-
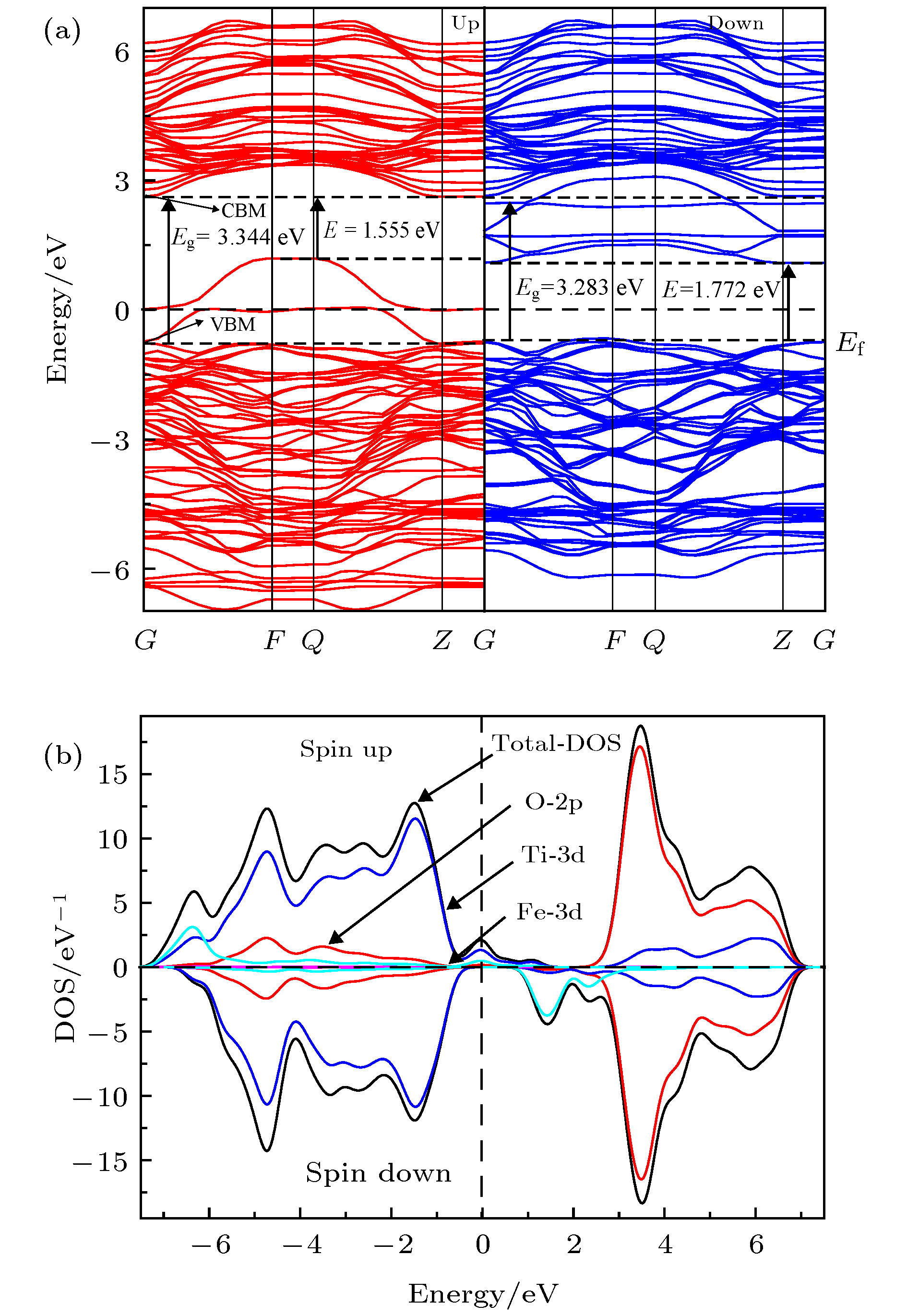
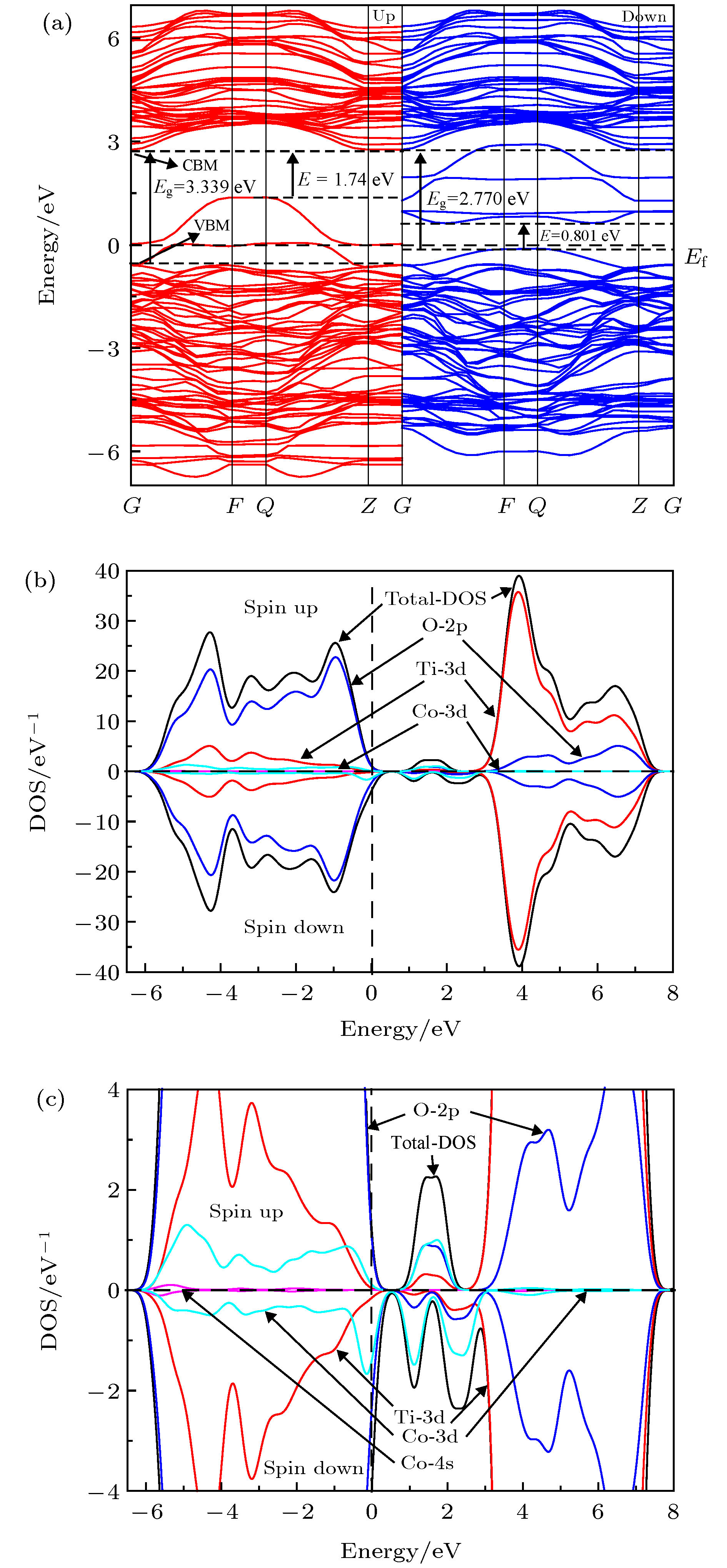
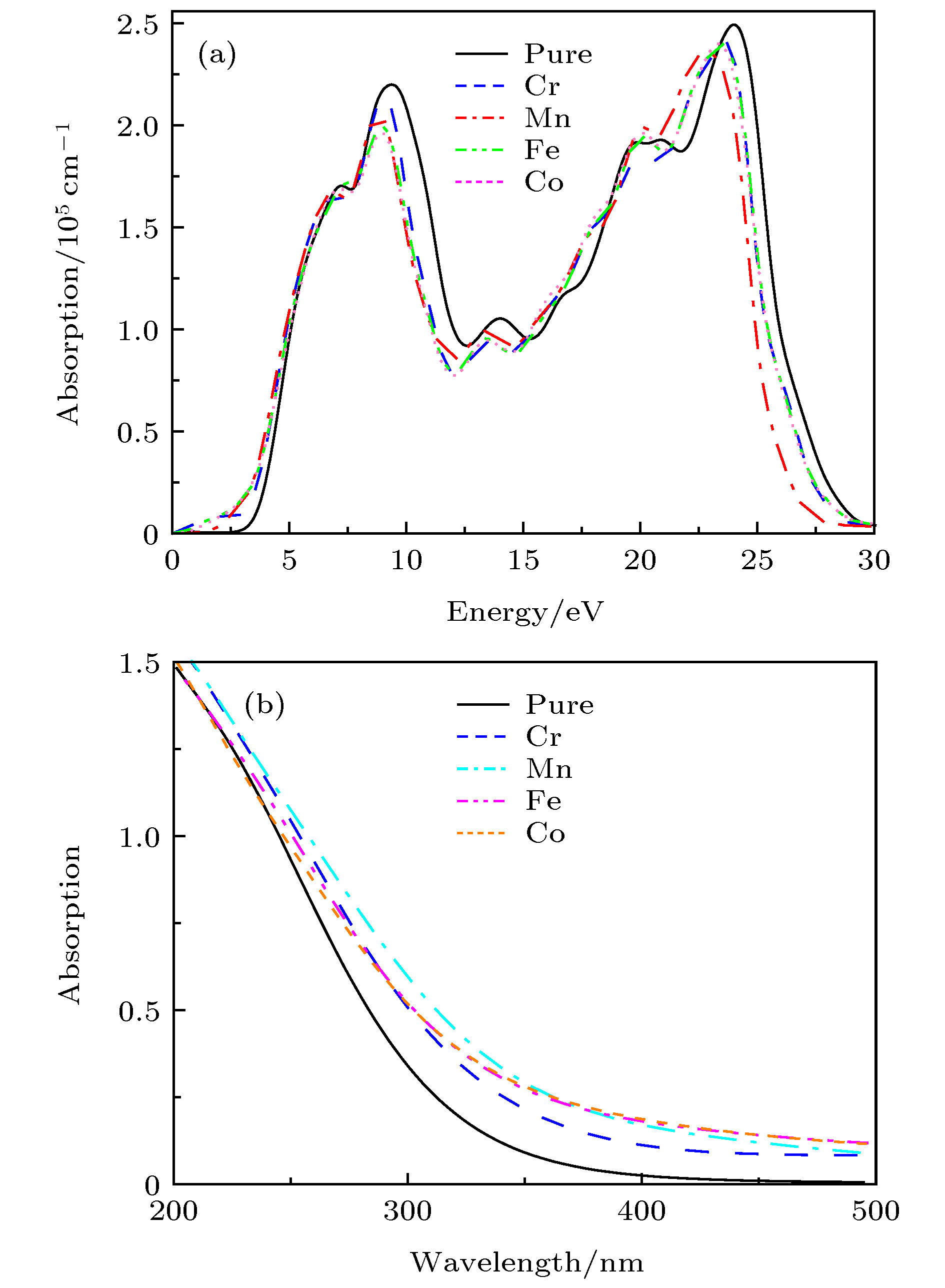
关于过渡金属掺杂TiO2是否会产生室温铁磁性及其磁性的来源存在争议, 为了解决此问题, 本文采用基于密度泛函理论下的GGA+U方法对体系Ti0.875X0.125O2 (X = Cr, Mn, Fe, Co)的磁学性质及光学性质进行了第一性原理的研究. 首先计算了铁磁和反铁磁的基态能量, 比较后推测出铁磁态为它们的基态; 分析能带结构发现Ti0.875Cr0.125O2和Ti0.875Mn0.125O2两种体系保持半导体性质, Ti0.875Fe0.125O2和Ti0.875Co0.125O2两种体系表现金属特性; 掺杂体系都产生了室温铁磁性, 磁性来源主要是过渡金属元素(Cr, Mn, Fe, Co)3d电子轨道诱导极化了周围的O-2p态自旋电子, 导致体系产生净磁矩而呈现铁磁性; 掺杂体系的吸收光谱均发生了红移, 有效扩展了对可见光的吸收范围.It is still in controversy whether the transition metal doped TiO2 will generate room temperature ferromagnetism and where its magnetism originates from. In order to solve this problem, in this paper we use the GGA+U method based on density functional theory to conduct a first-principle study of the magnetic and optical properties for each of the systems of Ti0.875X0.125O2, X = Cr, Mn, Fe, Co. We calculate the ground state energy of each system, on the supposition that they are ferromagnetic or antiferromagnetic. After comparison, the ferromagnetic state is speculated to be its ground state. The binding energy and magnetism calculation results show that Ti0.875Cr0.125O2 has the best stability in all doped systems, that the transition metal element doped TiO2 system has a net magnetic moment, and that the doped systems are ferrimagnetic. In comparison, the net magnetic moment produced by Cr, Mn and Fe doped with TiO2 are substantial, showing that these three systems have good ferromagnetic properties. The Curie temperatures of all doped systems are above room temperature, which is of great significance for the electron spin to retain the information in dilute magnetic semiconductors (DMS), and also greatly helps with the practical application of magnetic materials. The analysis of the energy band structure reveals that intrinsic TiO2 is non ferromagnet, Ti0.875Cr0.125O2 and Ti0.875Mn0.125O2 maintain semiconductor properties, and Ti0.875Fe0.125O2 and Ti0.875Co0.125O2 exhibit metal characteristics. The doped systems produce room temperature ferromagnetism, the main magnetic source is the transition metal elements (Cr, Mn, Fe, Co) 3d electron orbit induced polarization of the surrounding O-2p state spin electrons, causing the systems to produce a net magnetic moment and be ferromagnetic. The absorption spectrum of the doped system is red-shifted, which shows that the doping causes the range of its absorption spectrum to extend to the visible range. At the same time, in all the doped systems in this article, Fe and Co doped TiO2 have the best light absorption intensity, and the magnetic property of the Fe doped system is the strongest, which indicates that when the system is ferromagnetic, the spin up and spin down splitting will occur in the local magnetic field, which will change the electronic structure of TiO2 and enhance its photocatalytic performance. The calculation results in this paper are of theoretical significance for preparing TiO2 with curie temperature above room temperature by being doped with transition metal elements of Cr, Mn, Fe, and Co.
-
Keywords:
- TiO2 /
- electronic structure /
- magnetism /
- optical properties
[1] Yang K, Dai Y, Huang B 2007 J. Phys. Chem. C 111 12086
 Google Scholar
Google Scholar
[2] Yang K, Dai Y, Huang B, Han S 2006 J. Phys. Chem. B 110 24011
 Google Scholar
Google Scholar
[3] Gao Y, Hou Q Y, Liu Q L 2019 J. Supercond. Nov. Magn. 32 2877
 Google Scholar
Google Scholar
[4] Liu Y, Wei L, Zhang W, Zhang J, Han P 2013 Solid State. Commun. 164 27
 Google Scholar
Google Scholar
[5] Yao X, Wang X, Su L, Yan H, Yao M 2011 J. Mol. Catal. A-Chem. 351 11
 Google Scholar
Google Scholar
[6] Matsumoto Y, Murakami M, Shono T, Hasegawa T, Fukumura T, Kawasaki M, Ahmet P, Chikyow T, Koshihara S, Koinuma H 2001 Science 291 854
 Google Scholar
Google Scholar
[7] Hoa N T Q, Huyen D N 2013 J. Mater. Sci.-Mater. El. 24 793
 Google Scholar
Google Scholar
[8] Chanda A, Rout K, Vasundhara M, Joshi S R, Singh J 2018 RSC Adv. 8 10939
 Google Scholar
Google Scholar
[9] Sharma S, Chaudhary S, Kashyap S C, Sharma S K 2011 J. Appl. Phys. 109 951
 Google Scholar
Google Scholar
[10] Tao J G, Guan L X, Pan J S, Huan C H A, Wang L, Kuo J L, Zhang Z, Chai J W, Wang S J 2009 Appl. Phys. Lett. 95 062505
 Google Scholar
Google Scholar
[11] Kim R, Cho S, Park W, Cho D, Oh S, Saint-Martin R, Berthet P, Park J, Yu J 2014 J. Phys. Condens. Matter 26 146003
 Google Scholar
Google Scholar
[12] Xu J, Li L, Lv L, Zhang X, Chen X, Wang J, Zhang F, Zhong W, Du Y W 2009 Chin. Phys. Lett. 26 097502
 Google Scholar
Google Scholar
[13] Wang Y, Zhang R, Li J, Li L, Lin S 2014 Nanoscale Res. Lett. 9 46
 Google Scholar
Google Scholar
[14] Rumaiz A K, Woicik J C, Cockayne E, Lin H Y, Jaffari G H, Shah S I 2009 Appl. Phys. Lett. 95 262111
 Google Scholar
Google Scholar
[15] Peng H, Li J, Li S, Xia J 2008 J. Phys. Condens. Matter 20 125207
 Google Scholar
Google Scholar
[16] Fujishima A, Honda K 1972 Nature 238 37
 Google Scholar
Google Scholar
[17] 李聪, 郑友进, 付斯年, 姜宏伟, 王丹 2016 65 037102
 Google Scholar
Google Scholar
Li C, Zheng Y J, Fu S N, Jiang H W, Wang D 2016 Acta Phys. Sin. 65 037102
 Google Scholar
Google Scholar
[18] Burdett J K, Hughbanks T, Miller G J, Richardson J W, Smith J V 1987 J. Am. Chem. Soc. 109 3639
 Google Scholar
Google Scholar
[19] 张丽丽, 夏桐, 刘桂安, 雷博程, 赵旭才, 王少霞, 黄以能 2019 68 017401
 Google Scholar
Google Scholar
Zhang L L, Xia T, Liu G A, Lei B C, Zhao X C, Wang S X, Huang Y N 2019 Acta Phys. Sin. 68 017401
 Google Scholar
Google Scholar
[20] Clark S J, Segall M D, Pickard C J, Hasnip P J, Probert M J, Refson K, Payne M C 2005 Z. Krist.-Cryst. Mater. 220 567
 Google Scholar
Google Scholar
[21] Segall M D, Lindan P, Probert M J, Pickard C J, Hasnip P J, Clark S J, Payne M C 2002 J. Phys. Condens. Matter 14 2717
 Google Scholar
Google Scholar
[22] Di Valentin C, Finazzi E, Pacchioni G, Selloni A, Livraghi S, Paganini M C, Giamello E 2007 Chem. Phys. 339 44
 Google Scholar
Google Scholar
[23] Pickett W E, Erwin S C, Ethridge E C 1998 Phys. Rev. B 58 1201
 Google Scholar
Google Scholar
[24] Perdew J P, Burke K, Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[25] Morgan B J, Watson G W 2010 J. Phys. Chem. C 114 2328
 Google Scholar
Google Scholar
[26] Cui X Y, Medvedeva J E, Delley B, Freeman A J, Newman N, Stampfl C 2005 Phys. Rev. Lett. 95 256404
 Google Scholar
Google Scholar
[27] 王闯, 赵永红, 刘永 2019 68 176301
 Google Scholar
Google Scholar
Wang C, Zhao Y H, Liu Y 2019 Acta Phys. Sin. 68 176301
 Google Scholar
Google Scholar
[28] Cheng Y C, Zhu Z Y, Mi W B, Guo Z B, Schwingenschloegl U 2013 Phys. Rev. B 87 100401
 Google Scholar
Google Scholar
[29] Sasioglu E, Sandratskii L M, Bruno P 2004 Phys. Rev. B 70 024427
 Google Scholar
Google Scholar
[30] Fukushima T, Sato K, Katayama-Yoshida H, Dederichs P H 2004 Jpn. J. Appl. Phys. 43 L1416
 Google Scholar
Google Scholar
[31] Salmani E M, Laghrissi A, Lamouri R, Dehmani M, Benchafia E M, Ez-Zahraouy H, Benyoussef A 2017 Opt. Quantum Electron. 49 97
 Google Scholar
Google Scholar
[32] Fukumura T, Kawasaki M 2013 Functional Metal Oxides: New Science and Novel Appl. (Tokyo: Weinheim, Germany) pp91−131
[33] Roy S, Luitel H, Sanyal D 2019 Comput. Condens. Matter 18 e00349
 Google Scholar
Google Scholar
[34] Sun J, Wang H T, He J L, Tian Y J 2005 Phys. Rev. B 71 125132
 Google Scholar
Google Scholar
[35] Brown G F, Wu J Q 2009 Laser Photonics Rev. 3 394
 Google Scholar
Google Scholar
[36] 周诗文, 彭平, 陈文钦, 庾名槐, 郭惠, 袁珍 2019 68 037101
 Google Scholar
Google Scholar
Zhou S W, Peng P, Chen W Q, Yu M H, Guo H, Yuan Z 2019 Acta Phys.Sin. 68 037101
 Google Scholar
Google Scholar
[37] 侯清玉, 曲灵丰, 赵春旺 2016 65 057401
 Google Scholar
Google Scholar
Hou Q Y, Qu L F, Zhao C W 2016 Acta Phys. Sin. 65 057401
 Google Scholar
Google Scholar
[38] 赵宗彦, 柳清菊, 张瑾, 朱忠其 2007 56 6592
 Google Scholar
Google Scholar
Zhao Z Y, Liu Q J, Zhang J, Zhu Z Q 2007 Acta Phys. Sin. 56 6592
 Google Scholar
Google Scholar
-
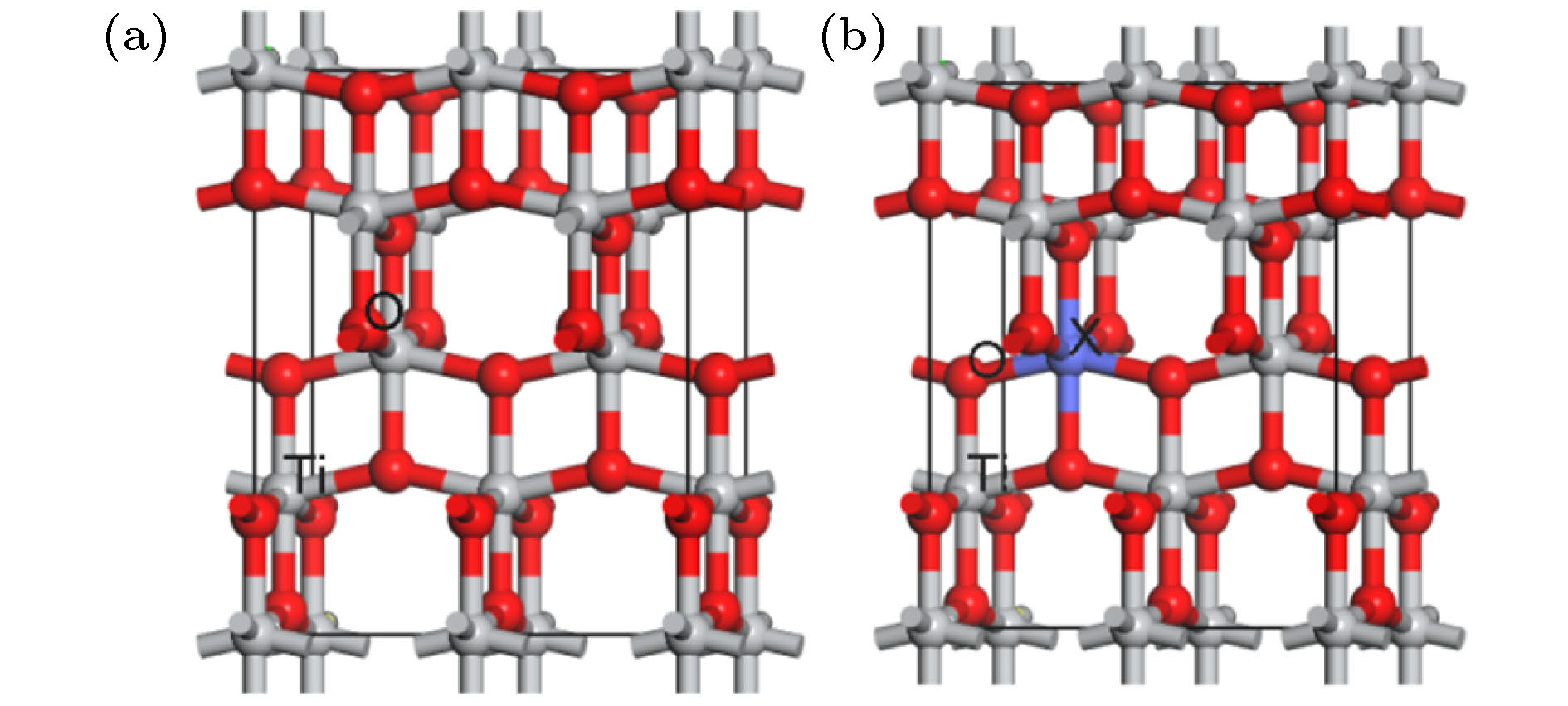
表 1 掺杂前后体系结构参数计算结果
Table 1. Calculation results of system parameters before and after doping.
模型 Eb EFM/eV EAFM/eV ∆E/meV 2※Integrated spin density/μB 2※Integrated|spin density|/μB TiO2(2 × 1 × 1) –7457.281 Ti0.875Cr0.125O2 –7727.549 –20572.796 –20572.767 29 3 3.316 Ti0.875Mn0.125O2 –7063.942 –18797.713 –18797.674 39 3 3.668 Ti0.875Fe0.125O2 –7158.347 –19034.057 –19034.017 40 4 4.296 Ti0.875Co0.125O2 –7253.848 –19269.779 –19269.689 90 1.667 2.056 -
[1] Yang K, Dai Y, Huang B 2007 J. Phys. Chem. C 111 12086
 Google Scholar
Google Scholar
[2] Yang K, Dai Y, Huang B, Han S 2006 J. Phys. Chem. B 110 24011
 Google Scholar
Google Scholar
[3] Gao Y, Hou Q Y, Liu Q L 2019 J. Supercond. Nov. Magn. 32 2877
 Google Scholar
Google Scholar
[4] Liu Y, Wei L, Zhang W, Zhang J, Han P 2013 Solid State. Commun. 164 27
 Google Scholar
Google Scholar
[5] Yao X, Wang X, Su L, Yan H, Yao M 2011 J. Mol. Catal. A-Chem. 351 11
 Google Scholar
Google Scholar
[6] Matsumoto Y, Murakami M, Shono T, Hasegawa T, Fukumura T, Kawasaki M, Ahmet P, Chikyow T, Koshihara S, Koinuma H 2001 Science 291 854
 Google Scholar
Google Scholar
[7] Hoa N T Q, Huyen D N 2013 J. Mater. Sci.-Mater. El. 24 793
 Google Scholar
Google Scholar
[8] Chanda A, Rout K, Vasundhara M, Joshi S R, Singh J 2018 RSC Adv. 8 10939
 Google Scholar
Google Scholar
[9] Sharma S, Chaudhary S, Kashyap S C, Sharma S K 2011 J. Appl. Phys. 109 951
 Google Scholar
Google Scholar
[10] Tao J G, Guan L X, Pan J S, Huan C H A, Wang L, Kuo J L, Zhang Z, Chai J W, Wang S J 2009 Appl. Phys. Lett. 95 062505
 Google Scholar
Google Scholar
[11] Kim R, Cho S, Park W, Cho D, Oh S, Saint-Martin R, Berthet P, Park J, Yu J 2014 J. Phys. Condens. Matter 26 146003
 Google Scholar
Google Scholar
[12] Xu J, Li L, Lv L, Zhang X, Chen X, Wang J, Zhang F, Zhong W, Du Y W 2009 Chin. Phys. Lett. 26 097502
 Google Scholar
Google Scholar
[13] Wang Y, Zhang R, Li J, Li L, Lin S 2014 Nanoscale Res. Lett. 9 46
 Google Scholar
Google Scholar
[14] Rumaiz A K, Woicik J C, Cockayne E, Lin H Y, Jaffari G H, Shah S I 2009 Appl. Phys. Lett. 95 262111
 Google Scholar
Google Scholar
[15] Peng H, Li J, Li S, Xia J 2008 J. Phys. Condens. Matter 20 125207
 Google Scholar
Google Scholar
[16] Fujishima A, Honda K 1972 Nature 238 37
 Google Scholar
Google Scholar
[17] 李聪, 郑友进, 付斯年, 姜宏伟, 王丹 2016 65 037102
 Google Scholar
Google Scholar
Li C, Zheng Y J, Fu S N, Jiang H W, Wang D 2016 Acta Phys. Sin. 65 037102
 Google Scholar
Google Scholar
[18] Burdett J K, Hughbanks T, Miller G J, Richardson J W, Smith J V 1987 J. Am. Chem. Soc. 109 3639
 Google Scholar
Google Scholar
[19] 张丽丽, 夏桐, 刘桂安, 雷博程, 赵旭才, 王少霞, 黄以能 2019 68 017401
 Google Scholar
Google Scholar
Zhang L L, Xia T, Liu G A, Lei B C, Zhao X C, Wang S X, Huang Y N 2019 Acta Phys. Sin. 68 017401
 Google Scholar
Google Scholar
[20] Clark S J, Segall M D, Pickard C J, Hasnip P J, Probert M J, Refson K, Payne M C 2005 Z. Krist.-Cryst. Mater. 220 567
 Google Scholar
Google Scholar
[21] Segall M D, Lindan P, Probert M J, Pickard C J, Hasnip P J, Clark S J, Payne M C 2002 J. Phys. Condens. Matter 14 2717
 Google Scholar
Google Scholar
[22] Di Valentin C, Finazzi E, Pacchioni G, Selloni A, Livraghi S, Paganini M C, Giamello E 2007 Chem. Phys. 339 44
 Google Scholar
Google Scholar
[23] Pickett W E, Erwin S C, Ethridge E C 1998 Phys. Rev. B 58 1201
 Google Scholar
Google Scholar
[24] Perdew J P, Burke K, Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[25] Morgan B J, Watson G W 2010 J. Phys. Chem. C 114 2328
 Google Scholar
Google Scholar
[26] Cui X Y, Medvedeva J E, Delley B, Freeman A J, Newman N, Stampfl C 2005 Phys. Rev. Lett. 95 256404
 Google Scholar
Google Scholar
[27] 王闯, 赵永红, 刘永 2019 68 176301
 Google Scholar
Google Scholar
Wang C, Zhao Y H, Liu Y 2019 Acta Phys. Sin. 68 176301
 Google Scholar
Google Scholar
[28] Cheng Y C, Zhu Z Y, Mi W B, Guo Z B, Schwingenschloegl U 2013 Phys. Rev. B 87 100401
 Google Scholar
Google Scholar
[29] Sasioglu E, Sandratskii L M, Bruno P 2004 Phys. Rev. B 70 024427
 Google Scholar
Google Scholar
[30] Fukushima T, Sato K, Katayama-Yoshida H, Dederichs P H 2004 Jpn. J. Appl. Phys. 43 L1416
 Google Scholar
Google Scholar
[31] Salmani E M, Laghrissi A, Lamouri R, Dehmani M, Benchafia E M, Ez-Zahraouy H, Benyoussef A 2017 Opt. Quantum Electron. 49 97
 Google Scholar
Google Scholar
[32] Fukumura T, Kawasaki M 2013 Functional Metal Oxides: New Science and Novel Appl. (Tokyo: Weinheim, Germany) pp91−131
[33] Roy S, Luitel H, Sanyal D 2019 Comput. Condens. Matter 18 e00349
 Google Scholar
Google Scholar
[34] Sun J, Wang H T, He J L, Tian Y J 2005 Phys. Rev. B 71 125132
 Google Scholar
Google Scholar
[35] Brown G F, Wu J Q 2009 Laser Photonics Rev. 3 394
 Google Scholar
Google Scholar
[36] 周诗文, 彭平, 陈文钦, 庾名槐, 郭惠, 袁珍 2019 68 037101
 Google Scholar
Google Scholar
Zhou S W, Peng P, Chen W Q, Yu M H, Guo H, Yuan Z 2019 Acta Phys.Sin. 68 037101
 Google Scholar
Google Scholar
[37] 侯清玉, 曲灵丰, 赵春旺 2016 65 057401
 Google Scholar
Google Scholar
Hou Q Y, Qu L F, Zhao C W 2016 Acta Phys. Sin. 65 057401
 Google Scholar
Google Scholar
[38] 赵宗彦, 柳清菊, 张瑾, 朱忠其 2007 56 6592
 Google Scholar
Google Scholar
Zhao Z Y, Liu Q J, Zhang J, Zhu Z Q 2007 Acta Phys. Sin. 56 6592
 Google Scholar
Google Scholar
计量
- 文章访问数: 17195
- PDF下载量: 588
- 被引次数: 0













 下载:
下载: