-
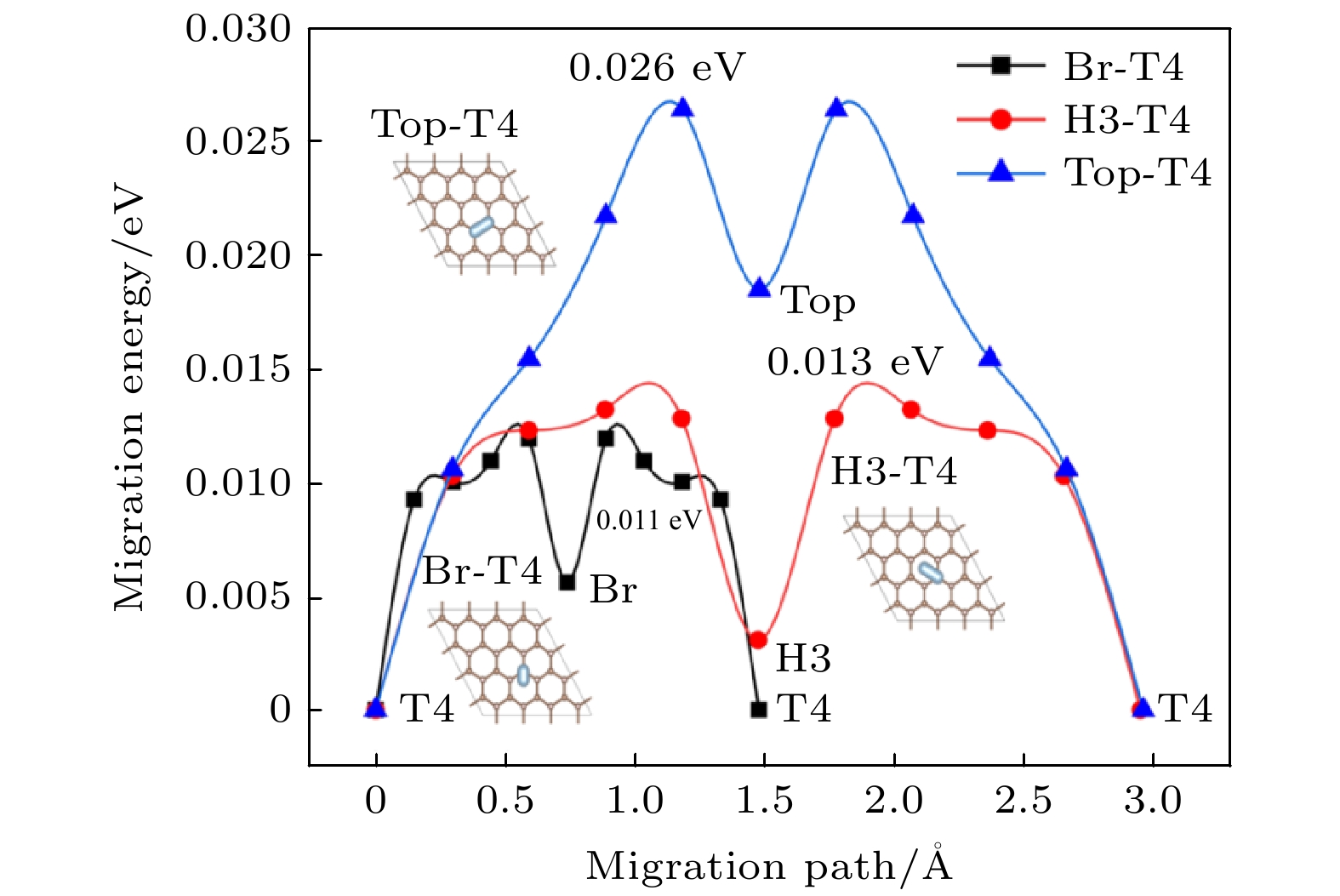
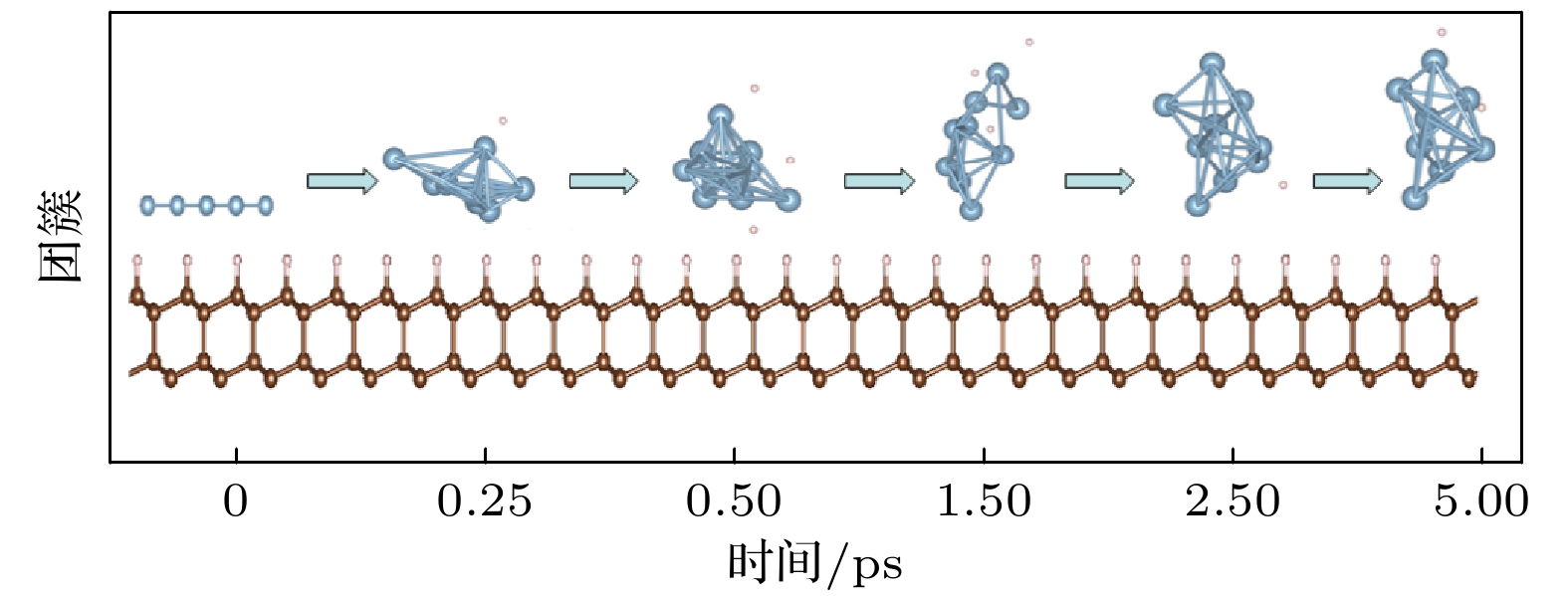
The simple and convenient metallic mask method is a significant method of preparing diamond nanostructures. The metallic mask method has poor repeatability and can not give the ideal results, because it is supported by no theory about formation of surface mental nanoparticles and its technological parameters are optimized by no experimental techniques that are expensive either. Aiming at the formation and performance of the diamond/Al interface, this paper adopts the first-principles to study the adsorption and migration behavior of Al atoms on the H-terminated diamond surface and the structure of the diamond/Al interface. The results show that the highest adsorption energy is at the T4 position, which is only 0.181 eV, through comparing the adsorption energies of Al atoms at the highly symmetrical positions (Top, Br, H3 and T4) on the surface of the H-terminated diamond (111). The adsorption energies at these different positions are similar and the maximum difference is only 0.019 eV. There is formed no chemical bond, although Al has partial charge transfer on the H-terminated surface through the analysis of differential charge density and worse layout distribution. This phenomenon can be considered as electrostatic adsorption. That is to say, the adsorption of Al atoms are physical adsorption. The smooth potential energy surface also makes it easier for Al atoms to migrate on the diamond surface. The calculation results reveal that the migration activation energies of the two possible migration paths (from T4 position to Br position and from T4 to Top position) are 0.011 eV and 0.026 eV respectively. The above results imply that the metal Al and diamond are mainly connected by weak force, so the adhesion work of the three diamond/Al interface structures is compared based on the geometric stacking structure. The results show that the adhesion work of the three interfaces is around 0. These results indicate that the stability of the diamond/Al interface is not high and the stable structure of the interface is easily destroyed when the external environment changes. This speculation can be confirmed in molecular dynamics. When the simulated temperature is 300 ℃, the liquefied metal Al obviously accumulates into spheres. According to the above research results, we deduce that the metallic mask method does not require high requirements for the relationship between the metal and the substrate material, which depends mainly on the surface topography of the base material. This research provides an important theoretical reference for understanding the formation mechanism of metal nanomasks.
-
Keywords:
- diamond/Al formation mechanism /
- adsorptivity /
- activation energy /
- first-principles
[1] Zhou Y, Zhi J, Zou Y 2008 Anal. Chem. 80 4141
 Google Scholar
Google Scholar
[2] Gu H, Su X D 2006 J. Phys. Chem. B 109 3611
 Google Scholar
Google Scholar
[3] Smirnov W, Kriele A, Yang N 2010 Diamond Relat. Mater. 19 186
 Google Scholar
Google Scholar
[4] Loginov P A, Zhassay U A, Bychkova M Y 2020 Int. J. Refract. Met. Hard Mater. 92 105289
 Google Scholar
Google Scholar
[5] 陆延青, 肖敏, 彭茹雯 2020 中国基础科学 22 11
 Google Scholar
Google Scholar
Lu Y Q, Xiao M, Peng R W 2020 China Basic. Sci. 22 11
 Google Scholar
Google Scholar
[6] Yu Y, Wu L, Zhi J 2015 Angew. Chem. 46 14326
 Google Scholar
Google Scholar
[7] Zhang J, Cao J X, Chen X 2015 Phys. Rev. B 91 045417
 Google Scholar
Google Scholar
[8] Liao M, Hishita S, Watanabe E 2010 Adv. Mater. 22 47
 Google Scholar
Google Scholar
[9] Masuda H, Yanagishita T, Yasui K 2001 Adv. Mater. 13 247
 Google Scholar
Google Scholar
[10] Yang N, Uetsuka H, Williams O A 2009 Phys. Status Solidi R 206 9
 Google Scholar
Google Scholar
[11] Okuyama S, Matsushita S I, Fujishima A 2002 Langmuir 18 8282
 Google Scholar
Google Scholar
[12] Scholze A, Schmidt W G, Kgckell P 1996 Mater. Sci. Eng., C 37 158
 Google Scholar
Google Scholar
[13] Stampfl C, Derry T E, Makau N W 2010 J. Phys. Condens. Matter 22 475005
 Google Scholar
Google Scholar
[14] Larsson K, Lunell S 1997 J. Phys. Chem. A 101 76
 Google Scholar
Google Scholar
[15] Nie J L, Xiao H Y, Zu X T 2006 Chem. Phys. 326 308
 Google Scholar
Google Scholar
[16] Hong X Y, Xu L, Gu C 2007 Appl. Surf. Sci. 253 4260
 Google Scholar
Google Scholar
[17] 于洋 2004 硕士学位论文 (长春: 吉林大学)
Yu Y 2004 M. S. Thesis (Changchun: Jilin University) (in Chinese)
[18] 徐力方, 顾长志, 于洋 2004 53 2710
 Google Scholar
Google Scholar
Xu L F, Gu C Z, Yu Y 2004 Acta Phys. Sin. 53 2710
 Google Scholar
Google Scholar
[19] 刘峰斌, 金秀婷, 张畅 2020 有色金属工程 10 21
 Google Scholar
Google Scholar
Liu F B, Jin X T, Zhang C 2020 Nonferrous Metal Engineering. 10 21
 Google Scholar
Google Scholar
[20] Kresse G, Furthmüller J B 1996 Comput. Mater. Sci. 6 15
 Google Scholar
Google Scholar
[21] Blöchl P E 1994 Phys. Rev. B 50 17953
 Google Scholar
Google Scholar
[22] Perdew J P, Burke K 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[23] Payne M C, Teter M P, Allan D C, Arias T A, Joannopoulos J D 1992 Rev. Mod. Phys. 64 1045
 Google Scholar
Google Scholar
[24] Methfessel M, Paxton A T 1989 Phys. Rev. B 40 3616
 Google Scholar
Google Scholar
[25] Hendrik J, Monkhorst H J, James D P 1976 Phys. Rev. B 13 5188
 Google Scholar
Google Scholar
[26] Maniopoulou A, Davidson E 2012 Comput. Phys. Commun. 183 1696
 Google Scholar
Google Scholar
[27] 刘峰斌, 汪家道, 陈大融 2010 59 6556
 Google Scholar
Google Scholar
Liu B F, Wang J D, Chen D R 2010 Acta Phys. Sin. 59 6556
 Google Scholar
Google Scholar
[28] Kawarada H. 1996 Surf. Sci. Rep. 26 205
 Google Scholar
Google Scholar
[29] Siegel D J, Hector L G, Adams J B 2002 Acta Mater. 50 619
 Google Scholar
Google Scholar
[30] Taubin G 1991 IEEE. Trans. Pattern Anal. 13 1115
 Google Scholar
Google Scholar
[31] Almtoft K P, Ejsing A M, Bøttiger J 2007 J. Mater. Res. 22 1018
 Google Scholar
Google Scholar
[32] Voigt W 1966 Lehrbuch der Kristallphysik (Vol. 1) (Germany: Springer Fachmedien Wiesbaden Gmbh) p3
[33] Reuss A 1929 Appl. Math. Mech. 9 49
 Google Scholar
Google Scholar
[34] Hill R 1952 Proc. Phys. Soc. 65 349
 Google Scholar
Google Scholar
[35] 基泰尔 1979 固体物理导论(北京: 科学出版社) 第341页
Kittel C 1979 Introduction to Solid State Physics (Beijing: Science Press) p341 (in Chinese)
-
图 1 H终止金刚石(111)表面模型, 其中棕色球表示C原子, 白色小球表示表面H原子 (a)模型原子分布的立体结构示意图; (b)金刚石(111)表面格点位置示意图
Fig. 1. H-Ter diamond (111) surface model: (a) Three-dimensional structure diagram of model atom distribution; (b) location of diamond (111) surface grid points. Brown spheres represent C atoms, white spheres represent surface H atoms.
图 3 Al原子在Br (a), H3 (b), Top (c), T4 (d)吸附位置的电荷密度分布图, 图中银白色球为Al原子, 颜色从蓝到红表示电荷密度从高到低
Fig. 3. Charge density distribution of single Al atom adsorbed on Br (a), H3 (b), Top (c), and T4 (d) sites, respectively. The big silver ball in the picture is Al atom. The color from blue to red indicates the charge density from high to low.
表 1 金刚石弹性常数及弹性模量
Table 1. Elastic constants and modulus of diamond.
a/Å C11/GPa C12/GPa C44/GPa B/GPa E/GPa G/GPa Text value 3.523 10.311 1.104 5.464 668.621 102.606 34.821 Experimental value [35] 3.567 10.764 1.252 5.961 708.082 99.324 33.601 表 2 单个Al原子的吸附能
Table 2. Adsorption energies of a single Al atom.
Top T4 H3 Br Etot /eV –892.761 –892.779 –892.776 –892.775 Ead/eV 0.162 0.181 0.176 0.177 $ {E}_{\mathrm{a}\mathrm{d}}^{{*}} $/eV 0.145 0.162 0.159 0.157 表 3 不同吸附结构中Al原子及其周边原子电荷转移量
Table 3. Amount of charge transfer of Al atom and its surrounding atoms in different adsorption structures.
site Element C1/e C2/e H1/e H2/e Al/e Br –0.022 –0.029 0.045 0.045 –0.117 H3 –0.025 –0.020 0.047 0.046 –0.138 T4 –0.026 –0.028 0.047 0.046 –0.137 Top –0.027 –0.024 0.023 0.072 –0.130 表 4 由吸附位置形成界面的黏附功
Table 4. Adhesion work of the interface formed by the adsorption position.
Top T4 H3 Wad/(J·m–2) 0.019 0.023 0.034 -
[1] Zhou Y, Zhi J, Zou Y 2008 Anal. Chem. 80 4141
 Google Scholar
Google Scholar
[2] Gu H, Su X D 2006 J. Phys. Chem. B 109 3611
 Google Scholar
Google Scholar
[3] Smirnov W, Kriele A, Yang N 2010 Diamond Relat. Mater. 19 186
 Google Scholar
Google Scholar
[4] Loginov P A, Zhassay U A, Bychkova M Y 2020 Int. J. Refract. Met. Hard Mater. 92 105289
 Google Scholar
Google Scholar
[5] 陆延青, 肖敏, 彭茹雯 2020 中国基础科学 22 11
 Google Scholar
Google Scholar
Lu Y Q, Xiao M, Peng R W 2020 China Basic. Sci. 22 11
 Google Scholar
Google Scholar
[6] Yu Y, Wu L, Zhi J 2015 Angew. Chem. 46 14326
 Google Scholar
Google Scholar
[7] Zhang J, Cao J X, Chen X 2015 Phys. Rev. B 91 045417
 Google Scholar
Google Scholar
[8] Liao M, Hishita S, Watanabe E 2010 Adv. Mater. 22 47
 Google Scholar
Google Scholar
[9] Masuda H, Yanagishita T, Yasui K 2001 Adv. Mater. 13 247
 Google Scholar
Google Scholar
[10] Yang N, Uetsuka H, Williams O A 2009 Phys. Status Solidi R 206 9
 Google Scholar
Google Scholar
[11] Okuyama S, Matsushita S I, Fujishima A 2002 Langmuir 18 8282
 Google Scholar
Google Scholar
[12] Scholze A, Schmidt W G, Kgckell P 1996 Mater. Sci. Eng., C 37 158
 Google Scholar
Google Scholar
[13] Stampfl C, Derry T E, Makau N W 2010 J. Phys. Condens. Matter 22 475005
 Google Scholar
Google Scholar
[14] Larsson K, Lunell S 1997 J. Phys. Chem. A 101 76
 Google Scholar
Google Scholar
[15] Nie J L, Xiao H Y, Zu X T 2006 Chem. Phys. 326 308
 Google Scholar
Google Scholar
[16] Hong X Y, Xu L, Gu C 2007 Appl. Surf. Sci. 253 4260
 Google Scholar
Google Scholar
[17] 于洋 2004 硕士学位论文 (长春: 吉林大学)
Yu Y 2004 M. S. Thesis (Changchun: Jilin University) (in Chinese)
[18] 徐力方, 顾长志, 于洋 2004 53 2710
 Google Scholar
Google Scholar
Xu L F, Gu C Z, Yu Y 2004 Acta Phys. Sin. 53 2710
 Google Scholar
Google Scholar
[19] 刘峰斌, 金秀婷, 张畅 2020 有色金属工程 10 21
 Google Scholar
Google Scholar
Liu F B, Jin X T, Zhang C 2020 Nonferrous Metal Engineering. 10 21
 Google Scholar
Google Scholar
[20] Kresse G, Furthmüller J B 1996 Comput. Mater. Sci. 6 15
 Google Scholar
Google Scholar
[21] Blöchl P E 1994 Phys. Rev. B 50 17953
 Google Scholar
Google Scholar
[22] Perdew J P, Burke K 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[23] Payne M C, Teter M P, Allan D C, Arias T A, Joannopoulos J D 1992 Rev. Mod. Phys. 64 1045
 Google Scholar
Google Scholar
[24] Methfessel M, Paxton A T 1989 Phys. Rev. B 40 3616
 Google Scholar
Google Scholar
[25] Hendrik J, Monkhorst H J, James D P 1976 Phys. Rev. B 13 5188
 Google Scholar
Google Scholar
[26] Maniopoulou A, Davidson E 2012 Comput. Phys. Commun. 183 1696
 Google Scholar
Google Scholar
[27] 刘峰斌, 汪家道, 陈大融 2010 59 6556
 Google Scholar
Google Scholar
Liu B F, Wang J D, Chen D R 2010 Acta Phys. Sin. 59 6556
 Google Scholar
Google Scholar
[28] Kawarada H. 1996 Surf. Sci. Rep. 26 205
 Google Scholar
Google Scholar
[29] Siegel D J, Hector L G, Adams J B 2002 Acta Mater. 50 619
 Google Scholar
Google Scholar
[30] Taubin G 1991 IEEE. Trans. Pattern Anal. 13 1115
 Google Scholar
Google Scholar
[31] Almtoft K P, Ejsing A M, Bøttiger J 2007 J. Mater. Res. 22 1018
 Google Scholar
Google Scholar
[32] Voigt W 1966 Lehrbuch der Kristallphysik (Vol. 1) (Germany: Springer Fachmedien Wiesbaden Gmbh) p3
[33] Reuss A 1929 Appl. Math. Mech. 9 49
 Google Scholar
Google Scholar
[34] Hill R 1952 Proc. Phys. Soc. 65 349
 Google Scholar
Google Scholar
[35] 基泰尔 1979 固体物理导论(北京: 科学出版社) 第341页
Kittel C 1979 Introduction to Solid State Physics (Beijing: Science Press) p341 (in Chinese)
计量
- 文章访问数: 9460
- PDF下载量: 237
- 被引次数: 0














 下载:
下载: