-
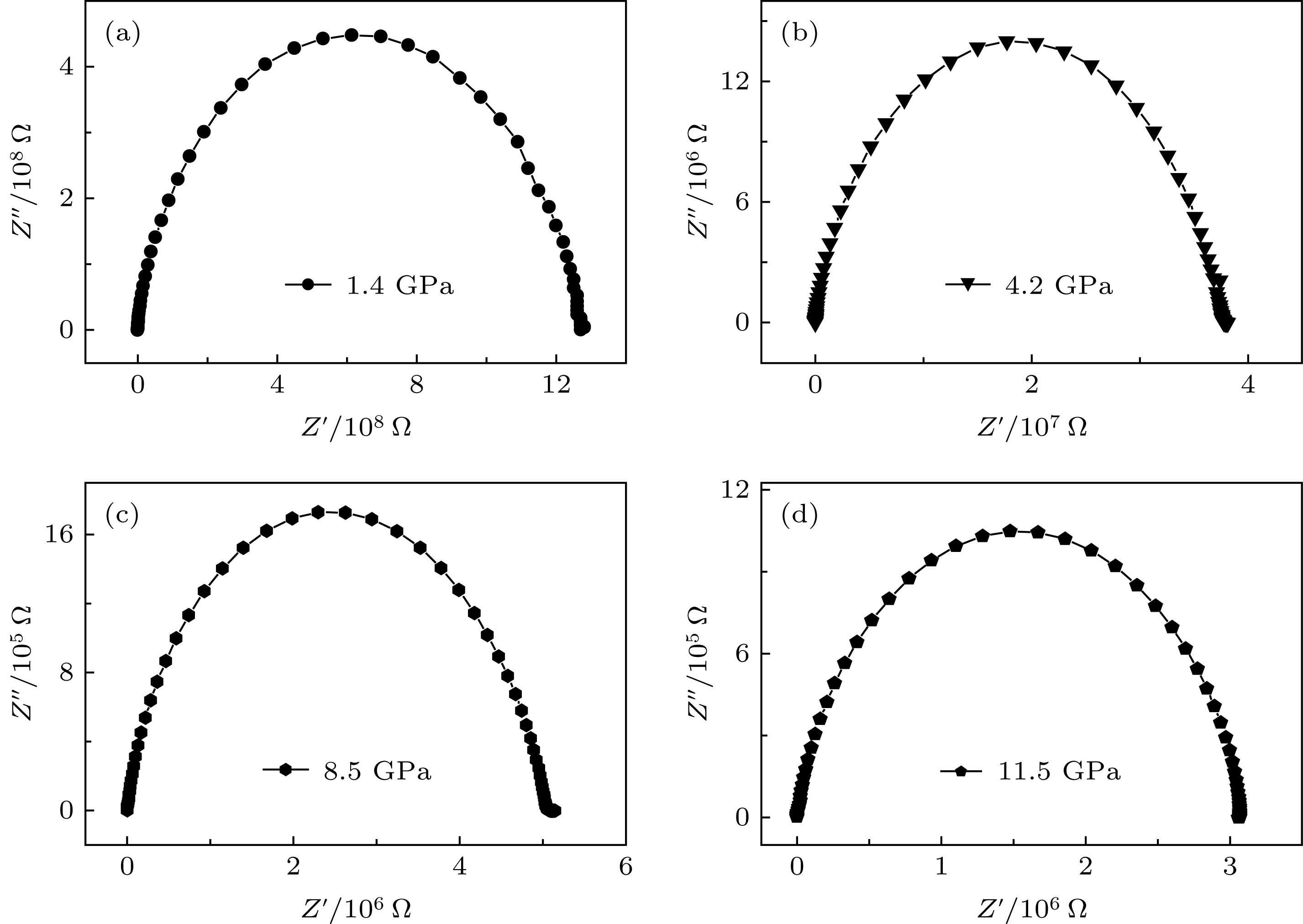
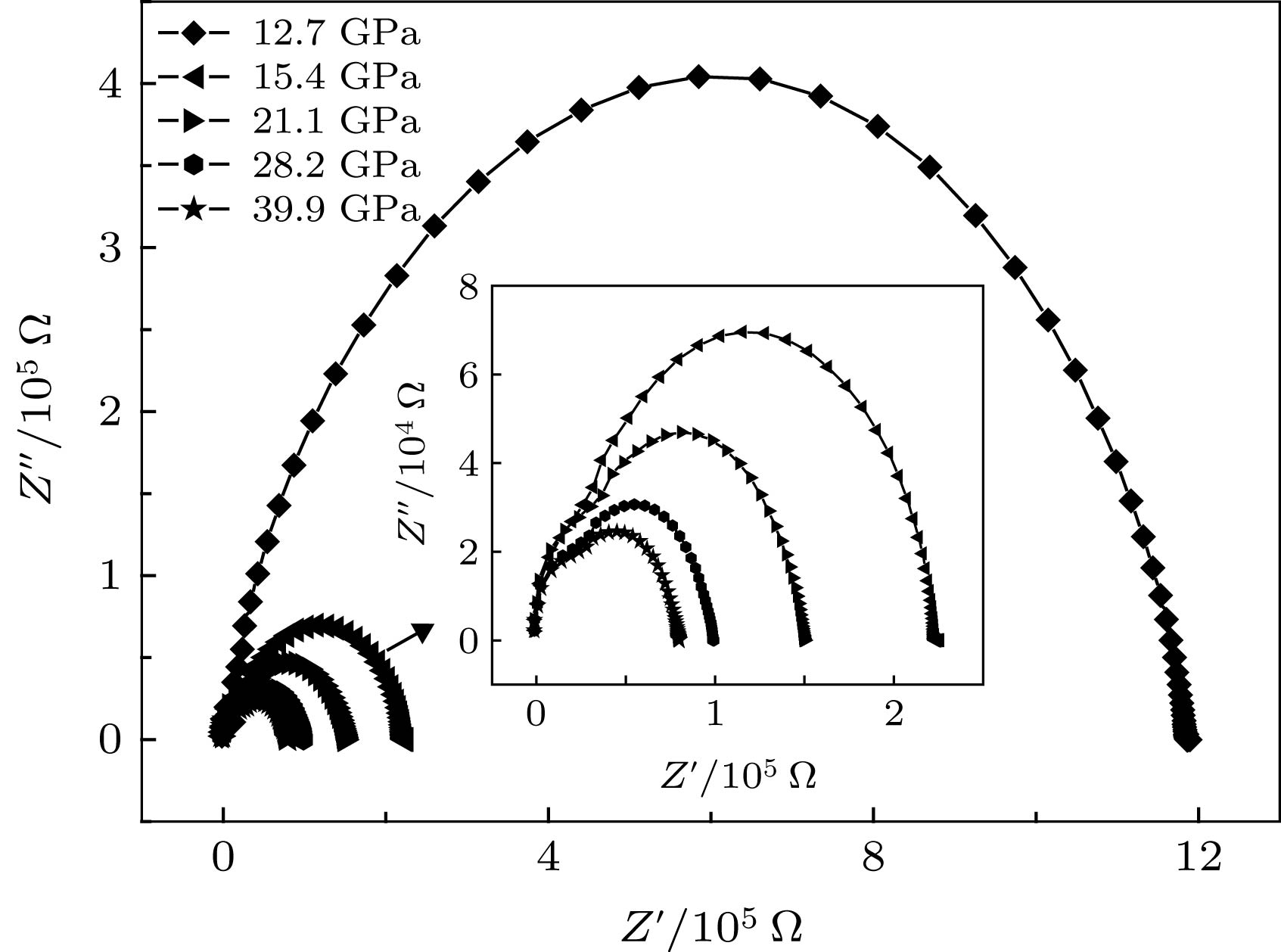
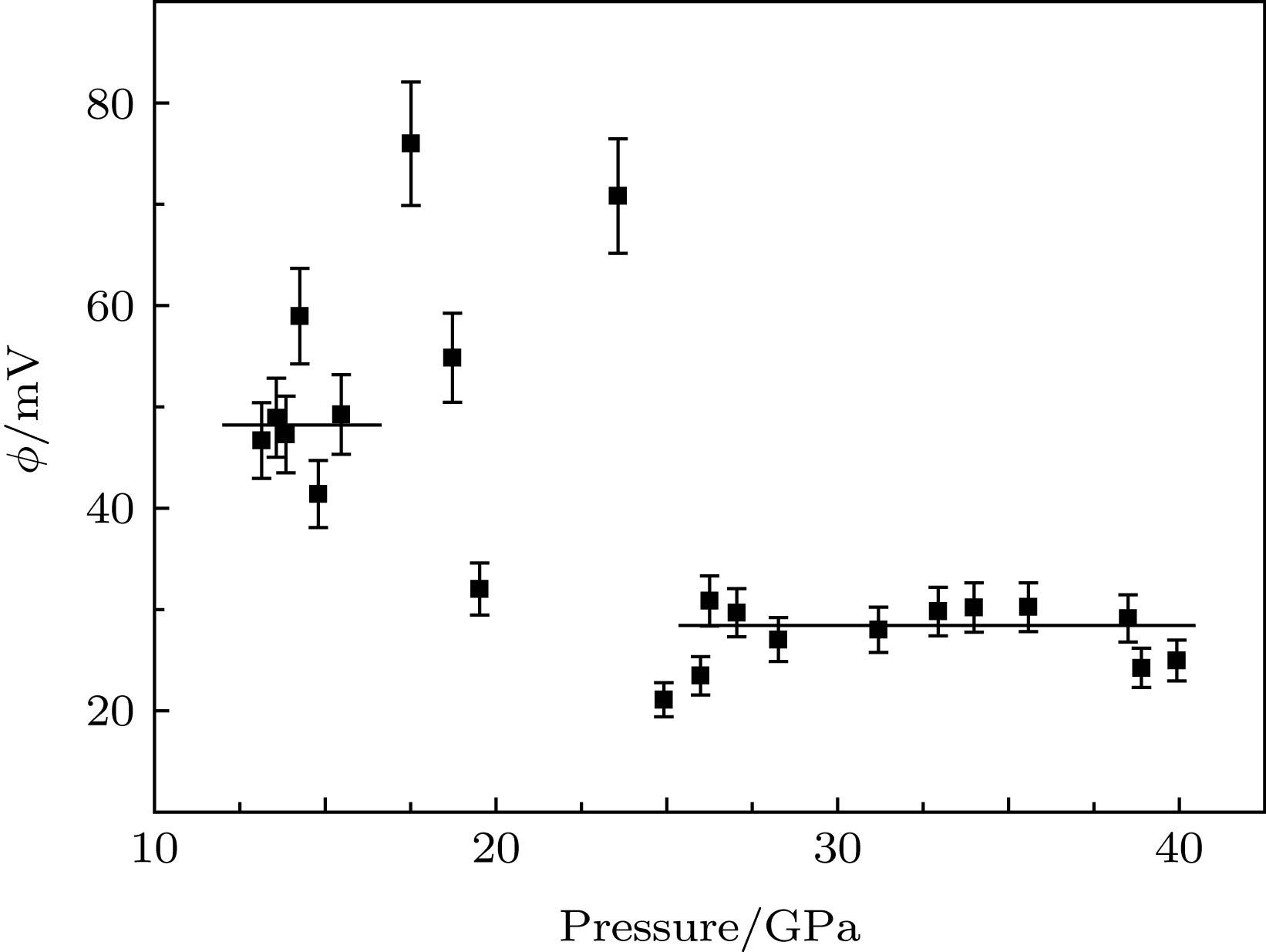
应用电化学阻抗谱法研究了高压下金红石相TiO2的晶粒和晶界电学性质. 随着压力的升高, TiO2的电阻降低, 在相变区域内(11.5 GPa附近), 表现出了无规则的变化. 通过对阻抗谱的测量发现, 在较低的压力下(常压到11.5 GPa范围内), TiO2的晶界特性不明显. 但是随着压力(大于11.5 GPa)的升高, 相变后TiO2的晶界特性变得显著. 这说明压力作用下晶粒和晶界的行为与TiO2相结构的转变有着密切的联系. 通过计算得到, 当压力高于25.2 GPa时, TiO2的晶粒边界空间电荷势稳定存在, 其值约为30 mV. 分析表明高压下TiO2晶界空间电荷势来源于静电相互作用和弹性相互作用两部分共同作用的影响.In this paper, the grain and grain boundary characteristics of pure rutile TiO2 under pressure are investigated by electrochemical impedance spectroscopy equipped with diamond anvil cell (DAC). Only one semi-circle can be detected under each pressure in a range of 1.4–11.5 GPa. With the pressure increasing, the shape of semi-circle is unchanged, while the size of semi-circle gradually decreases, which can be attributed to the decrease of bulk resistance due to the reduction of band gap under pressure. The absence of grain boundary characteristic in the impedance spectra signifying that Schottky barrier is not present at the grain boundaries. With further increasing pressure, an interesting phenomenon can be observed above 12.7 GPa. The shape of semi-circle is distorted, and exhibits two overlapping semi-circles. The first semi-circle (high frequency) originates from the contribution of bulk, and the second one (low frequency) can be ascribed to the effect of grain boundary. The occurrence of grain boundary semicircle indicates that the aggregation of space charges at the grain boundary. In this case, the phase transformation from rutile to baddeleyite structure occurs, the electric transport mechanism is changed, and new lattice defects are formed. Also, two discontinuous points (11.5 and 15.4 GPa) can be detected in the resistance curve. The remarkable change of resistance occurs at 12.7 GPa which is corresponding to the phase transition from rutile to baddeleyite phase. The occurrence of phase transition leads the new interfacial energy to occur, the total energy of system to increase, and the movement of carriers to impede. Thus, the resistance increases significantly, and the maximum value occurs at 15 GPa. Further analysis indicates that the space charge potential is modified with pressure increasing, implying that the electrical transport properties of TiO2 are related closely to phase transition. With the pressure increasing from 12.7 to 25.2 GPa, the irregular change of space charge potential can be attributed to the rutile and baddeleyite phase coexisting. When the pressure is higher than 25.2 GPa, the space charge potential is a constant (about 30 mV). According to the investigations, the TiO2 grain boundary space charge potential under pressure is mainly contributed from two parts: the electrostatic interaction and the elastic interaction.
-
Keywords:
- high pressure /
- TiO2 /
- impedance spectroscopy /
- grain boundary
[1] Langlet M, Burgos M, Coutier C, Jimenez C, Morant C, Manso M 2001 J. Sol-Gel Sci. Technol. 22 139
 Google Scholar
Google Scholar
[2] Dubrovinsky L S, Dubrovinskaia N A, Swamy V, Muscat J, Harrison N M, Ahuja R, Holm B, Johansson B 2001 Nature 410 653
 Google Scholar
Google Scholar
[3] Goresy A E, Chen M, Dubrovinsky L, Gillet P, Graup G 2001 Science 293 1467
 Google Scholar
Google Scholar
[4] Hiroshi K, Monami Y, Meiko K, Yoshiyuki I, Daisuke M, Masak A 2018 Phys. Chem. Miner. 45 963
 Google Scholar
Google Scholar
[5] Arlt T, Bermejo M, Blanco M A, Gerward L, Jiang J Z, Staun O, Recio J M 2000 Phys. Rev. B 61 14414
 Google Scholar
Google Scholar
[6] Dong Z H, Xiao F P, Zhao A K, Liu L J, Tsun-Kong S A, Song Y 2016 RSC Adv. 6 76142
 Google Scholar
Google Scholar
[7] Swamy V, Kuznetsov A, Dubrovinsky L S, Caruso R A, Shchukin D G, Muddle B C 2005 Phys. Rev. B 71 184302
 Google Scholar
Google Scholar
[8] Navrotsky A 2003 Geochem. Trans. 4 34
 Google Scholar
Google Scholar
[9] Mei Z G, Wang Y, Shang S L, Liu Z K 2011 Inorg. Chem. 50 6996
 Google Scholar
Google Scholar
[10] Varghese S, Muddle B C 2007 Phys. Rev. Lett. 98 035502
 Google Scholar
Google Scholar
[11] Zhang G, Wu B, Wang J, Zhang H, Liu H, Zhang J, Liu C, Gu G, Tian L, Ma Y, Gao C 2017 Sci. Rep. 7 2656
 Google Scholar
Google Scholar
[12] Li Y, Gao Y, Han Y, Liu C L, Peng G, Wang Q L, Ke F, Ma Y Z, Gao C X 2015 Appl. Phys. Lett. 107 142103
 Google Scholar
Google Scholar
[13] Qu T J, Liu C L, Yan H C, Han Y H, Wang Q L, Liu X Z, Ma Y Z, Gao C X 2019 Appl. Phys. Lett. 114 062105
 Google Scholar
Google Scholar
[14] 王月, 张凤霞, 王春杰, 高春晓 2014 63 216401
 Google Scholar
Google Scholar
Wang Y, Zhang F X, Wang C J, Gao C X 2014 Acta Phys. Sin. 63 216401
 Google Scholar
Google Scholar
[15] Ohsaka T, Yamaoka S, Shimomura O 1979 Solid State Commun. 30 345
[16] Lagarec K, Desgreniers S 1995 Solid State Commun. 94 519
 Google Scholar
Google Scholar
[17] Olse J S, Gerward L, Jiang J 1999 J. Phys. Chem. Solids 60 229
 Google Scholar
Google Scholar
[18] Wang Q, Liu C, Han Y, Gao C, Ma Y 2016 Rev. Sci. Instrum. 87 123904
 Google Scholar
Google Scholar
[19] Wang Q L, Varghese O, Grimes C A, Dickey E C 2007 Solid State Ionics 178 187
 Google Scholar
Google Scholar
[20] Wang Q, Lian G D, Dickey E C 2004 Acta Mater. 52 809
 Google Scholar
Google Scholar
[21] Al-Khatatbeh Y, Lee K M, Kiefer B 2009 Phys. Rev. B 79 134114
 Google Scholar
Google Scholar
[22] Sato H, Endo S, Sugiyama M, Kikegawa T, Shimomura O, Kusaba K 1991 Science 251 78
 Google Scholar
Google Scholar
[23] 曹楚南, 张鉴清 2002 电化学阻抗谱导论 (典藏版1) (北京: 科学出版社)第21页
Cao C N, Zhang J Q 2002 Introduction to Electrochemical Impedance Spectroscopy (Vol. 1) (Beijing: Science Press) p21 (in Chinese)
[24] Kliewer K, Koehler J 1965 Phys. Rev. A 140 1226
[25] Ikeda J A S, Chiang Y M 1993 J. Am. Ceram. Soc. 76 2437
 Google Scholar
Google Scholar
[26] Fleig J, Rodewald S, Maier J 2000 J. Appl. Phys. 87 2372
 Google Scholar
Google Scholar
[27] Eshelby J D 1956 Solid. State. Phys. 3 79
 Google Scholar
Google Scholar
[28] Yan M F, Cannon R M, Bowen H K 1983 J. Appl. Phys. 54 764
 Google Scholar
Google Scholar
[29] Yan M F, Rhodes W W 1987 Materials Science Research (Vol. 21) (US: Springer) p519
-
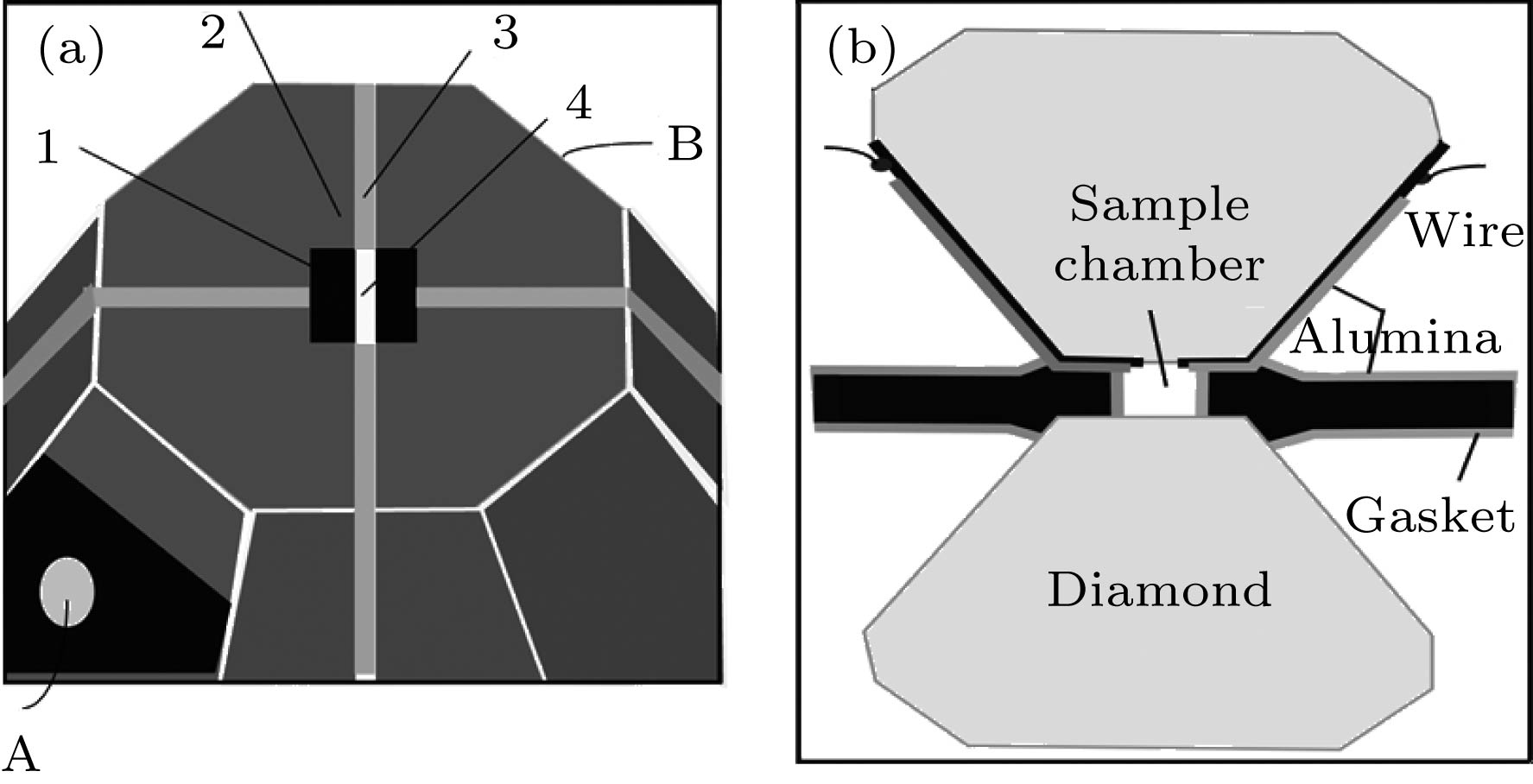
图 1 (a) 金刚石砧面上微电路结构示意图, 1-钼电极, 2-在钼膜上沉积的Al2O3绝缘层, 3-沉积到金刚石砧面上的Al2O3, 4-裸露的金刚石砧面, A和B为微电路的接触端; (b) 金刚石对顶砧的剖面示意图
Fig. 1. (a) The configuration of a complete microcircuit on a diamond anvil: 1-the Mo electrodes, 2-the Al2O3 layer deposited on the Mo film, 3-the Al2O3 layer deposited on the diamond anvil, 4-exposed diamond anvil, A and B are the contact ends of the microcircuit; (b) the cross section of the designed diamond-anvil-cell.
-
[1] Langlet M, Burgos M, Coutier C, Jimenez C, Morant C, Manso M 2001 J. Sol-Gel Sci. Technol. 22 139
 Google Scholar
Google Scholar
[2] Dubrovinsky L S, Dubrovinskaia N A, Swamy V, Muscat J, Harrison N M, Ahuja R, Holm B, Johansson B 2001 Nature 410 653
 Google Scholar
Google Scholar
[3] Goresy A E, Chen M, Dubrovinsky L, Gillet P, Graup G 2001 Science 293 1467
 Google Scholar
Google Scholar
[4] Hiroshi K, Monami Y, Meiko K, Yoshiyuki I, Daisuke M, Masak A 2018 Phys. Chem. Miner. 45 963
 Google Scholar
Google Scholar
[5] Arlt T, Bermejo M, Blanco M A, Gerward L, Jiang J Z, Staun O, Recio J M 2000 Phys. Rev. B 61 14414
 Google Scholar
Google Scholar
[6] Dong Z H, Xiao F P, Zhao A K, Liu L J, Tsun-Kong S A, Song Y 2016 RSC Adv. 6 76142
 Google Scholar
Google Scholar
[7] Swamy V, Kuznetsov A, Dubrovinsky L S, Caruso R A, Shchukin D G, Muddle B C 2005 Phys. Rev. B 71 184302
 Google Scholar
Google Scholar
[8] Navrotsky A 2003 Geochem. Trans. 4 34
 Google Scholar
Google Scholar
[9] Mei Z G, Wang Y, Shang S L, Liu Z K 2011 Inorg. Chem. 50 6996
 Google Scholar
Google Scholar
[10] Varghese S, Muddle B C 2007 Phys. Rev. Lett. 98 035502
 Google Scholar
Google Scholar
[11] Zhang G, Wu B, Wang J, Zhang H, Liu H, Zhang J, Liu C, Gu G, Tian L, Ma Y, Gao C 2017 Sci. Rep. 7 2656
 Google Scholar
Google Scholar
[12] Li Y, Gao Y, Han Y, Liu C L, Peng G, Wang Q L, Ke F, Ma Y Z, Gao C X 2015 Appl. Phys. Lett. 107 142103
 Google Scholar
Google Scholar
[13] Qu T J, Liu C L, Yan H C, Han Y H, Wang Q L, Liu X Z, Ma Y Z, Gao C X 2019 Appl. Phys. Lett. 114 062105
 Google Scholar
Google Scholar
[14] 王月, 张凤霞, 王春杰, 高春晓 2014 63 216401
 Google Scholar
Google Scholar
Wang Y, Zhang F X, Wang C J, Gao C X 2014 Acta Phys. Sin. 63 216401
 Google Scholar
Google Scholar
[15] Ohsaka T, Yamaoka S, Shimomura O 1979 Solid State Commun. 30 345
[16] Lagarec K, Desgreniers S 1995 Solid State Commun. 94 519
 Google Scholar
Google Scholar
[17] Olse J S, Gerward L, Jiang J 1999 J. Phys. Chem. Solids 60 229
 Google Scholar
Google Scholar
[18] Wang Q, Liu C, Han Y, Gao C, Ma Y 2016 Rev. Sci. Instrum. 87 123904
 Google Scholar
Google Scholar
[19] Wang Q L, Varghese O, Grimes C A, Dickey E C 2007 Solid State Ionics 178 187
 Google Scholar
Google Scholar
[20] Wang Q, Lian G D, Dickey E C 2004 Acta Mater. 52 809
 Google Scholar
Google Scholar
[21] Al-Khatatbeh Y, Lee K M, Kiefer B 2009 Phys. Rev. B 79 134114
 Google Scholar
Google Scholar
[22] Sato H, Endo S, Sugiyama M, Kikegawa T, Shimomura O, Kusaba K 1991 Science 251 78
 Google Scholar
Google Scholar
[23] 曹楚南, 张鉴清 2002 电化学阻抗谱导论 (典藏版1) (北京: 科学出版社)第21页
Cao C N, Zhang J Q 2002 Introduction to Electrochemical Impedance Spectroscopy (Vol. 1) (Beijing: Science Press) p21 (in Chinese)
[24] Kliewer K, Koehler J 1965 Phys. Rev. A 140 1226
[25] Ikeda J A S, Chiang Y M 1993 J. Am. Ceram. Soc. 76 2437
 Google Scholar
Google Scholar
[26] Fleig J, Rodewald S, Maier J 2000 J. Appl. Phys. 87 2372
 Google Scholar
Google Scholar
[27] Eshelby J D 1956 Solid. State. Phys. 3 79
 Google Scholar
Google Scholar
[28] Yan M F, Cannon R M, Bowen H K 1983 J. Appl. Phys. 54 764
 Google Scholar
Google Scholar
[29] Yan M F, Rhodes W W 1987 Materials Science Research (Vol. 21) (US: Springer) p519
计量
- 文章访问数: 12205
- PDF下载量: 75
- 被引次数: 0













 下载:
下载: