Azevedo I, Santos L A, Tadeu V 2020 J. Mater. Res. Technol. 9 10121
 Google Scholar
Google Scholar
[18] Chen C C, Hu S H, Fu Y P 2015 J. Alloys Compd. 632 326
 Google Scholar
Google Scholar
[19] Ahadi S, Moalej N S, Sheibani S 2019 Solid State Sci. 96 105975
 Google Scholar
Google Scholar
[20] Yoon Y H, Lee S Y, Gwon J, Cho H J, Wu Q, Kim Y H, Lee W H 2018 Ceram. Int. 44 16647
 Google Scholar
Google Scholar
[21] Qu X, Lin J B, Wei Q, Chen C T, Sun D P 2022 Chemosphere 308 136239
 Google Scholar
Google Scholar
[22] Jaimy K B, Safeena V P, Ghosh S, Hebalkar N, Warrier K 2012 Dalton Trans. 41 4824
 Google Scholar
Google Scholar
[23] Chen K T, Hsu C H, Jiang S C, Liang L S, Gao P, Qiu Y, Wu W Y, Zhang S, Zhu W Z, Lien S Y 2022 IEEE Trans. Electron Devices 69 1149
 Google Scholar
Google Scholar
[24] Nair P K, Mizukami F, Nair J, Salou M, Oosawa Y, Izutsu H, Maeda K, Okubo T 1998 Mater. Res. Bull. 33 1495
 Google Scholar
Google Scholar
[25] Chen P C, Chen C C, Chen S H 2017 Curr. Nanosci. 13 373
 Google Scholar
Google Scholar
[26] Ren T Z, Yuan Z Y, Su B L 2004 Colloids Surf., A 241 67
 Google Scholar
Google Scholar
[27] Lang J, Takahashi K, Kubo M, Shimada M 2022 Catalysts 12 365
 Google Scholar
Google Scholar
[28] Mercado C C, Seeley Z M, Bandyopadhyay A, Bose S, McHale J L 2011 ACS Appl. Mater. Interfaces 3 2281
 Google Scholar
Google Scholar
[29] Henderson M 2002 Surf. Sci. Rep. 46 1
 Google Scholar
Google Scholar
[30] Dikici T, Yılmaz O, Akalin A S, Demirci S, Gültekin S, Yıldırım S, Yurddaşkal M 2022 J. Aust. Ceram. Soc. 58 1415
 Google Scholar
Google Scholar
[31] Xu W, Li X J, Zhang S J, Wang J G, Xu Z K 2005 Trans. Nonferrous Met. Soc. China 15 119
 Google Scholar
Google Scholar
[32] Wang C T, Lin H S, Wang W P 2019 Mater. Sci. Semicond. Process. 99 85
 Google Scholar
Google Scholar
[33] Liao C Z, Li Y C, Tjong S C 2020 Nanomaterials 10 124
 Google Scholar
Google Scholar
[34] Ismael M 2020 Sol. Energy 211 522
 Google Scholar
Google Scholar
[35] Matsumoto Y, Katayama M, Abe T, Ohsawa T, Ohkubo I, Kumigashira H, Oshima M, Koinuma H 2010 J. Ceram. Soc. Jpn. 118 993
 Google Scholar
Google Scholar
[36] Ribao P, Rivero M J, Ortiz I 2017 Environ. Sci. Pollut. Res. 24 12628
 Google Scholar
Google Scholar
[37] Tang T, Wang T, Gao Y, Xiao H, Xu J H 2019 J. Mater. Sci. -Mater. Electron. 30 8471
 Google Scholar
Google Scholar
[38] Xavier A M, Jacob I D, Surender S, Saravana kumaar M S S, Elangovan P 2022 Inorg. Chem. Commun. 146 110168
 Google Scholar
Google Scholar
[39] Pecherskaya M D, Butanov K T, Ruzimuradov O N, Mamatkulov S I, Parpiev O 2022 Glass Phys. Chem. 48 327
 Google Scholar
Google Scholar
[40] Heiba Z K, Mohamed M B, Badawi A, Abdellatief M 2022 J. Mater. Sci. -Mater. Electron. 33 10399
 Google Scholar
Google Scholar
[41] Castellón E, Zayat M, Lévy D 2017 Adv. Funct. Mater. 28 04717
 Google Scholar
Google Scholar
[42] Chu J Y, Sun Y C, Han X J, Zhang B, Du Y C, Song B, Xu P 2019 ACS Appl. Mater. Interfaces 11 18475
 Google Scholar
Google Scholar
[43] Maleki K, Abdizadeh H, Golobostanfard M R, Adelfar R 2017 Appl. Surf. Sci. 394 37
 Google Scholar
Google Scholar
计量
- 文章访问数: 3473
- PDF下载量: 47
- 被引次数: 0













 下载:
下载: