-
采用第一性原理计算和实验相结合的方法, 研究了金刚石/铝复合材料的界面性质及界面反应. 计算结果表明: 金刚石(100)/铝(111)界面粘附功更大, 相比金刚石(111)/铝(111)的界面粘附功4.14 J/m2提高了41%. 同时, 金刚石(100)/铝(111)界面处形成Al—C键合的趋势更强. Al—C键的引入能够促进金刚石(100)/铝(111)界面处C—C键的形成, 提高界面粘附功. 利用真空气压浸渗法制备金刚石/铝复合材料, 并对金刚石/铝复合材料的界面结构进行多尺度表征. 在金刚石{100}面观察到界面产物Al4C3, 且界面脱粘多发生在金刚石{111}面,实验现象与计算结果相一致. 湿热实验研究了界面反应对金刚石/铝复合材料的影响, 进一步表明抑制Al4C3生成、改善界面选择性结合对于提高金刚石/铝复合材料性能及稳定性具有重要意义. 本文的研究为第一性原理计算金刚石/金属的界面性质提供了新的思路, 也对金刚石/金属复合材料的设计具有重要的指导意义.First-principles calculation and experimental methods are used to study the interfacial properties and reaction of diamond/Al composites. Based on the first-principles method, the interfacial adhesion work (Wad), electronic structure and charge transfer of diamond/Al models are calculated systematically. The results show that the adhesion work of diamond(100)/Al(111) is 41% higher than that of diamond(111)/Al(111), therefore, the interface bonding of diamond(100)/Al(111) interface is stronger. According to the analysis of the electronic structure, there are more charges transferring at the diamond(100)/Al(111) interface, and the high charge density is distributed on the side of C atoms. The redistribution of charges at the interface is conducive to the formation of Al—C bond, so that the tendency of forming Al—C bonds is greater. The introduction of Al—C bond can promote the formation of C—C bond at the diamond(100)/Al(111) interface and improve the interfacial adhesion work. In addition, the diamond/Al composites are fabricated by vacuum gas pressure infiltration, and multi-scale characterization of the interface structure of diamond/Al composites is carried out. The interfacial debonding occurs mainly on the diamond {111}. Meanwhile, the interface product Al4C3 is easier to form on the diamond {100}. The experimental phenomenon is consistent with the calculated results. Moreover, the influence of the interfacial reaction on the properties and stability of diamond/Al composites are further discussed through heat-moisture treatment. The study finds that the performance degradation in heat-moisture environment is related mainly to the hydrolysis of the interface product Al4C3. After 60 days’ heat-moisture, the thermal conductivity of the diamond/Al composites decreases by 29.9%, and the bending strength is reduced by 40.1%. The large attenuation of performance is not conducive to the stability of composites in complex environments. Therefore, inhibiting the formation of Al4C3 and improving interfacial selectivity are of great importance in developing the performance and stability of diamond/Al composites. The research in this paper not only lays a theoretical foundation for the first-principles calculation of the interface properties of diamond/metal, but also possesses important guidance significance in designing the diamond/metal composites.
-
Keywords:
- diamond/Al /
- first-principles /
- interface properties /
- heat-moisture treatment
[1] Edtmaier C, Segl J, Koos R, Schöbel M, Feldbaumer C 2020 Diamond Relat. Mater. 106 107842
 Google Scholar
Google Scholar
[2] Guo B S, Chen B, Zhang X M, Cen X, Wang X H, Song M, Ni S, Yi J H, Shen T, Du Y 2018 Carbon 135 224
 Google Scholar
Google Scholar
[3] Lu Y F, Wang X T, Zhang Y, Wang J G, Kim M J, Zhang H L 2018 J. Compos. Mater. 52 2709
 Google Scholar
Google Scholar
[4] Li N, Wang L H, Dai J J, Wang X T, Wang J G, Kim M J, Zhang H L 2019 Diamond Relat. Mater. 100 107565
 Google Scholar
Google Scholar
[5] Chen G Q, Yang W S, Xin L, Wang P P, Liu S F, Qiao J, Hu F J, Zhang Q, Wu G H 2018 J. Alloys Compd. 735 777
 Google Scholar
Google Scholar
[6] Edtmaier C, Segl J, Rosenberg E, Liedl G, Pospichal R, Steiger-Thirsfeld A 2018 J. Mater. Sci. 53 15514
 Google Scholar
Google Scholar
[7] Tan Z Q, Li Z Q, Xiong D B, Fan G L, Ji G, Zhang D 2014 Mater. Des. 55 257
 Google Scholar
Google Scholar
[8] Che Z F, Wang Q X, Wang L H, Li J W, Zhang H L, Zhang Y, Wang X T, Wang J G, Kim M J 2017 Composites Part B 113 285
 Google Scholar
Google Scholar
[9] Ji G, Tan Z Q, Li X P, Li Z Q, Kruth J P 2018 Mater. Sci. Forum 941 2184
 Google Scholar
Google Scholar
[10] Guo C Y, He X B, Ren S B, Qu X H 2016 J. Alloys Compd. 664 777
 Google Scholar
Google Scholar
[11] Tan Z Q, Ji G, Addad A, Li Z Q, Silvain J F, Zhang D 2016 Composites Part A 91 9
 Google Scholar
Google Scholar
[12] Monje I E, Louis E, Molina J M 2013 Composites Part A 48 9
 Google Scholar
Google Scholar
[13] Wu J H, Zhang H L, Zhang Y, Li J W, Wang X T 2012 Mater. Des. 39 87
 Google Scholar
Google Scholar
[14] Monje I E, Louis E, Molina J M 2016 Scr. Mater. 115 159
 Google Scholar
Google Scholar
[15] Li X J, Yang W L, Sang J Q, Zhu J J, Fu L C, Li DY, Zhou L P 2020 J. Alloys Compd. 846 156258
 Google Scholar
Google Scholar
[16] 张恒, 黄燕, 石旺舟, 周孝好, 陈效双 2019 68 207302
 Google Scholar
Google Scholar
Zhang H, Huang Y, Shi W Z, Zhou X H, Chen X S 2019 Acta Phys. Sin. 68 207302
 Google Scholar
Google Scholar
[17] 董珊, 张岩星, 张喜林, 许晓培, 毛建军, 李东霖, 陈志明, 马款, 范政权, 魏丹丹, 杨宗献 2016 65 068201
 Google Scholar
Google Scholar
Dong S, Zhang Y X, Zhang X L, Xu X P, Mao J J, Li D L, Chen Z M, Ma K, Fan Z Q, Wei D D, Yang Z X 2016 Acta Phys. Sin. 65 068201
 Google Scholar
Google Scholar
[18] Zhao Z Y, Zhao W J, Bai P K, Wu L Y, Huo P C 2019 Mater. Lett. 255 126559
 Google Scholar
Google Scholar
[19] Chen L, Chen S T, Hou Y 2019 Carbon 148 249
 Google Scholar
Google Scholar
[20] Xie H N, Chen Y T, Zhang T B, Zhao N Q, Shi C S, He C N, Liu E Z 2020 Appl. Surf. Sci. 527 146817
 Google Scholar
Google Scholar
[21] Fathzadeh M, Fahrvandi H, Nadimi E 2020 Nanotechnology 31 025710
 Google Scholar
Google Scholar
[22] Qi Y, Hector L G 2003 Phys. Rev. B 68 201403
 Google Scholar
Google Scholar
[23] 吴孔平, 孙昌, 马文飞, 王杰, 魏巍, 蔡俊, 陈昌兆, 任斌, 桑立雯, 廖梅勇 2017 66 088102
 Google Scholar
Google Scholar
Wu K P, Sun C X, Ma W F, Wang J, Wei W, Cai J, Chen C Z, Ren B, Sang L W, Liao M Y 2017 Acta Phys. Sin. 66 088102
 Google Scholar
Google Scholar
[24] Vanderbilt, David 1990 Phys. Rev. B 41 7892
 Google Scholar
Google Scholar
[25] Perdew J P, Burke K, Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[26] Monkhorst H J, Pack J D 1976 Phys. Rev. B 13 5188
 Google Scholar
Google Scholar
[27] Monachon C, Schusteritsch G, Kaxiras E, Weber L 2014 J. Appl. Phys. 115 123509
 Google Scholar
Google Scholar
[28] Riley D P 1944 Nature 153 587
 Google Scholar
Google Scholar
[29] Liu L M, Wang S Q, Ye H Q 2004 Surf. Sci. 550 46
 Google Scholar
Google Scholar
[30] Xu X Y, Wang H Y, Zha M, Wang C, Yang Z Z, Jiang Q C 2018 Appl. Surf. Sci. 437 103
 Google Scholar
Google Scholar
[31] Liu R, Yin X M, Feng K X, Xu R 2018 Comput. Mater. Sci. 149 373
 Google Scholar
Google Scholar
[32] Che Z F, Zhang Y, Li J W, Zhang H L, Wang X T, Sun C, Wang J G, Kim M J 2016 J. Alloys Compd. 657 81
 Google Scholar
Google Scholar
[33] Monje I E, Louis E, Molina J M 2016 J. Mater. Sci. 51 8027
 Google Scholar
Google Scholar
-
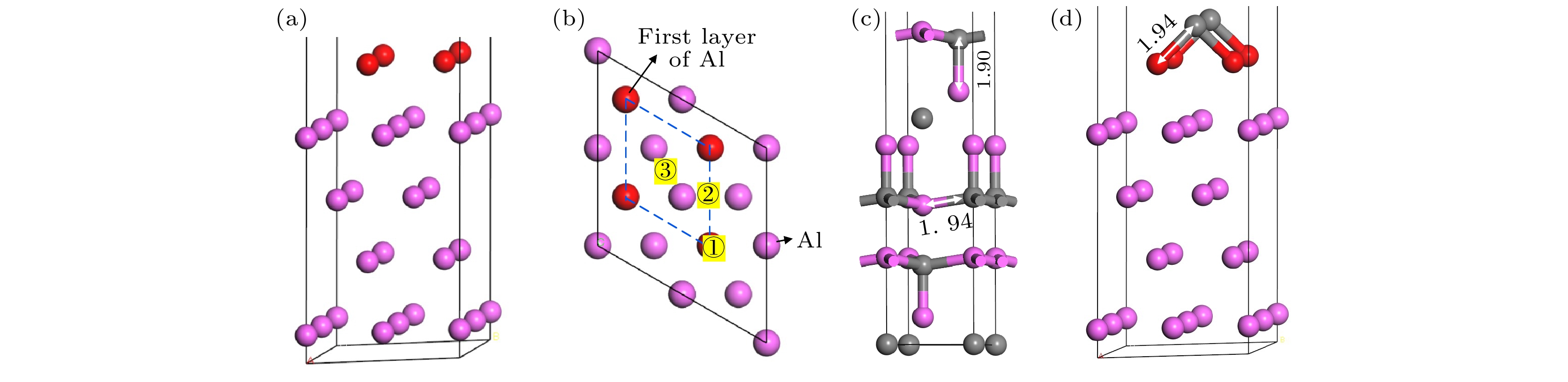
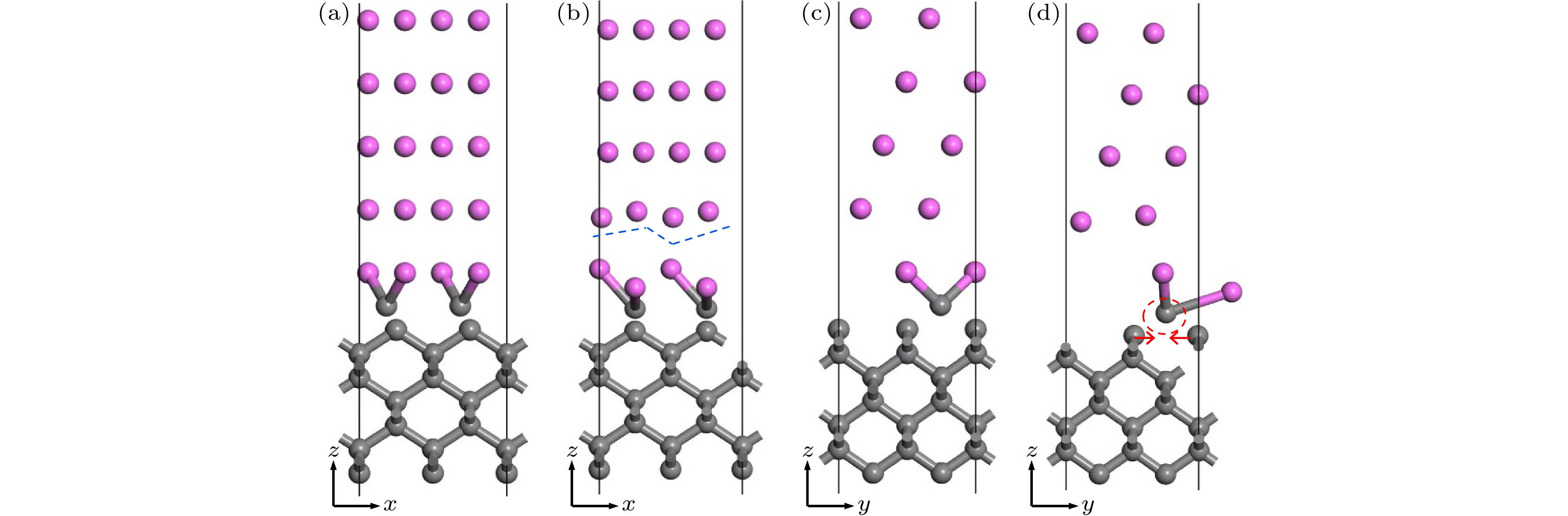
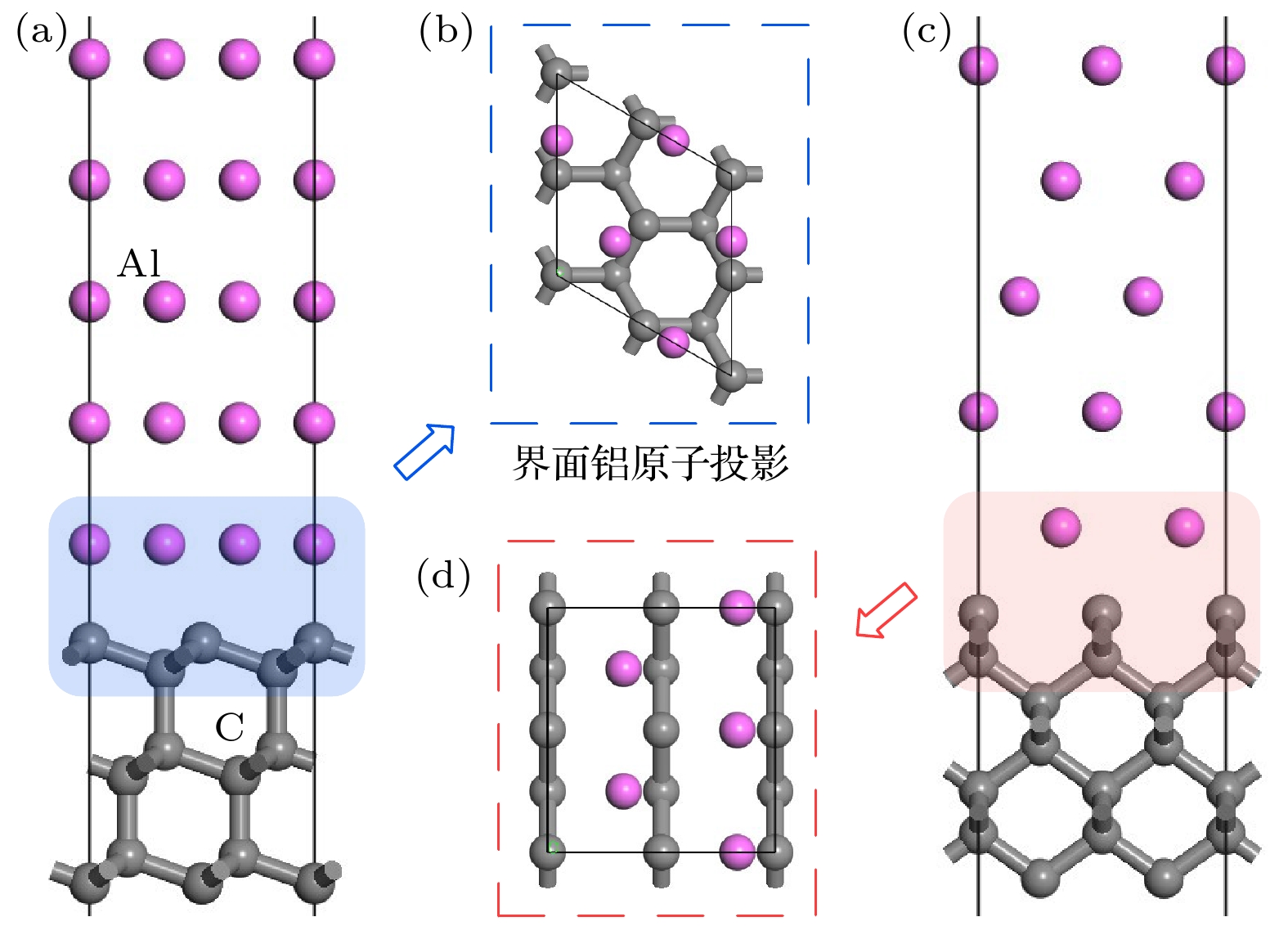
图 5 C原子在Al(111)表面吸附位置确定 (a) Al(111)表面模型; (b) C原子在Al(111)表面吸附位置俯视图; (c) Al4C3模型局部图; (d) 优化的C原子在Al(111)表面的位置
Fig. 5. Determination of adsorption position of C atom on Al (111) surface: (a) Al (111) surface model; (b) top view of the adsorption position of C atom on Al (111) surface; (c) the local map of Al4C3 model; (d) the position of the optimized C atom on Al (111) surface.
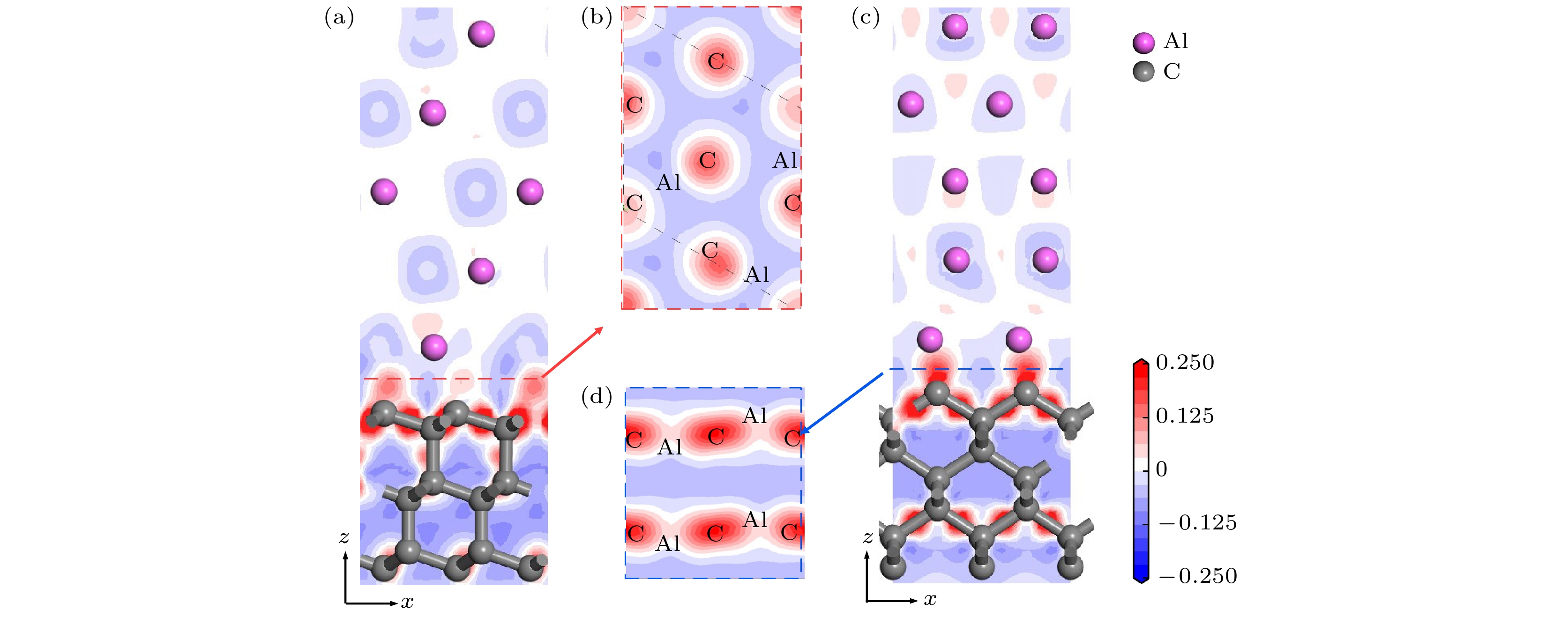
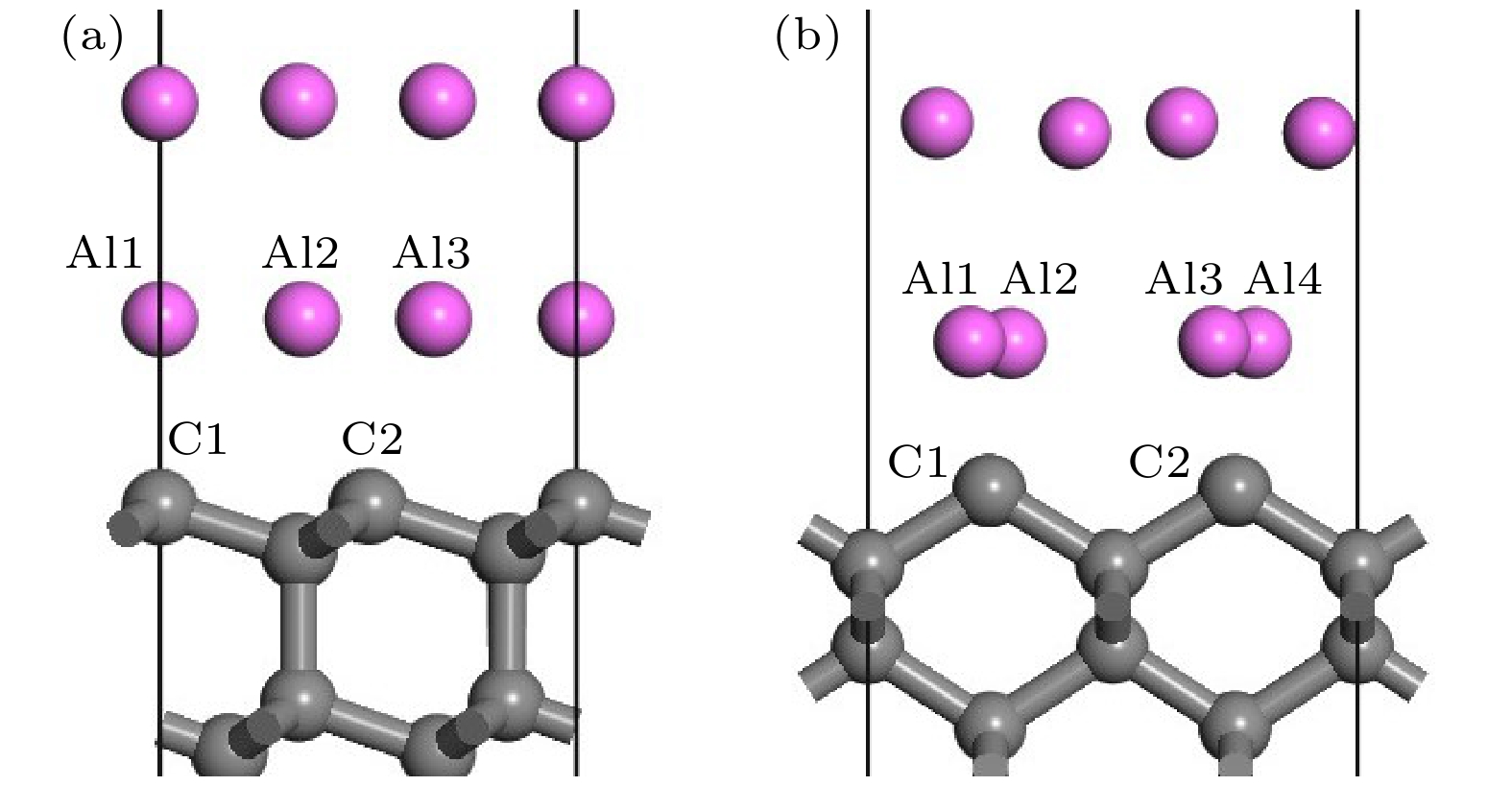
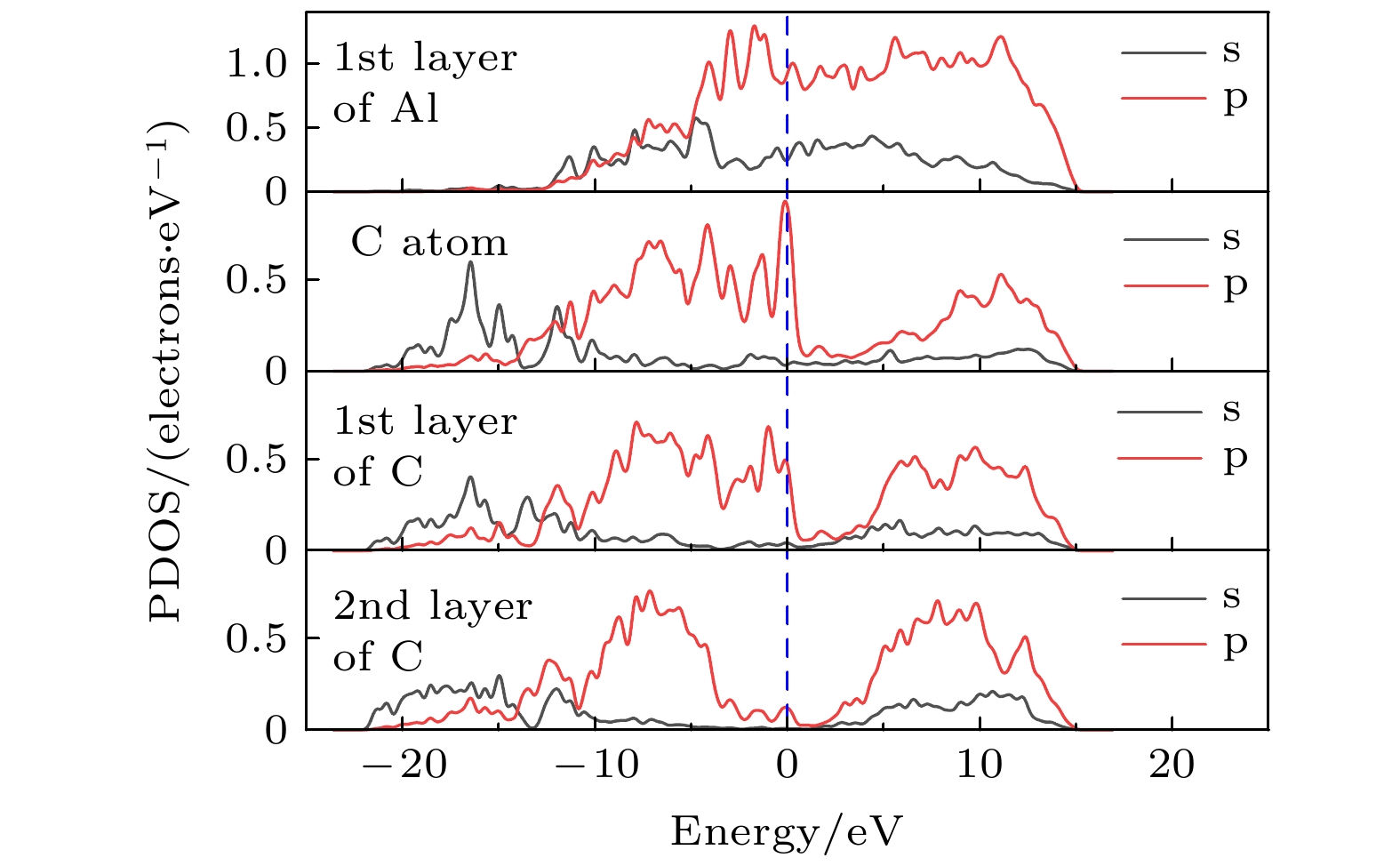
图 7 修正的金刚石(100)/铝(111)模型差分电荷密度分析 (a) 结构优化后模型俯视图; (b) (a)中①截面差分电荷密度图; (c) (a)中②截面差分电荷密度图; (d) (b)中虚线部分的差分电荷密度图
Fig. 7. Differential charge density analysis of diamond(100)/Al(111) modified model: (a) Top view of the model after structure optimization; (b) cross section differential charge density diagram of ① in Fig. (a); (c) cross section differential charge density diagram of ② in Fig. (a); (d) the differential charge density diagram of the dotted line in Fig. (b).
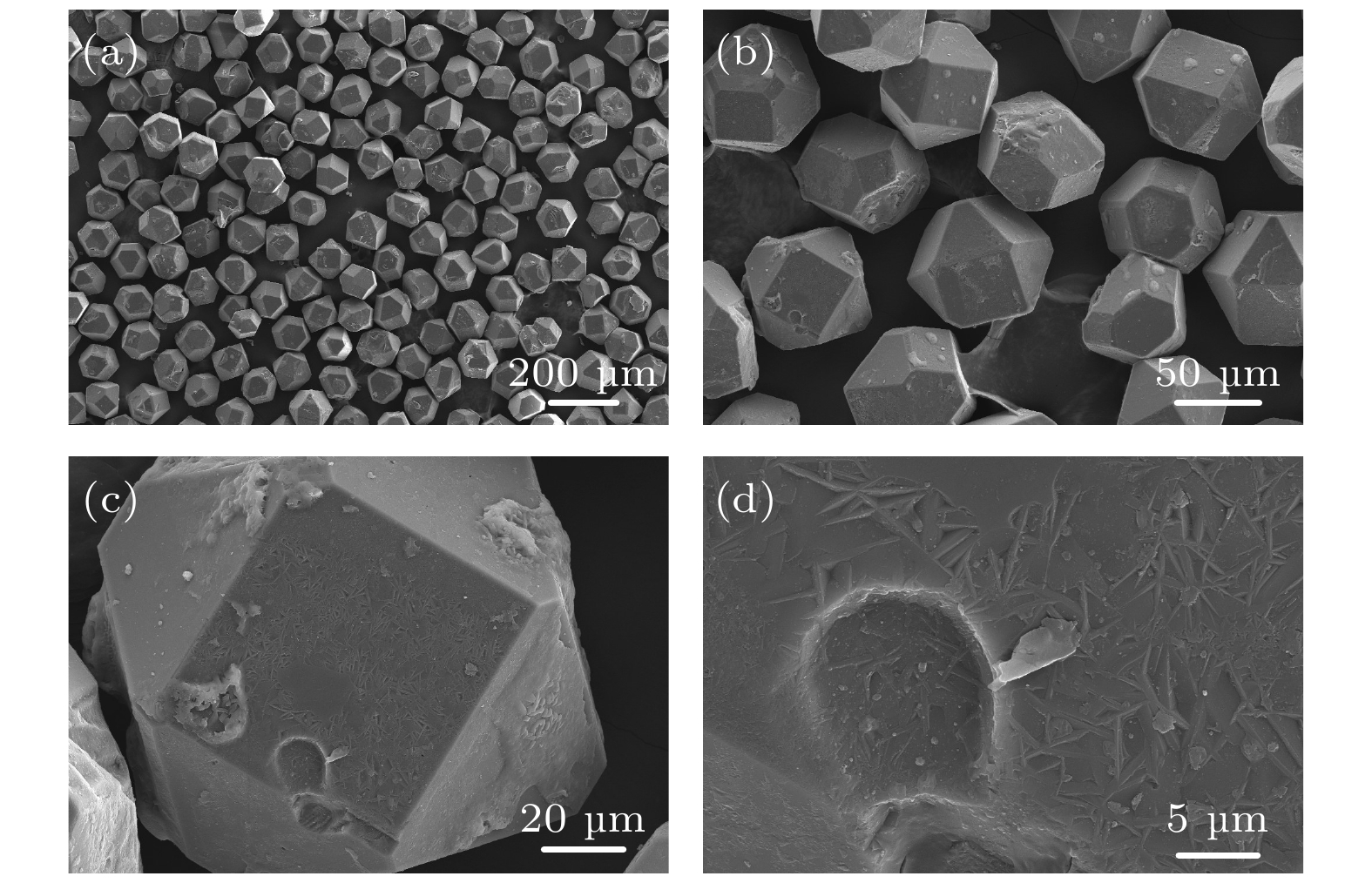
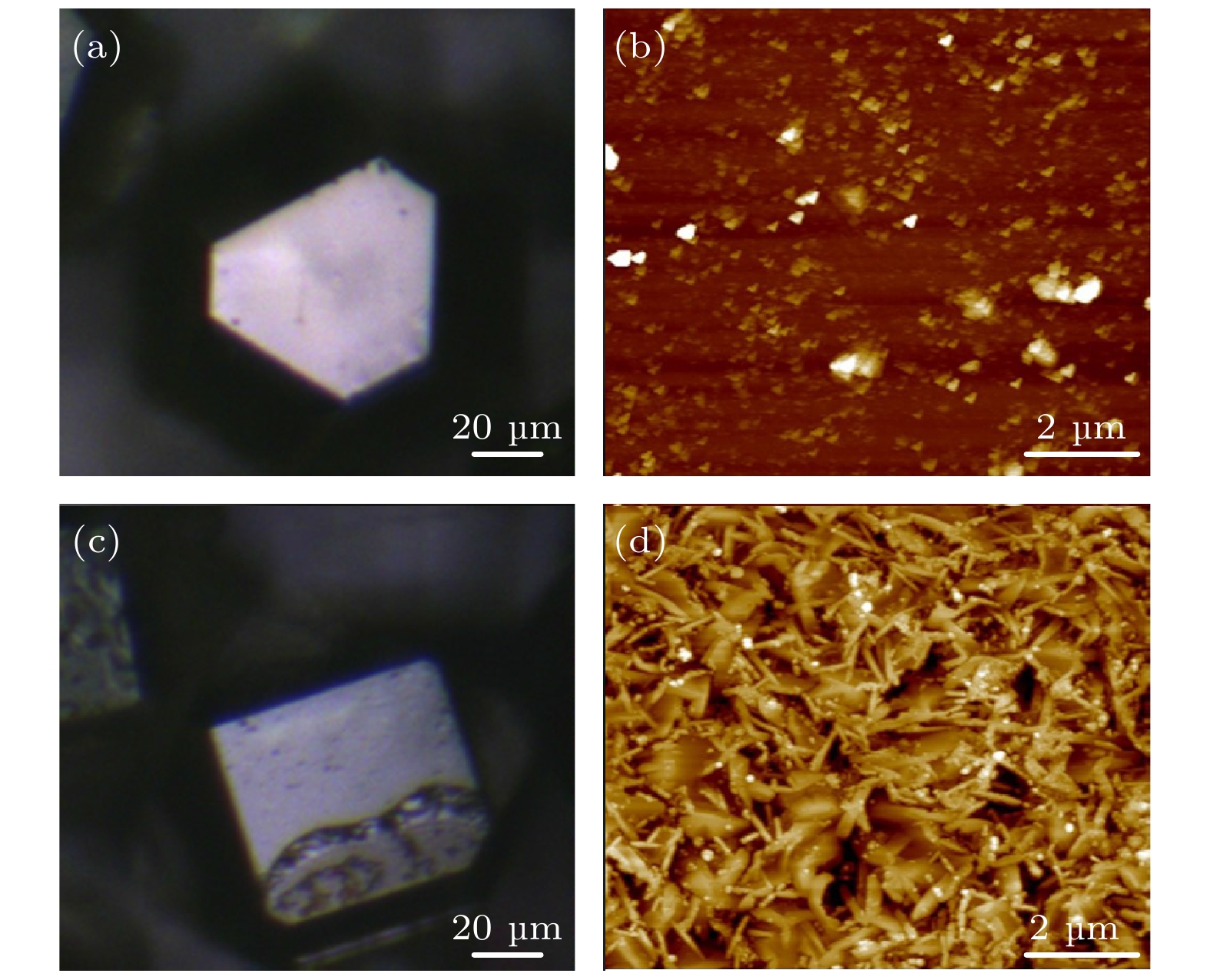
图 9 复合材料中提取金刚石的SEM形貌 (a) 提取金刚石颗粒整体形貌; (b) 提取金刚石颗粒局部形貌; (c) 单个金刚石颗粒形貌; (d) {100}晶面上的Al4C3蚀坑
Fig. 9. SEM morphology of diamond extracted from diamond/Al composites: (a) Overall morphology of extracted diamond particles; (b) local morphology of extracted diamond particles; (c) morphology of single diamond particle; (d) Al4C3 pits on {100} plane.
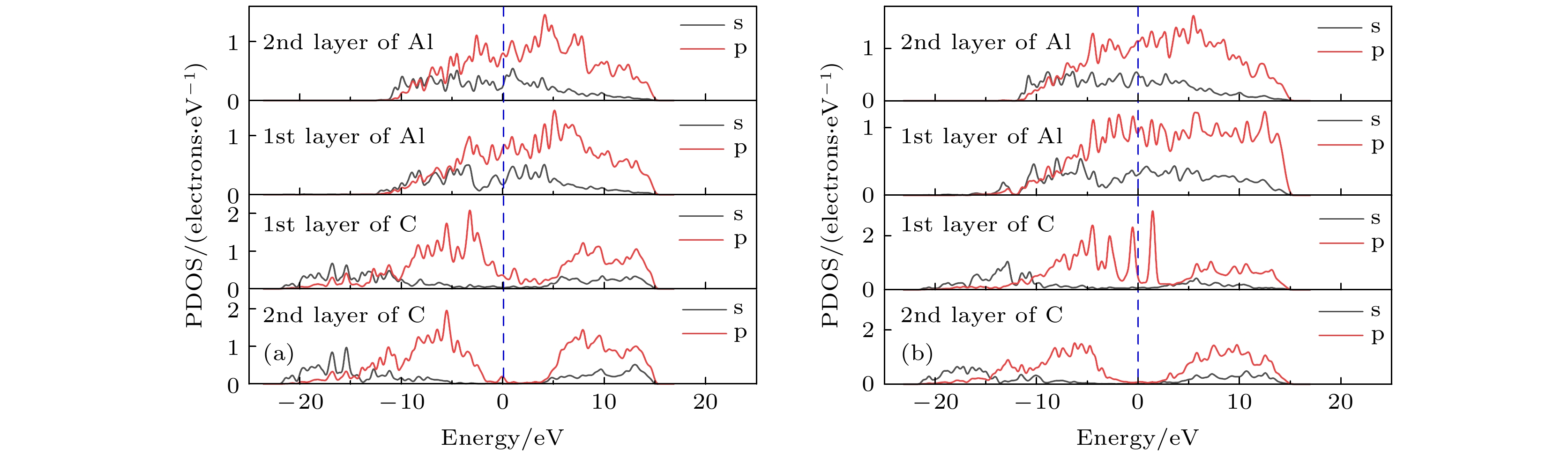
表 1 金刚石/铝界面原子轨道布居分析
Table 1. Atomic orbital population analysis of diamond/Al interface.
Interface model Position s p Total Charge Diamond(111)/Al(111) Al1 1.03 1.61 2.64 0.36 Al2 1.03 1.61 2.64 0.36 Al3 1.03 1.61 2.64 0.36 C1 1.18 3.07 4.25 –0.25 Diamond(100)/Al(111) C2 1.18 3.09 4.26 –0.26 Al1 0.88 1.73 2.61 0.39 Al2 0.89 1.72 2.60 0.40 Al3 0.88 1.73 2.61 0.39 Al4 0.89 1.72 2.61 0.39 C1 1.33 3.10 4.43 0.43 C2 1.33 3.10 4.43 0.43 表 2 不同覆盖率及不同吸附位置时C原子在Al(111)表面的吸附能 (eV·atom–1)
Table 2. Adsorption energy of C atom on Al (111) surface with different coverage and adsorption position (eV·atom–1).
Coverage/ML ① ② ③ 0.25 4.12 7.96 7.63 0.50 6.89 8.62 8.21 1.00 4.27 7.41 5.76 -
[1] Edtmaier C, Segl J, Koos R, Schöbel M, Feldbaumer C 2020 Diamond Relat. Mater. 106 107842
 Google Scholar
Google Scholar
[2] Guo B S, Chen B, Zhang X M, Cen X, Wang X H, Song M, Ni S, Yi J H, Shen T, Du Y 2018 Carbon 135 224
 Google Scholar
Google Scholar
[3] Lu Y F, Wang X T, Zhang Y, Wang J G, Kim M J, Zhang H L 2018 J. Compos. Mater. 52 2709
 Google Scholar
Google Scholar
[4] Li N, Wang L H, Dai J J, Wang X T, Wang J G, Kim M J, Zhang H L 2019 Diamond Relat. Mater. 100 107565
 Google Scholar
Google Scholar
[5] Chen G Q, Yang W S, Xin L, Wang P P, Liu S F, Qiao J, Hu F J, Zhang Q, Wu G H 2018 J. Alloys Compd. 735 777
 Google Scholar
Google Scholar
[6] Edtmaier C, Segl J, Rosenberg E, Liedl G, Pospichal R, Steiger-Thirsfeld A 2018 J. Mater. Sci. 53 15514
 Google Scholar
Google Scholar
[7] Tan Z Q, Li Z Q, Xiong D B, Fan G L, Ji G, Zhang D 2014 Mater. Des. 55 257
 Google Scholar
Google Scholar
[8] Che Z F, Wang Q X, Wang L H, Li J W, Zhang H L, Zhang Y, Wang X T, Wang J G, Kim M J 2017 Composites Part B 113 285
 Google Scholar
Google Scholar
[9] Ji G, Tan Z Q, Li X P, Li Z Q, Kruth J P 2018 Mater. Sci. Forum 941 2184
 Google Scholar
Google Scholar
[10] Guo C Y, He X B, Ren S B, Qu X H 2016 J. Alloys Compd. 664 777
 Google Scholar
Google Scholar
[11] Tan Z Q, Ji G, Addad A, Li Z Q, Silvain J F, Zhang D 2016 Composites Part A 91 9
 Google Scholar
Google Scholar
[12] Monje I E, Louis E, Molina J M 2013 Composites Part A 48 9
 Google Scholar
Google Scholar
[13] Wu J H, Zhang H L, Zhang Y, Li J W, Wang X T 2012 Mater. Des. 39 87
 Google Scholar
Google Scholar
[14] Monje I E, Louis E, Molina J M 2016 Scr. Mater. 115 159
 Google Scholar
Google Scholar
[15] Li X J, Yang W L, Sang J Q, Zhu J J, Fu L C, Li DY, Zhou L P 2020 J. Alloys Compd. 846 156258
 Google Scholar
Google Scholar
[16] 张恒, 黄燕, 石旺舟, 周孝好, 陈效双 2019 68 207302
 Google Scholar
Google Scholar
Zhang H, Huang Y, Shi W Z, Zhou X H, Chen X S 2019 Acta Phys. Sin. 68 207302
 Google Scholar
Google Scholar
[17] 董珊, 张岩星, 张喜林, 许晓培, 毛建军, 李东霖, 陈志明, 马款, 范政权, 魏丹丹, 杨宗献 2016 65 068201
 Google Scholar
Google Scholar
Dong S, Zhang Y X, Zhang X L, Xu X P, Mao J J, Li D L, Chen Z M, Ma K, Fan Z Q, Wei D D, Yang Z X 2016 Acta Phys. Sin. 65 068201
 Google Scholar
Google Scholar
[18] Zhao Z Y, Zhao W J, Bai P K, Wu L Y, Huo P C 2019 Mater. Lett. 255 126559
 Google Scholar
Google Scholar
[19] Chen L, Chen S T, Hou Y 2019 Carbon 148 249
 Google Scholar
Google Scholar
[20] Xie H N, Chen Y T, Zhang T B, Zhao N Q, Shi C S, He C N, Liu E Z 2020 Appl. Surf. Sci. 527 146817
 Google Scholar
Google Scholar
[21] Fathzadeh M, Fahrvandi H, Nadimi E 2020 Nanotechnology 31 025710
 Google Scholar
Google Scholar
[22] Qi Y, Hector L G 2003 Phys. Rev. B 68 201403
 Google Scholar
Google Scholar
[23] 吴孔平, 孙昌, 马文飞, 王杰, 魏巍, 蔡俊, 陈昌兆, 任斌, 桑立雯, 廖梅勇 2017 66 088102
 Google Scholar
Google Scholar
Wu K P, Sun C X, Ma W F, Wang J, Wei W, Cai J, Chen C Z, Ren B, Sang L W, Liao M Y 2017 Acta Phys. Sin. 66 088102
 Google Scholar
Google Scholar
[24] Vanderbilt, David 1990 Phys. Rev. B 41 7892
 Google Scholar
Google Scholar
[25] Perdew J P, Burke K, Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[26] Monkhorst H J, Pack J D 1976 Phys. Rev. B 13 5188
 Google Scholar
Google Scholar
[27] Monachon C, Schusteritsch G, Kaxiras E, Weber L 2014 J. Appl. Phys. 115 123509
 Google Scholar
Google Scholar
[28] Riley D P 1944 Nature 153 587
 Google Scholar
Google Scholar
[29] Liu L M, Wang S Q, Ye H Q 2004 Surf. Sci. 550 46
 Google Scholar
Google Scholar
[30] Xu X Y, Wang H Y, Zha M, Wang C, Yang Z Z, Jiang Q C 2018 Appl. Surf. Sci. 437 103
 Google Scholar
Google Scholar
[31] Liu R, Yin X M, Feng K X, Xu R 2018 Comput. Mater. Sci. 149 373
 Google Scholar
Google Scholar
[32] Che Z F, Zhang Y, Li J W, Zhang H L, Wang X T, Sun C, Wang J G, Kim M J 2016 J. Alloys Compd. 657 81
 Google Scholar
Google Scholar
[33] Monje I E, Louis E, Molina J M 2016 J. Mater. Sci. 51 8027
 Google Scholar
Google Scholar
计量
- 文章访问数: 14762
- PDF下载量: 500
- 被引次数: 0













 下载:
下载: