-
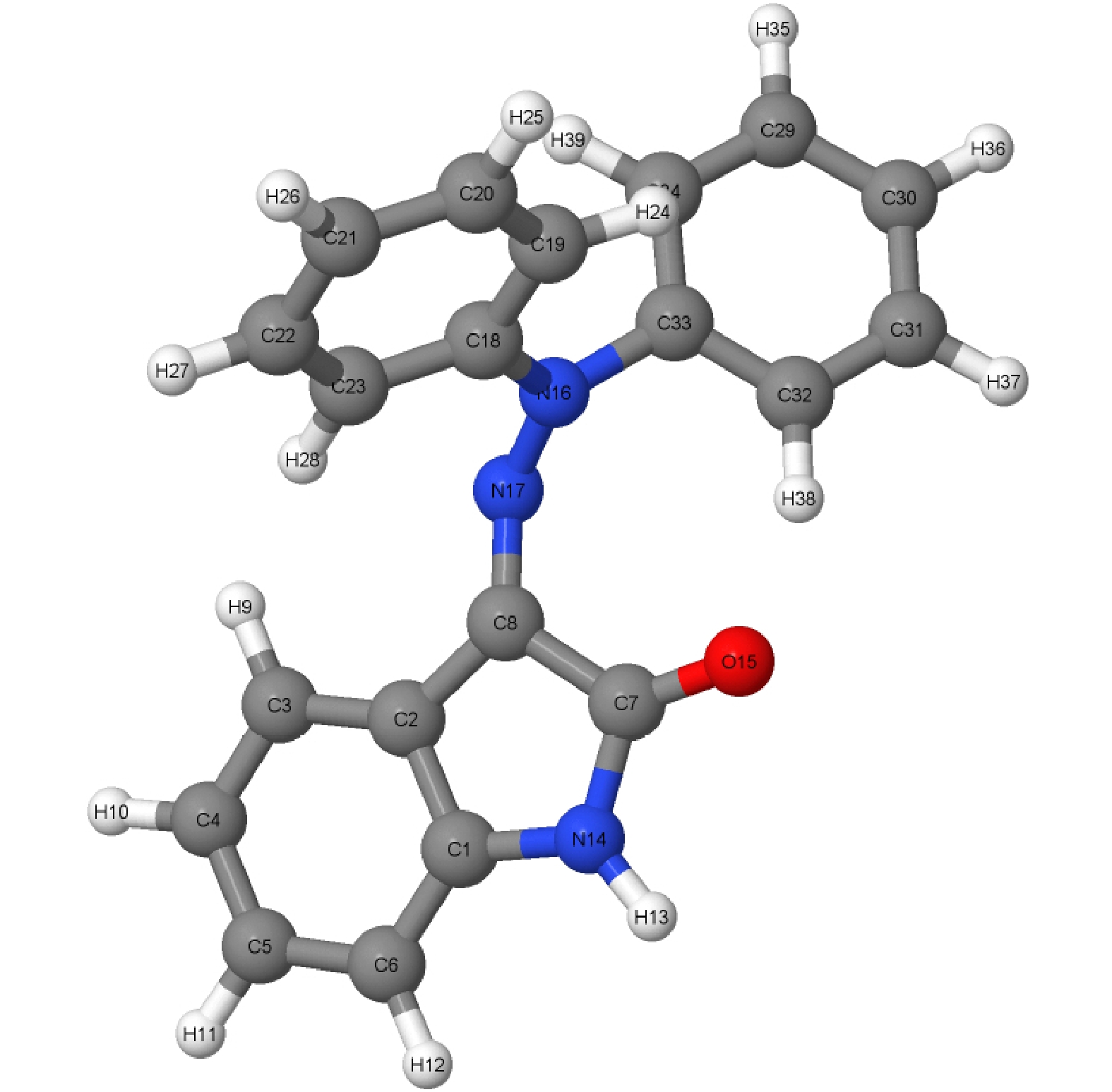
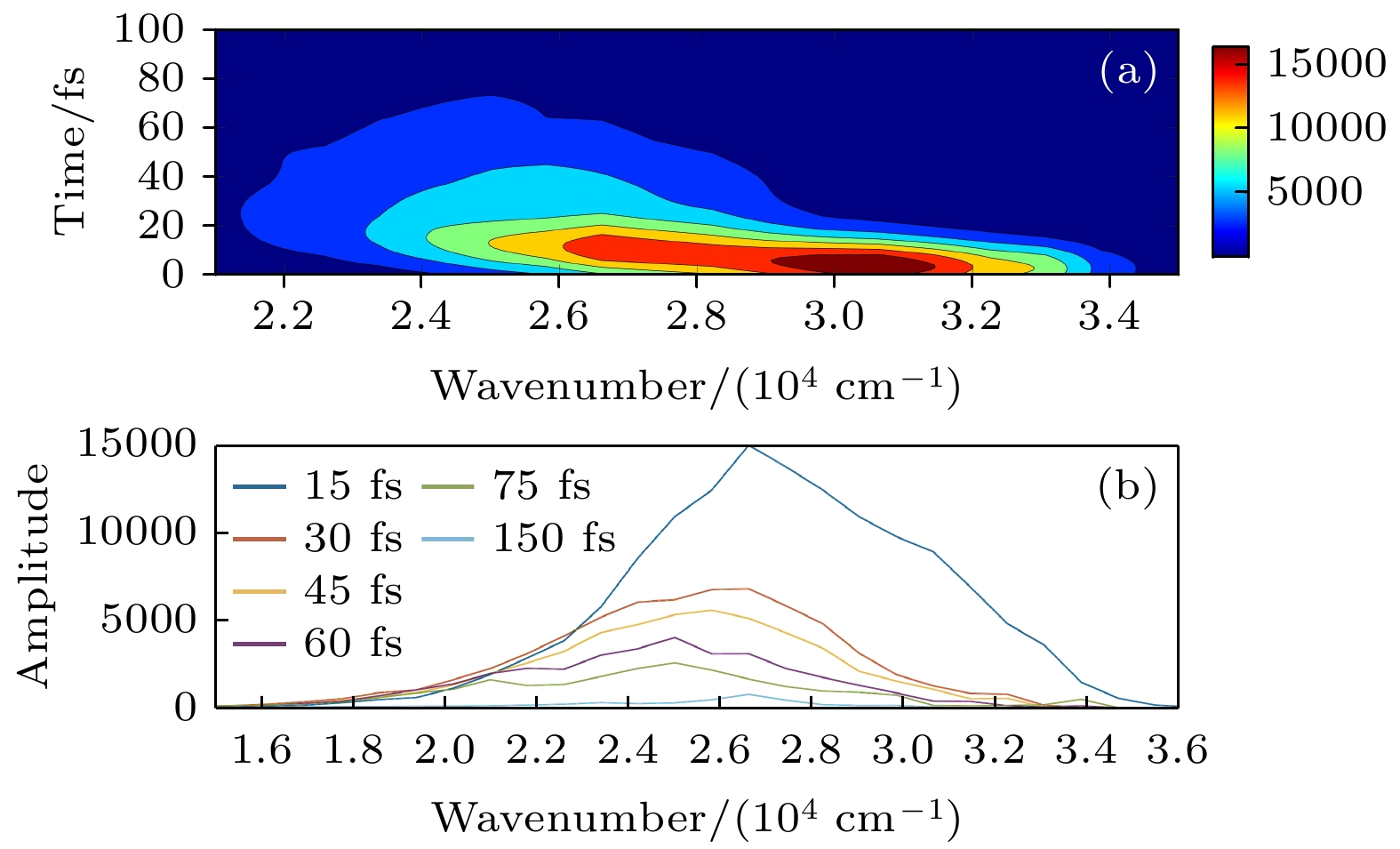
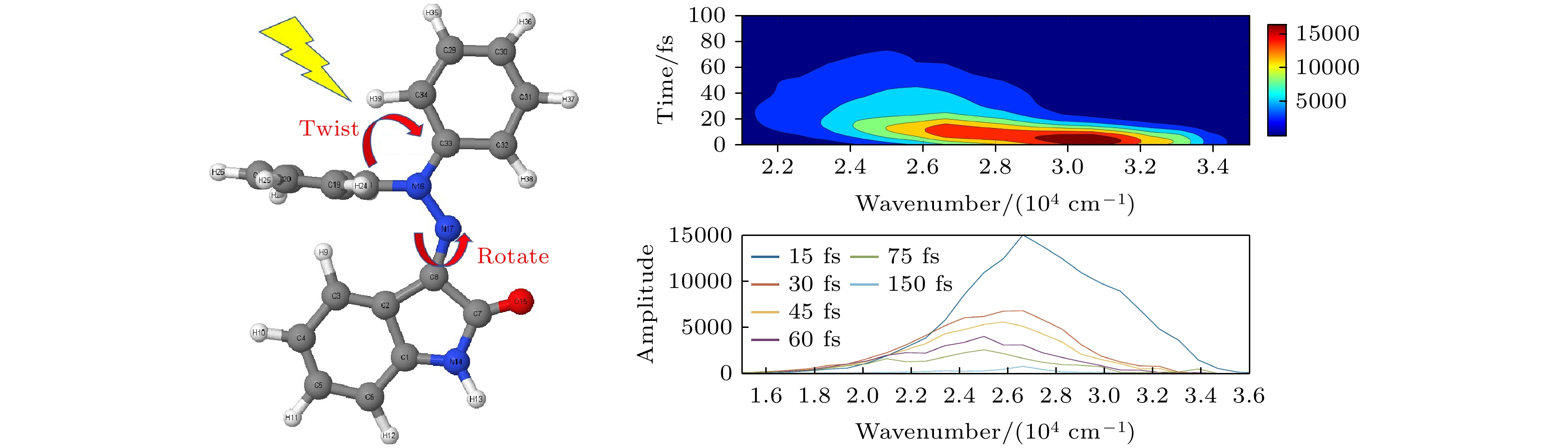
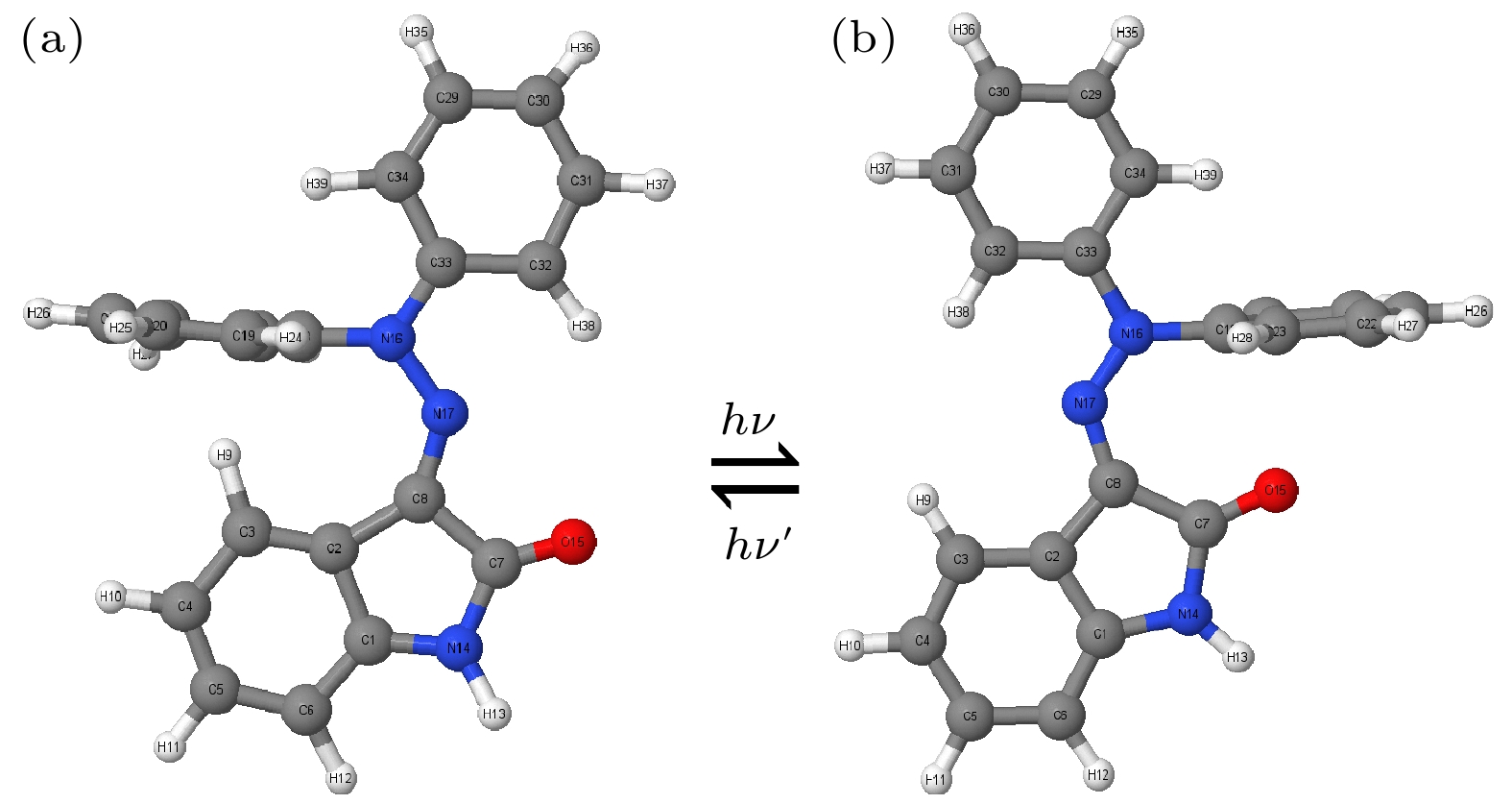
Hydrazone molecular switches have significant application value in supramolecular chemistry. A new type of hydrazone molecular switch, named isatin N2-diphenylhydrazone, has been synthesized. Owing to its cis-trans isomerization characteristics under visible light excitation, ease of synthesizing of derivatives, and sensitivity to external stimuli, it has important application value in the field of biochemistry. Because of its forward and backward visible light excitation characteristics, it is considered a class of compound that is very suitable for molecular switches, and it has a wide application value in fields such as biotechnology. In addition, the derivatives compound exhibits strong interactions with negative ions, which enhances its function as a molecular switch, making it a four-state molecular switch that can be achieved by a single molecule. However, the photo-induced isomerization mechanism of these new molecular switches is not yet clear, and whether there are novel phenomena in the isomerization process is also unknown. In this work, a semi empirical OM2/MRCI based trajectory surface hopping dynamics method is adopted to systematically study a photo induced isomerization mechanism based on the E-Z isomerization process of the isatin N2-diphenylhydrazones molecular switch. Optimization configuration and the average lifetime of the first excited S1 state are obtained by using the semi-empirical OM2/MRCI method of molecular switch. It is found that the average lifetime of the S1 excited state of the E-configuration molecular switch is about 107 fs, and the quantum yield of E-Z isomerization of the molecular switch is 16.01%. By calculating the photo induced isomerization process of the molecular switch, two different isomerization mechanisms of the molecular switch are identified. In addition to the traditional molecular switch isomerization mechanism revolving around the C=N bond, a new isomerization mechanism, i.e. the face-to-face twisting of the molecular switch rotor part is elucidated. By calculating the time-resolved fluorescence radiation spectrum, it is predicted that there may be a very fast fluorescence quenching phenomenon occurring in about 75 fs in the isomerization process, slightly faster than the S1 average decay events (107 fs). The information about wavelength-resolved attenuation at different times is also calculated, which reflects the ultrafast fluorescence quenching process accompanied by fluorescence red shift, ranging from 2.1 × 104 cm–1 to 3.4 × 104 cm–1. By comparing the calculated fluorescence spectra with the average lifetime of excited states, the existence of “dark states” is proposed, and possible explanations for the existence of “dark states” are provided, and those “dark states” may be related to lower quantum yields. The research results can provide theoretical guidance for the design and application of new molecular switches. The ease of synthesis and sensitivity to external stimuli of its derivatives make those compounds extremely valuable in molecular switching and light measurement applications.
[1] Li R J, Mou B Z, Yamada M, Li W, Nakashima T, Kawai T 2024 Molecules 29 25
 Google Scholar
Google Scholar
[2] Jago D, Gaschk E E, Koutsantonis G A 2023 Aust. J. Chem. 76 635
 Google Scholar
Google Scholar
[3] Yu Z, Hecht S 2016 Chem. Commun. 52 6639
 Google Scholar
Google Scholar
[4] Feringa B L, Van Delden R A, Koumura N, Geertsema E M 2000 Chem. Rev. 100 1789
 Google Scholar
Google Scholar
[5] Rice A M, Martin C R, Galitskiy V A, Berseneva A A, Leith G A, Shustova N B 2020 Chem. Rev. 120 8790
 Google Scholar
Google Scholar
[6] Zhang X Y, Hou L L, Samorì P 2016 Nat. Commun. 7 11118
 Google Scholar
Google Scholar
[7] Alenazi M H, Mubarak A T, Abboud M 2024 Nanotechnol. Rev. 13 20240032
 Google Scholar
Google Scholar
[8] Goulet-Hanssens A, Eisenreich F, Hecht S 2020 Adv. Mater. 32 1905966
 Google Scholar
Google Scholar
[9] Pios S V, Gelin M F, Ullah A, Dral P O, Chen L 2024 J. Phys. Chem. Lett. 15 2325
 Google Scholar
Google Scholar
[10] Conti I, Cerullo G, Nenov A, Garavelli M 2020 J. Am. Chem. Soc. 142 16117
 Google Scholar
Google Scholar
[11] Towns A 2021 Phys. Sci. Rev. 6 477
 Google Scholar
Google Scholar
[12] Cheng H B, Zhang S, Bai E, Cao X, Wang J, Qi J, Liu J, Zhao J, Zhang L, Yoon J 2022 Adv. Mater. 34 2108289
 Google Scholar
Google Scholar
[13] Bertarelli C, Bianco A, Castagna R, Pariani G 2011 J. Photoch. Photobio. C 12 106
 Google Scholar
Google Scholar
[14] Bléger D, Hecht S 2015 Angew. Chem. Int. Ed. 54 11338
 Google Scholar
Google Scholar
[15] Shao B, Aprahamian I 2020 Chem 6 2162
 Google Scholar
Google Scholar
[16] Van Dijken D J, KovaříčEk P, Ihrig S P, Hecht S 2015 J. Am. Chem. Soc. 137 14982
 Google Scholar
Google Scholar
[17] Schnetz M, Meier J K, Rehwald C, Mertens C, Urbschat A, Tomat E, Akam E A, Baer P, Roos F C, Brüne B 2020 Cancers 12 530
 Google Scholar
Google Scholar
[18] Ferreira I P, Piló E D, Recio-Despaigne A A, Da Silva J G, Ramos J P, Marques L B, Prazeres P H, Takahashi J A, Souza-Fagundes E M, Rocha W 2016 Bioorg. Med. Chem. 24 2988
 Google Scholar
Google Scholar
[19] Vantomme G, Lehn J M 2014 Chem. Eur. J. 20 16188
 Google Scholar
Google Scholar
[20] Vantomme G, Lehn J M 2013 Angew. Chem. Int. Edit. 52 3940
 Google Scholar
Google Scholar
[21] Vantomme G, Jiang S M, Lehn J M 2015 J. Am. Chem. Soc. 137 3138
 Google Scholar
Google Scholar
[22] Su X, Aprahamian I 2014 Chem. Soc. Rev. 43 1963
 Google Scholar
Google Scholar
[23] Chaur M N, Collado D, Lehn J M 2011 Chem. Eur. J. 17 248
 Google Scholar
Google Scholar
[24] Vantomme G, Jiang S, Lehn J M 2014 J. Am. Chem. Soc. 136 9509
 Google Scholar
Google Scholar
[25] 刘同力, 黄从树, 王晶晶, 梁宇, 谢志鹏, 庄海燕, 李九龙, 朱绪飞 2024 精细化工 https://doi.org/10.13550/j.jxhg.20230989
Liu T L, Huang C S, Wang J J, Liang Y, Xie Z P, Zhuang H Y, Li J L, Zhu X F 2024 Fine. Chem https://doi.org/ 10.13550/ j.jxhg.20230989
[26] Siewertsen R, Neumann H, Buchheim-Stehn B, Herges R, Näther C, Renth F, Temps F 2009 J. Am. Chem. Soc. 131 15594
 Google Scholar
Google Scholar
[27] Beharry A A, Sadovski O, Woolley G A 2011 J. Am. Chem. Soc. 133 19684
 Google Scholar
Google Scholar
[28] Poloni C, Szymanski W, Hou L L, Browne W R, Feringa B L 2014 Chem. Eur. J. 20 946
 Google Scholar
Google Scholar
[29] Zhang Z W, Yang W X, Zhang J J 2023 Chem. Ind. Eng. Prog 42 4058 [张志伟, 杨伟鑫, 张隽佶 2023 化工进展 42 4058]
 Google Scholar
Google Scholar
Zhang Z W, Yang W X, Zhang J J 2023 Chem. Ind. Eng. Prog 42 4058
 Google Scholar
Google Scholar
[30] Ye H 2020 M. S. Thesie (Wuhan: Huazhong University of Science and Technology) [叶欢 2020 硕士学位论文(武汉: 华中科技大学)]
Ye H 2020 M. S. Thesie (Wuhan: Huazhong University of Science and Technology)
[31] Szymanski W, Beierle J M, Kistemaker H A, Velema W A, Feringa B L 2013 Chem. Rev. 113 6114
 Google Scholar
Google Scholar
[32] Cigán M, Gáplovsky M, Jakusová K, Donovalová J, Horváth M, Filo J, Gáplovsky A 2015 RSC Adv. 5 62449
 Google Scholar
Google Scholar
[33] Cigáň M, Jakusová K, Gáplovský M, Filo J, Donovalová J, Gáplovský A 2015 Photochem. Photobiol. Sci. 14 2064
 Google Scholar
Google Scholar
[34] Tisovský P, Donovalová J, Kožíšek J, Horváth M, Gáplovský A 2022 J. Photochem. Photobiol. A Chem. 427 113827
 Google Scholar
Google Scholar
[35] Seleem H S 2011 Chem. Cent. J. 5 8
 Google Scholar
Google Scholar
[36] Šandrik R, Tisovský P, Csicsai K, Donovalová J, Gáplovský M, Sokolík R, Filo J, Gáplovský A 2019 Molecules 24 2668
 Google Scholar
Google Scholar
[37] Liu H H, Chen Y 2009 J. Phys. Chem. A 113 5550
 Google Scholar
Google Scholar
[38] Tochitsky I, Polosukhina A, Degtyar V E, Gallerani N, Smith C M, Friedman A, Van Gelder R N, Trauner D, Kaufer D, Kramer R H 2014 Neuron 81 800
 Google Scholar
Google Scholar
[39] Van Herpt J T, Areephong J, Stuart M C, Browne W R, Feringa B L 2014 Chem. Eur. J. 20 1737
 Google Scholar
Google Scholar
[40] Nakagawa T, Ubukata T, Yokoyama Y 2018 J. Photochem. Photobiol. C-Photochem. Rev. 34 152
 Google Scholar
Google Scholar
[41] Kistemaker J C M, Stacko P, Roke D, Wolters A T, Heideman G H, Chang M C, Van Der Meulen P, Visser J, Otten E, Feringa B L 2017 J. Am. Chem. Soc. 139 9650
 Google Scholar
Google Scholar
[42] Kistemaker J C M, Stacko P, Visser J, Feringa B L 2015 Nature Chemistry 7 890
 Google Scholar
Google Scholar
[43] Thiel W 1981 J. Am. Chem. Soc. 103 1413
 Google Scholar
Google Scholar
[44] Wang J, Durbeej B 2018 ChemistryOpen 7 583
 Google Scholar
Google Scholar
[45] Ma J Z, Yang S J, Zhao D, Jiang C W, Lan Z G, Li F L 2022 Int. J. Mol. Sci. 23 3908
 Google Scholar
Google Scholar
[46] Pang X J, He H Y, Zhao K Y, Zhang N B, Zhong Q J 2023 Chem. Phys. Lett. 819 140439
 Google Scholar
Google Scholar
[47] Zhuang X H, Wang J, Lan Z G 2013 J. Phys. Chem. A 117 4785
 Google Scholar
Google Scholar
[48] Pang X J, Cui X Y, Hu D P, Jiang C W, Zhao D, Lan Z G, Li F L 2017 J. Phys. Chem. A 121 1240
 Google Scholar
Google Scholar
[49] Weber W, Thiel W 2000 Theor. Chem. Acc. 103 495
 Google Scholar
Google Scholar
[50] Otte N, Scholten M, Thiel W 2007 J. Phys. Chem. A 111 5751
 Google Scholar
Google Scholar
[51] Lan Z G, Lu Y, Weingart O, Thiel W 2012 J. Phys. Chem. A 116 1510
 Google Scholar
Google Scholar
[52] Jin H, Liang M, Arzhantsev S, Li X, Maroncelli M 2010 J. Phys. Chem. B 114 7565
 Google Scholar
Google Scholar
-
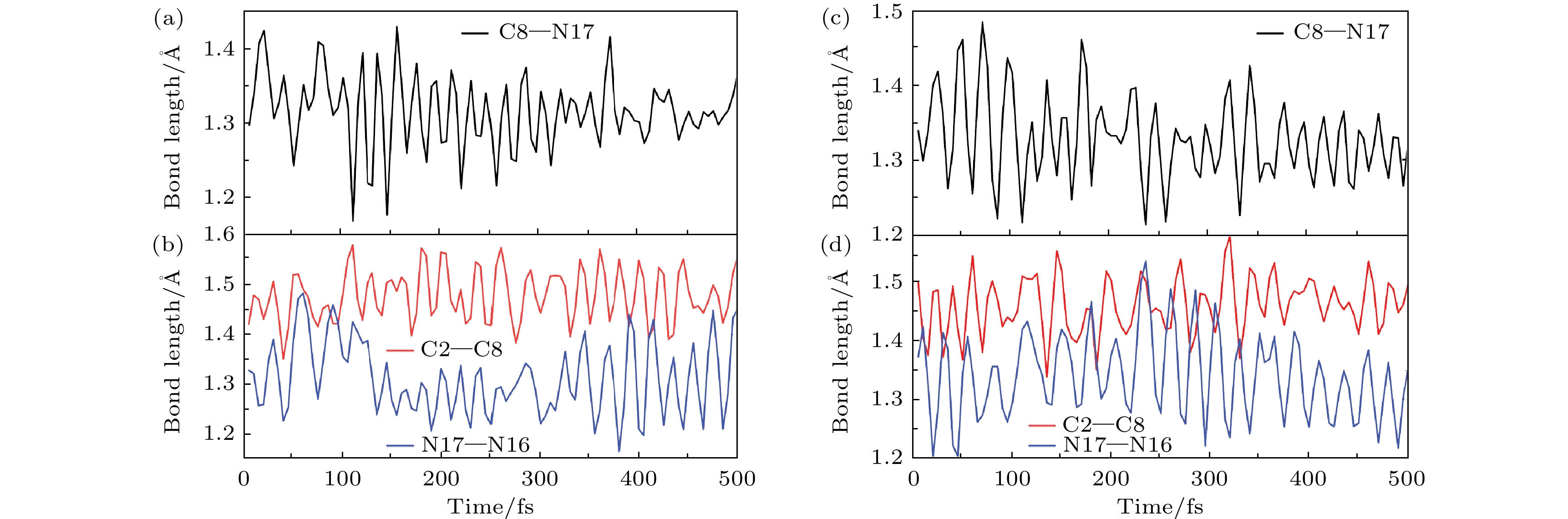
图 4 新型分子开关异构化过程中的键长的变化趋势图 (a), (b)扭转过程中C8—N17 与C2—C8, N17—N16的变化趋势; (c), (d)旋转过程中C8—N17 与C2—C8, N17—N16变化趋势
Figure 4. Trend chart of bond length changes during the isomerization process of the novel molecular switch: (a), (b) The changing trends of C8—N17 and C2—C8, N17—N16 during the twisting process; (c), (d) changes in C8—N17 and C2—C8, N17—N16 during rotation.
图 5 新型分子开关异构化过程中的二面角的变化趋势 (a), (b)扭转过程中C2—C8—N17—N16, C7—C8—N17—N16与C8—N17—N16—C33, C8—N17—N16—C18的变化趋势; (c), (d)旋转过程中C2—C8—N17—N16, C7—C8—N17—N16与C8—N17—N16—C33, C8—N17—N16—C18的变化趋势
Figure 5. The changing trend of dihedral angle during the isomerization process of the novel molecular switch: (a), (b) The changing trends of C2—C8—N17—N16, C7—C8—N17—N16, C8—N17—N16—C33, and C8—N17—N16—C18 during the twisting process; (c), (d) the changing trends of C2—C8—N17—N16, C7—C8—N17—N16, C8—N17—N16—C33, and C8—N17—N16—C18 during the rotation process.
-
[1] Li R J, Mou B Z, Yamada M, Li W, Nakashima T, Kawai T 2024 Molecules 29 25
 Google Scholar
Google Scholar
[2] Jago D, Gaschk E E, Koutsantonis G A 2023 Aust. J. Chem. 76 635
 Google Scholar
Google Scholar
[3] Yu Z, Hecht S 2016 Chem. Commun. 52 6639
 Google Scholar
Google Scholar
[4] Feringa B L, Van Delden R A, Koumura N, Geertsema E M 2000 Chem. Rev. 100 1789
 Google Scholar
Google Scholar
[5] Rice A M, Martin C R, Galitskiy V A, Berseneva A A, Leith G A, Shustova N B 2020 Chem. Rev. 120 8790
 Google Scholar
Google Scholar
[6] Zhang X Y, Hou L L, Samorì P 2016 Nat. Commun. 7 11118
 Google Scholar
Google Scholar
[7] Alenazi M H, Mubarak A T, Abboud M 2024 Nanotechnol. Rev. 13 20240032
 Google Scholar
Google Scholar
[8] Goulet-Hanssens A, Eisenreich F, Hecht S 2020 Adv. Mater. 32 1905966
 Google Scholar
Google Scholar
[9] Pios S V, Gelin M F, Ullah A, Dral P O, Chen L 2024 J. Phys. Chem. Lett. 15 2325
 Google Scholar
Google Scholar
[10] Conti I, Cerullo G, Nenov A, Garavelli M 2020 J. Am. Chem. Soc. 142 16117
 Google Scholar
Google Scholar
[11] Towns A 2021 Phys. Sci. Rev. 6 477
 Google Scholar
Google Scholar
[12] Cheng H B, Zhang S, Bai E, Cao X, Wang J, Qi J, Liu J, Zhao J, Zhang L, Yoon J 2022 Adv. Mater. 34 2108289
 Google Scholar
Google Scholar
[13] Bertarelli C, Bianco A, Castagna R, Pariani G 2011 J. Photoch. Photobio. C 12 106
 Google Scholar
Google Scholar
[14] Bléger D, Hecht S 2015 Angew. Chem. Int. Ed. 54 11338
 Google Scholar
Google Scholar
[15] Shao B, Aprahamian I 2020 Chem 6 2162
 Google Scholar
Google Scholar
[16] Van Dijken D J, KovaříčEk P, Ihrig S P, Hecht S 2015 J. Am. Chem. Soc. 137 14982
 Google Scholar
Google Scholar
[17] Schnetz M, Meier J K, Rehwald C, Mertens C, Urbschat A, Tomat E, Akam E A, Baer P, Roos F C, Brüne B 2020 Cancers 12 530
 Google Scholar
Google Scholar
[18] Ferreira I P, Piló E D, Recio-Despaigne A A, Da Silva J G, Ramos J P, Marques L B, Prazeres P H, Takahashi J A, Souza-Fagundes E M, Rocha W 2016 Bioorg. Med. Chem. 24 2988
 Google Scholar
Google Scholar
[19] Vantomme G, Lehn J M 2014 Chem. Eur. J. 20 16188
 Google Scholar
Google Scholar
[20] Vantomme G, Lehn J M 2013 Angew. Chem. Int. Edit. 52 3940
 Google Scholar
Google Scholar
[21] Vantomme G, Jiang S M, Lehn J M 2015 J. Am. Chem. Soc. 137 3138
 Google Scholar
Google Scholar
[22] Su X, Aprahamian I 2014 Chem. Soc. Rev. 43 1963
 Google Scholar
Google Scholar
[23] Chaur M N, Collado D, Lehn J M 2011 Chem. Eur. J. 17 248
 Google Scholar
Google Scholar
[24] Vantomme G, Jiang S, Lehn J M 2014 J. Am. Chem. Soc. 136 9509
 Google Scholar
Google Scholar
[25] 刘同力, 黄从树, 王晶晶, 梁宇, 谢志鹏, 庄海燕, 李九龙, 朱绪飞 2024 精细化工 https://doi.org/10.13550/j.jxhg.20230989
Liu T L, Huang C S, Wang J J, Liang Y, Xie Z P, Zhuang H Y, Li J L, Zhu X F 2024 Fine. Chem https://doi.org/ 10.13550/ j.jxhg.20230989
[26] Siewertsen R, Neumann H, Buchheim-Stehn B, Herges R, Näther C, Renth F, Temps F 2009 J. Am. Chem. Soc. 131 15594
 Google Scholar
Google Scholar
[27] Beharry A A, Sadovski O, Woolley G A 2011 J. Am. Chem. Soc. 133 19684
 Google Scholar
Google Scholar
[28] Poloni C, Szymanski W, Hou L L, Browne W R, Feringa B L 2014 Chem. Eur. J. 20 946
 Google Scholar
Google Scholar
[29] Zhang Z W, Yang W X, Zhang J J 2023 Chem. Ind. Eng. Prog 42 4058 [张志伟, 杨伟鑫, 张隽佶 2023 化工进展 42 4058]
 Google Scholar
Google Scholar
Zhang Z W, Yang W X, Zhang J J 2023 Chem. Ind. Eng. Prog 42 4058
 Google Scholar
Google Scholar
[30] Ye H 2020 M. S. Thesie (Wuhan: Huazhong University of Science and Technology) [叶欢 2020 硕士学位论文(武汉: 华中科技大学)]
Ye H 2020 M. S. Thesie (Wuhan: Huazhong University of Science and Technology)
[31] Szymanski W, Beierle J M, Kistemaker H A, Velema W A, Feringa B L 2013 Chem. Rev. 113 6114
 Google Scholar
Google Scholar
[32] Cigán M, Gáplovsky M, Jakusová K, Donovalová J, Horváth M, Filo J, Gáplovsky A 2015 RSC Adv. 5 62449
 Google Scholar
Google Scholar
[33] Cigáň M, Jakusová K, Gáplovský M, Filo J, Donovalová J, Gáplovský A 2015 Photochem. Photobiol. Sci. 14 2064
 Google Scholar
Google Scholar
[34] Tisovský P, Donovalová J, Kožíšek J, Horváth M, Gáplovský A 2022 J. Photochem. Photobiol. A Chem. 427 113827
 Google Scholar
Google Scholar
[35] Seleem H S 2011 Chem. Cent. J. 5 8
 Google Scholar
Google Scholar
[36] Šandrik R, Tisovský P, Csicsai K, Donovalová J, Gáplovský M, Sokolík R, Filo J, Gáplovský A 2019 Molecules 24 2668
 Google Scholar
Google Scholar
[37] Liu H H, Chen Y 2009 J. Phys. Chem. A 113 5550
 Google Scholar
Google Scholar
[38] Tochitsky I, Polosukhina A, Degtyar V E, Gallerani N, Smith C M, Friedman A, Van Gelder R N, Trauner D, Kaufer D, Kramer R H 2014 Neuron 81 800
 Google Scholar
Google Scholar
[39] Van Herpt J T, Areephong J, Stuart M C, Browne W R, Feringa B L 2014 Chem. Eur. J. 20 1737
 Google Scholar
Google Scholar
[40] Nakagawa T, Ubukata T, Yokoyama Y 2018 J. Photochem. Photobiol. C-Photochem. Rev. 34 152
 Google Scholar
Google Scholar
[41] Kistemaker J C M, Stacko P, Roke D, Wolters A T, Heideman G H, Chang M C, Van Der Meulen P, Visser J, Otten E, Feringa B L 2017 J. Am. Chem. Soc. 139 9650
 Google Scholar
Google Scholar
[42] Kistemaker J C M, Stacko P, Visser J, Feringa B L 2015 Nature Chemistry 7 890
 Google Scholar
Google Scholar
[43] Thiel W 1981 J. Am. Chem. Soc. 103 1413
 Google Scholar
Google Scholar
[44] Wang J, Durbeej B 2018 ChemistryOpen 7 583
 Google Scholar
Google Scholar
[45] Ma J Z, Yang S J, Zhao D, Jiang C W, Lan Z G, Li F L 2022 Int. J. Mol. Sci. 23 3908
 Google Scholar
Google Scholar
[46] Pang X J, He H Y, Zhao K Y, Zhang N B, Zhong Q J 2023 Chem. Phys. Lett. 819 140439
 Google Scholar
Google Scholar
[47] Zhuang X H, Wang J, Lan Z G 2013 J. Phys. Chem. A 117 4785
 Google Scholar
Google Scholar
[48] Pang X J, Cui X Y, Hu D P, Jiang C W, Zhao D, Lan Z G, Li F L 2017 J. Phys. Chem. A 121 1240
 Google Scholar
Google Scholar
[49] Weber W, Thiel W 2000 Theor. Chem. Acc. 103 495
 Google Scholar
Google Scholar
[50] Otte N, Scholten M, Thiel W 2007 J. Phys. Chem. A 111 5751
 Google Scholar
Google Scholar
[51] Lan Z G, Lu Y, Weingart O, Thiel W 2012 J. Phys. Chem. A 116 1510
 Google Scholar
Google Scholar
[52] Jin H, Liang M, Arzhantsev S, Li X, Maroncelli M 2010 J. Phys. Chem. B 114 7565
 Google Scholar
Google Scholar
Catalog
Metrics
- Abstract views: 5277
- PDF Downloads: 108
- Cited By: 0














 DownLoad:
DownLoad: