-
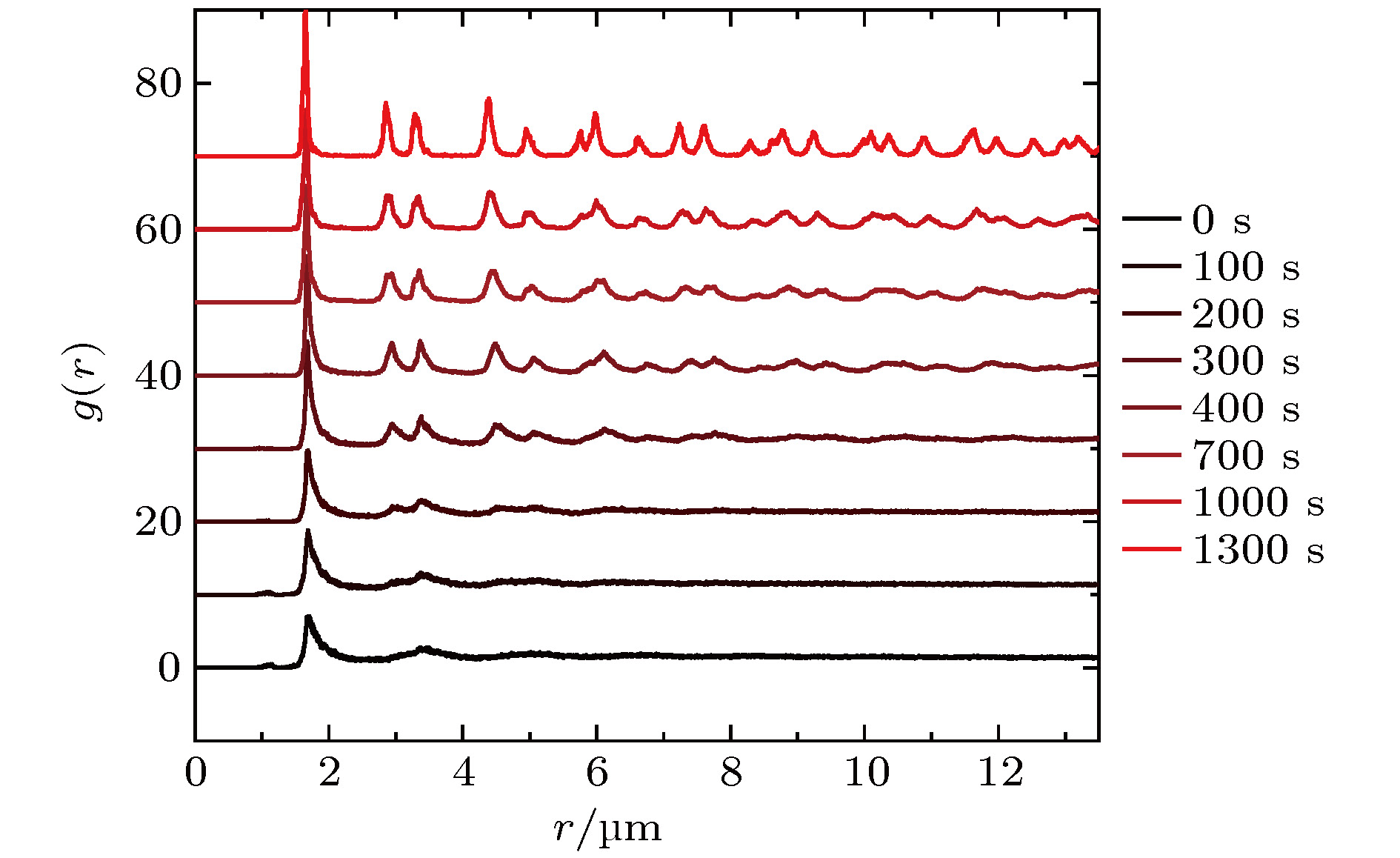
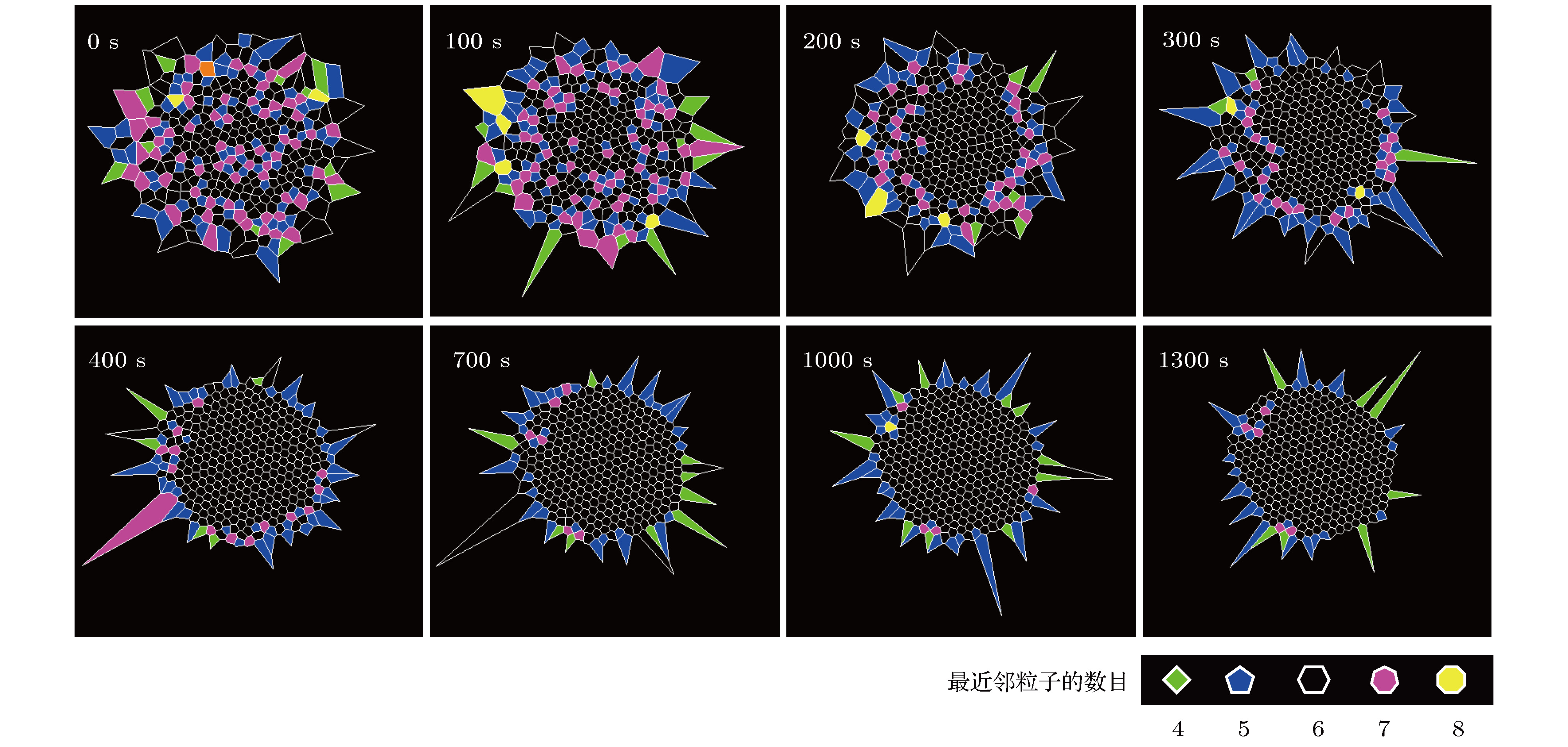
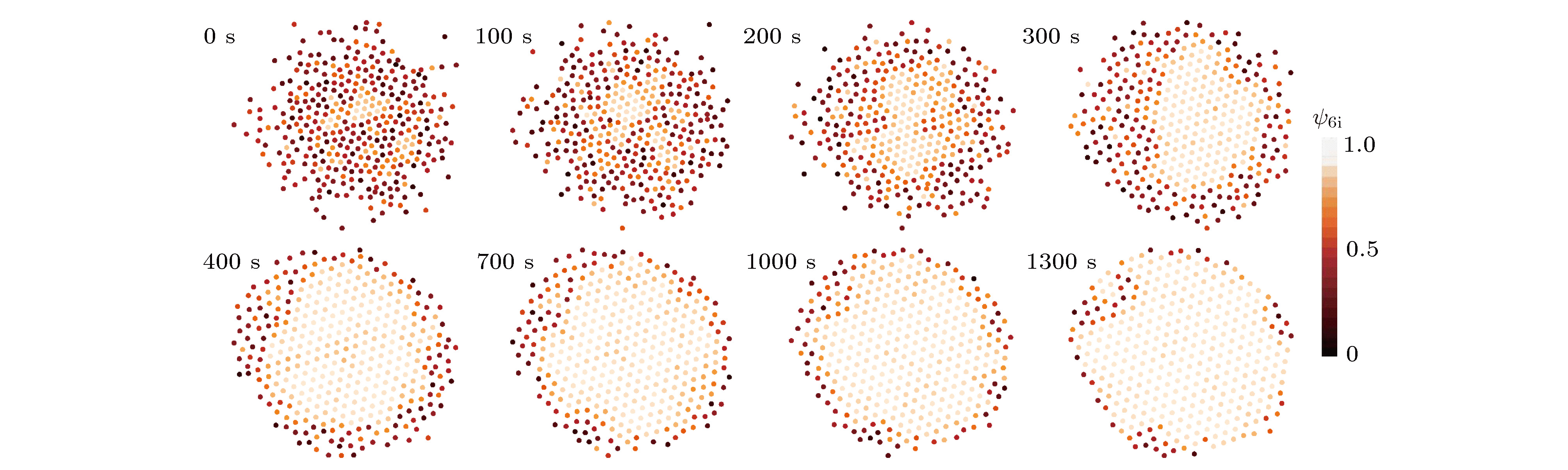
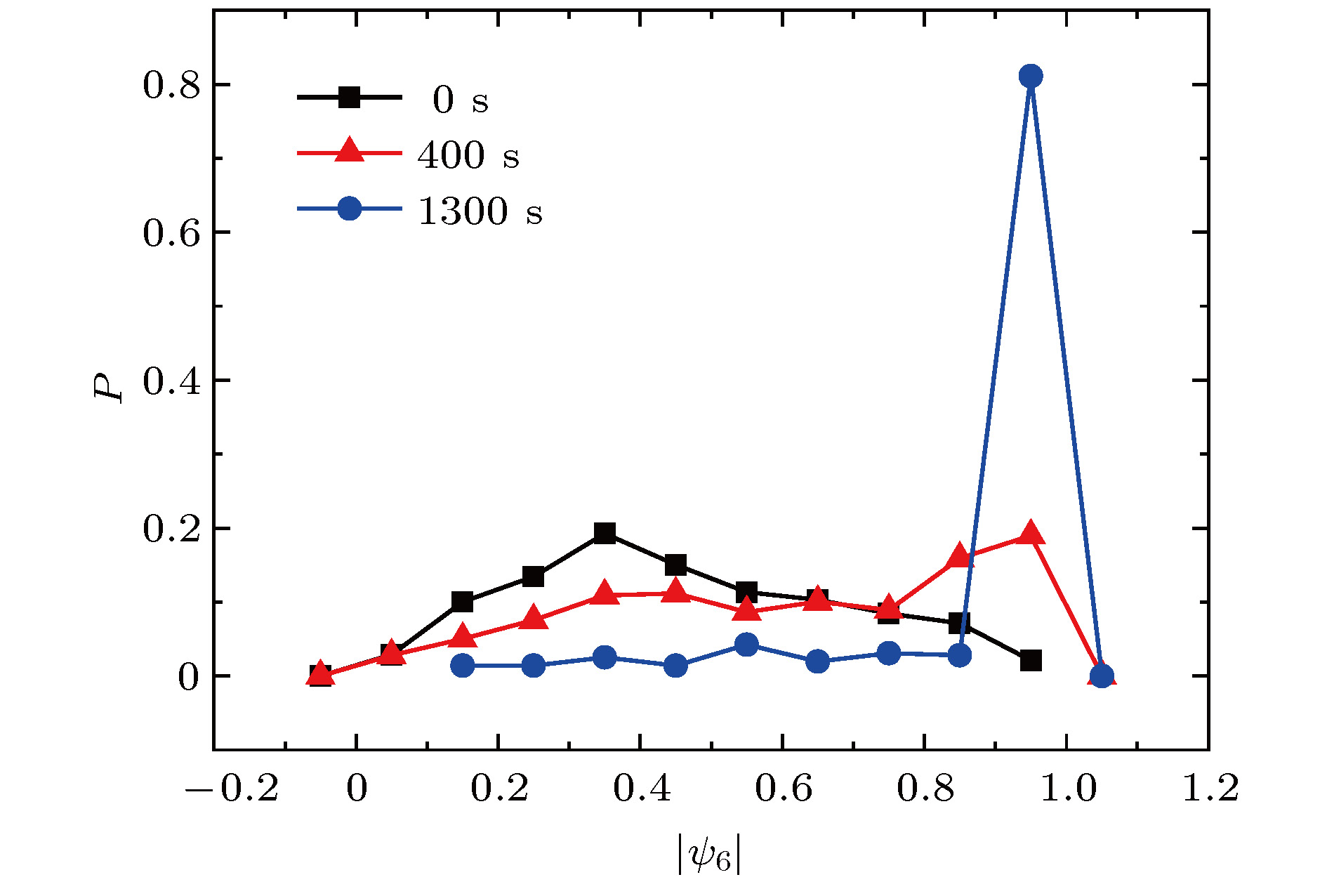
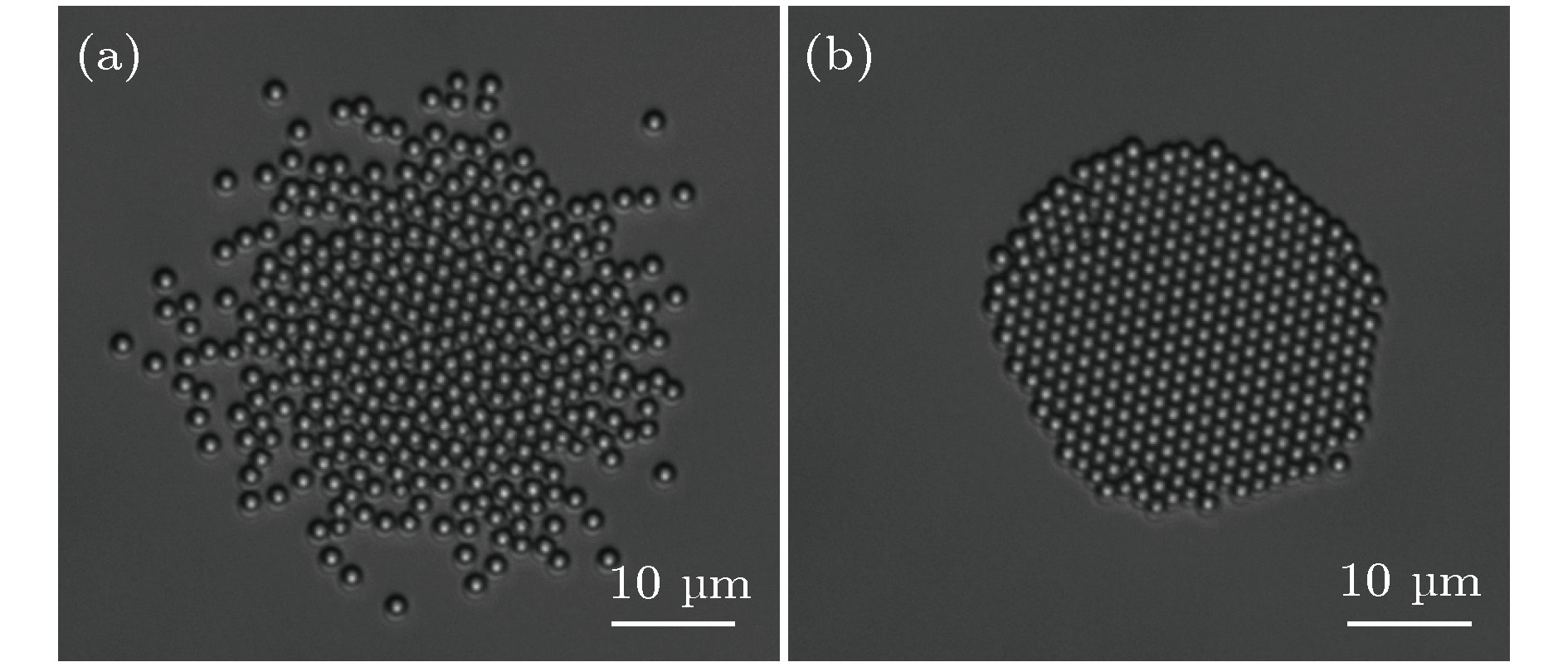
从工业上的大尺寸晶体生长到实验室中受限小体系的结晶, 结晶是普遍存在的物理现象, 也一直是物理学中的重要研究课题. 与大尺度结晶相变的研究相比, 对于有限小尺度体系结晶过程的研究相对较少. 本文通过设计具有吸引相互作用的胶体体系, 在实验上研究了有限小尺寸胶体体系的二维结晶相变. 通过计算和分析径向分布函数、泰森多边形以及取向序参量, 发现有限小尺寸体系的结晶过程是从中央高密度区域开始, 随着结晶的进行, 周围液相减小而晶相增加, 最后完全转变为晶态的过程. 体系结晶速率呈现两个阶段: 在结晶初期中央区域是高密度的亚稳态液体, 会降低结晶自由能能垒, 使得体系快速结晶; 随后晶相长大, 亚稳态液体消失, 体系结晶速率变慢. 此外, 通过统计有序度参量的分布发现: 在结晶过程中, 序参量出现双峰分布, 分别对应液相和晶相, 与大尺度胶体体系的二维结晶行为一致, 说明序参量分布的变化规律是二维结晶相变的重要特征.The nature of crystallization is considered to be one of the most fundamental research problems in condensed matter physics. With single particle resolution offered by video microscopy, colloidal suspensions provide a novel model system for studying crystallization, melting and other phase transitions, where the structures and dynamics of the particles during the transitions can be quantitatively probed. Traditional systems for studying the crystallization typically focus on the infinitely large systems in order to obtain the equilibrium state. However, studies of the crystallization in finite-sized systems such as crystallization in thin films and porous media, are rare despite the fact that they are the common phenomena in natural world. In this paper, we experimentally investigate the crystallization in a finite-sized colloidal system with attractive interactions. The colloidal suspension is composed of polystyrene microspheres dispersed in a mixture of water and 2, 6-lutidine, in which the interaction between the particles can be tuned by adjusting the temperature. We increase the temperature to 34 °C to induce attractions between the particles and thus producing a cluster, and then reduce the temperature to 33 °C to tune off the attractions. Thus we obtain a finite-sized liquid cluster of the colloidal particles. Crystallization is triggered by increasing the temperature to 34 °C. The crystallization process is recorded by video microscopy and the video data are analyzed by a standard particle tracking algorithm. Through the analysis of radial distribution function, Voronoi diagram, and local order parameter, we find that the crystallization of the finite colloidal system starts from the central dense region of the liquid cluster. This leads to a crystalline phase in the center and a liquid phase on the edge of the cluster. As time elapses, the central crystalline region grows while outer liquid region shrinks. The crystallization process exhibits a two-step scenario: a fast crystallization initially and a slow crystallization at the later stage. At the initial stage, the center of the system forms a dense metastable liquid phase, which lowers the free-energy barrier of crystallization and results in a fast crystallization. As the crystalline region grows, the metastable phase disappears, and thus the crystallization rate decreases. Moreover, a bimodal distribution of the orientational order parameter is observed during the crystallization in our finite-sized colloidal system, which is consistent with that in a large system. This indicates that the bimodal distribution is a common feature of the two-dimensional crystallization.
-
Keywords:
- colloids /
- crystallization /
- structure /
- phase transition
[1] Löwen H 1994 Phys. Rep. 237 249
 Google Scholar
Google Scholar
[2] Kosterlitz J M, Thouless D J 1973 J. Phys. C 6 1181
 Google Scholar
Google Scholar
[3] Nelson D R, Halperin B 1979 Phys. Rev. B 19 2457
 Google Scholar
Google Scholar
[4] Young A 1979 Phys. Rev. B 19 1855
 Google Scholar
Google Scholar
[5] Gasser U 2009 J. Phys.: Condes. Matter 21 203101
 Google Scholar
Google Scholar
[6] Dinsmore A D, Crocker J C, Yodh A G 1998 Curr. Opin. Colloid Interface Sci. 3 5
 Google Scholar
Google Scholar
[7] Gast A P, Russel W B 1998 Phys. Today 51 24
 Google Scholar
Google Scholar
[8] Pieranski P 1983 Contemp. Phys. 24 25
 Google Scholar
Google Scholar
[9] Gasser U, Weeks E R, Schofield A, Pusey P, Weitz D 2001 Science 292 258
 Google Scholar
Google Scholar
[10] Kegel W K, van Blaaderen A 2000 Science 287 290
 Google Scholar
Google Scholar
[11] Wu Y L, Derks D, van Blaaderen A, Imhof A 2009 Proc. Natl. Acad. Sci. U.S.A. 106 10564
 Google Scholar
Google Scholar
[12] Hynninen A P, Thijssen J H, Vermolen E C, Dijkstra M, van Blaaderen A 2007 Nat. Mater. 6 202
 Google Scholar
Google Scholar
[13] Wang Z, Alsayed A M, Yodh A G, Han Y 2010 J. Chem. Phys. 132 154501
 Google Scholar
Google Scholar
[14] Wang Z, Qi W, Peng Y, Alsayed A M, Chen Y, Tong P, Han Y 2011 J. Chem. Phys. 134 034506
 Google Scholar
Google Scholar
[15] Savage J, Blair D, Levine A, Guyer R, Dinsmore A 2006 Science 314 795
 Google Scholar
Google Scholar
[16] Savage J, Dinsmore A 2009 Phys. Rev. Lett. 102 198302
 Google Scholar
Google Scholar
[17] Moore L J, Dear R D, Summers M D, Dullens R P, Ritchie G A 2010 Nano Lett. 10 4266
 Google Scholar
Google Scholar
[18] Tanaka S, Oki Y, Kimura Y 2014 Phys. Rev. E 89 052305
 Google Scholar
Google Scholar
[19] Bonn D, Otwinowski J, Sacanna S, Guo H, Wegdam G, Schall P 2009 Phys. Rev. Lett. 103 156101
 Google Scholar
Google Scholar
[20] Zhang Z, Yunker P J, Habdas P, Yodh A G 2011 Phys. Rev. Lett. 107 208303
 Google Scholar
Google Scholar
[21] Xiao Y S, Yang L, Tian H Z, Yu Q M, Zhang Z X 2013 Langmuir 29 7216
 Google Scholar
Google Scholar
[22] 王华光, 张泽新 2016 65 219
Wang H G, Zhang Z Z 2016 Acta Phys. Sin. 65 219
[23] Zhang Z, Xu N, Chen D T N, Yunker P, Alsayed A M, Aptowicz K B, Habdas P, Liu A J, Nagel S R, Yodh A G 2009 Nature 459 230
 Google Scholar
Google Scholar
[24] Ten Wolde P R, Frenkel D 1997 Science 277 1975
 Google Scholar
Google Scholar
[25] Zhang K, Liu X 2004 Nature 429 739
 Google Scholar
Google Scholar
[26] Lutsko J F, Nicolis G 2006 Phys. Rev. Lett. 96 046102
 Google Scholar
Google Scholar
[27] Han Y, Ha N, Alsayed A, Yodh A 2008 Phys. Rev. E 77 041406
-
-
[1] Löwen H 1994 Phys. Rep. 237 249
 Google Scholar
Google Scholar
[2] Kosterlitz J M, Thouless D J 1973 J. Phys. C 6 1181
 Google Scholar
Google Scholar
[3] Nelson D R, Halperin B 1979 Phys. Rev. B 19 2457
 Google Scholar
Google Scholar
[4] Young A 1979 Phys. Rev. B 19 1855
 Google Scholar
Google Scholar
[5] Gasser U 2009 J. Phys.: Condes. Matter 21 203101
 Google Scholar
Google Scholar
[6] Dinsmore A D, Crocker J C, Yodh A G 1998 Curr. Opin. Colloid Interface Sci. 3 5
 Google Scholar
Google Scholar
[7] Gast A P, Russel W B 1998 Phys. Today 51 24
 Google Scholar
Google Scholar
[8] Pieranski P 1983 Contemp. Phys. 24 25
 Google Scholar
Google Scholar
[9] Gasser U, Weeks E R, Schofield A, Pusey P, Weitz D 2001 Science 292 258
 Google Scholar
Google Scholar
[10] Kegel W K, van Blaaderen A 2000 Science 287 290
 Google Scholar
Google Scholar
[11] Wu Y L, Derks D, van Blaaderen A, Imhof A 2009 Proc. Natl. Acad. Sci. U.S.A. 106 10564
 Google Scholar
Google Scholar
[12] Hynninen A P, Thijssen J H, Vermolen E C, Dijkstra M, van Blaaderen A 2007 Nat. Mater. 6 202
 Google Scholar
Google Scholar
[13] Wang Z, Alsayed A M, Yodh A G, Han Y 2010 J. Chem. Phys. 132 154501
 Google Scholar
Google Scholar
[14] Wang Z, Qi W, Peng Y, Alsayed A M, Chen Y, Tong P, Han Y 2011 J. Chem. Phys. 134 034506
 Google Scholar
Google Scholar
[15] Savage J, Blair D, Levine A, Guyer R, Dinsmore A 2006 Science 314 795
 Google Scholar
Google Scholar
[16] Savage J, Dinsmore A 2009 Phys. Rev. Lett. 102 198302
 Google Scholar
Google Scholar
[17] Moore L J, Dear R D, Summers M D, Dullens R P, Ritchie G A 2010 Nano Lett. 10 4266
 Google Scholar
Google Scholar
[18] Tanaka S, Oki Y, Kimura Y 2014 Phys. Rev. E 89 052305
 Google Scholar
Google Scholar
[19] Bonn D, Otwinowski J, Sacanna S, Guo H, Wegdam G, Schall P 2009 Phys. Rev. Lett. 103 156101
 Google Scholar
Google Scholar
[20] Zhang Z, Yunker P J, Habdas P, Yodh A G 2011 Phys. Rev. Lett. 107 208303
 Google Scholar
Google Scholar
[21] Xiao Y S, Yang L, Tian H Z, Yu Q M, Zhang Z X 2013 Langmuir 29 7216
 Google Scholar
Google Scholar
[22] 王华光, 张泽新 2016 65 219
Wang H G, Zhang Z Z 2016 Acta Phys. Sin. 65 219
[23] Zhang Z, Xu N, Chen D T N, Yunker P, Alsayed A M, Aptowicz K B, Habdas P, Liu A J, Nagel S R, Yodh A G 2009 Nature 459 230
 Google Scholar
Google Scholar
[24] Ten Wolde P R, Frenkel D 1997 Science 277 1975
 Google Scholar
Google Scholar
[25] Zhang K, Liu X 2004 Nature 429 739
 Google Scholar
Google Scholar
[26] Lutsko J F, Nicolis G 2006 Phys. Rev. Lett. 96 046102
 Google Scholar
Google Scholar
[27] Han Y, Ha N, Alsayed A, Yodh A 2008 Phys. Rev. E 77 041406
计量
- 文章访问数: 10236
- PDF下载量: 112
- 被引次数: 0













 下载:
下载: