-
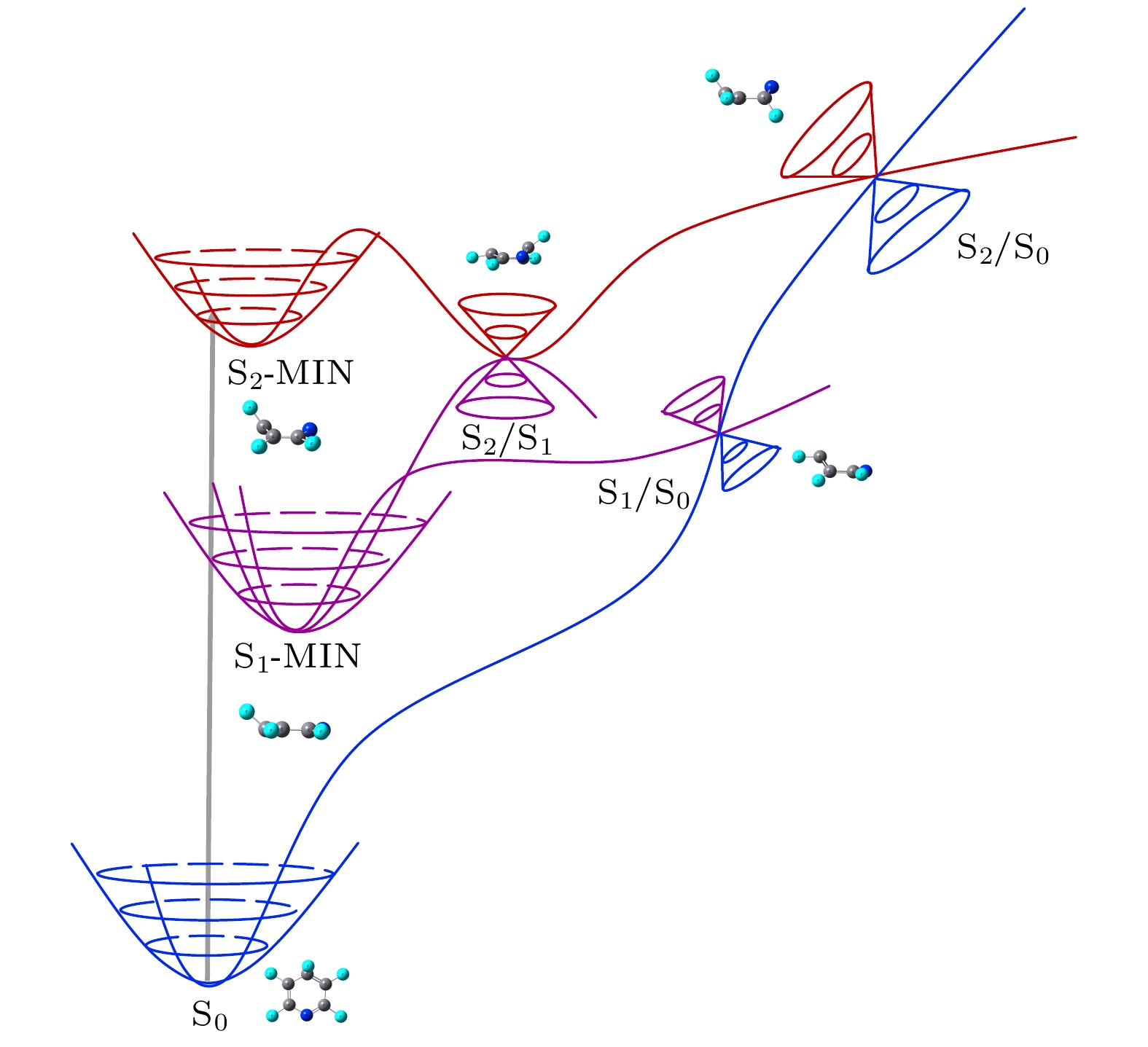
利用量子化学计算研究了五氟吡啶分子的激发态非绝热弛豫路径中一些关键点的分子结构和能量. 计算确定了五氟吡啶分子基态及两个最低激发态的结构和相应电子态的垂直和绝热激发能, 其中基态是具有C2v对称性的平面结构, 而激发态结构为平面外畸变的半船型结构. 同时确定了3个锥形交叉S2/S1, S1/S0, S2/S0的拓扑结构和能量. 在分支空间中, 锥形交叉S2/S1, S1/S0, S2/S0的结构都是尖峰不对称结构, 分别为船型、半船型和椅式结构, 其能量分别为6.39, 5.16和8.51 eV. 计算结果表明五氟吡啶分子的非辐射弛豫主要是S2态上的波包经锥形交叉S2/S1快速内转换到S1态, 再通过S1/S0弛豫到基态的路径, 而直接通过S2/S0衰减到基态的概率较小.In this work, the molecular structure and energy of some critical points in nonradiative relaxation process of the excited state of pentafluoropyridine are studied through quantum chemistry calculation. The structures and the vertical excitation energies and adiabatic excitation energies of the ground state and two lowest exited states are calculated. The geometry of the ground state is a planar structure with C2v symmetry, while the geometries of the two lowest excited states are half-boat structures with out-of-plane distortions. Furthermore, the topology structures and energy of the conical intersections of S2/S1, S1/S0 and S2/S0 are determined. The topology structures of the conical intersections S2/S1, S1/S0 and S2/S0 in the branching space are all peaked with asymmetric structures, and are determined to be structure of boat, half-boat, and chair, respectively. Their corresponding energy values are estimated at 6.39, 5.16 and 8.51 eV, respectively. The results show that the primary non-adiabatic relaxation pathway is the wavepacket of the S2 state rapidly evolving into the S1 state via the S2/S1, and then directly relaxing to the ground state via the S1/S0. In addition, the probability of directly relaxing to the ground state through S2/S0 is smaller.
-
Keywords:
- quantum chemical calculations /
- conical intersection /
- excited states /
- structure evolution
[1] Lim J S, Kim S K 2010 Nat. Chem. 2 627
 Google Scholar
Google Scholar
[2] Adachi S, Suzuki T 2020 Phys. Chem. Chem. Phys. 22 2814
 Google Scholar
Google Scholar
[3] Woo K C, Kang D H, Kim S K 2017 J. Am. Chem. Soc. 139 17152
 Google Scholar
Google Scholar
[4] Anand N, Isukapalli S V K, Vennapusa S R 2020 J. Comput. Chem. 41 1068
 Google Scholar
Google Scholar
[5] Zgrablic G, Novello A M, Parmigiani F 2012 J. Am. Chem. Soc. 134 955
 Google Scholar
Google Scholar
[6] Lee H, Kim S Y, Kim S K 2020 Chem. Sci. 11 6856
 Google Scholar
Google Scholar
[7] Adachi S, Schatteburg T, Humeniuk A, Mitric R, Suzuki T 2019 Phys. Chem. Chem. Phys. 21 13902
 Google Scholar
Google Scholar
[8] Chang K F, Reduzzi M, Wang H, Poullain S M, Kobayashi Y, Barreau L, Prendergast D, Neumark D M 2020 Nat. Commun. 11 4042
 Google Scholar
Google Scholar
[9] Pracht P, Bannwarth C 2022 J. Chem. Theory Comput. 18 6370
 Google Scholar
Google Scholar
[10] Benda Z, Jagau T C 2018 J. Chem. Theory Comput. 14 4216
 Google Scholar
Google Scholar
[11] De Sio A, Sommer E, Nguyen X T, Gross L, Popovic D, Nebgen B T, Fernandez-Alberti S, Pittalis S, Rozzi C A, Molinari E, Mena-Osteritz E, Bauerle P, Frauenheim T, Tretiak S, Lienau C 2021 Nat. Nanotechnol. 16 63
 Google Scholar
Google Scholar
[12] Bhebhe M N, De Eulate E A, Pei Y, Arrigan D W, Roth P J, Lowe A B 2017 Macromol. Rapid Comm. 38 1600450
 Google Scholar
Google Scholar
[13] Corley C A, Kobra K, Peloquin A J, Salmon K, Gumireddy L, Knoerzer T A, McMillen C D, Pennington W T, Schoffstall A M, Iacono S T 2019 J. Fluorine Chem. 228 109409
 Google Scholar
Google Scholar
[14] Houck M B, Fuhrer T J, Phelps C R, Brown L C, Iacono S T 2021 Macromolecules 54 5586
 Google Scholar
Google Scholar
[15] Iacono S T, Budy S M, Jin J, Smith D W 2007 J. Polym. Sci. Pol. Chem. 45 5705
 Google Scholar
Google Scholar
[16] Moore L M J, Greeson K T, Stewart K A, Kure D A, Corley C A, Jennings A R, Iacono S T, Ghiassi K B 2020 Macromol. Chem. Phys. 221 2000100
 Google Scholar
Google Scholar
[17] Seyb C, Kerres J 2013 Eur. Polym. J. 49 518
 Google Scholar
Google Scholar
[18] Miller W K, Samuel B, Roe A 1950 J. Am. Chem. Soc. 72 1629
 Google Scholar
Google Scholar
[19] Fuhrer T J, Houck M, Iacono S T 2021 ACS Omega. 48 32607
 Google Scholar
Google Scholar
[20] Hüter O, Sala M, Neumann H, Zhang S, Studzinski H, Egorova D, Temps F 2016 J. Chem. Phys. 145 014302
 Google Scholar
Google Scholar
[21] Studzinski H, Zhang S, Wang Y, Temps F 2008 J. Chem. Phys. 128 164314
 Google Scholar
Google Scholar
[22] Kus J A, Hüter O, Temps F 2017 J. Chem. Phys. 147 013938
 Google Scholar
Google Scholar
[23] Frisch M J, Trucks G W, Schlegel H B, et al. 2009 Gaussian Inc, Revision B.01, Wallingford CT
[24] Werner H J, Knowles P J, Knizia G, et al. 2010 MOLPRO
[25] Neese F 2022 Wires Comput. 12 1606
 Google Scholar
Google Scholar
[26] Dennington R, Keith T A, Millam J M 2016 Semichem Inc. Shawnee Mission, KS, GaussView, Version 6
[27] Lu T, Chen F 2012 J. Comput. Chem. 33 580
 Google Scholar
Google Scholar
[28] Schaftenaar G, Noordik J H 2000 J. Comput. Aid. Mol. Des. 14 123
 Google Scholar
Google Scholar
[29] Varras P C, Gritzapis P S, Fylaktakidou K C 2017 Mol. Phys. 116 154
 Google Scholar
Google Scholar
[30] Nagaoka S I, Nagashima U 1990 J. Chem. Phys. 94 4467
 Google Scholar
Google Scholar
[31] Chachisvilis M, Zewail A H 1999 J. Phys. Chem. A 103 7408
 Google Scholar
Google Scholar
[32] Cox J M, Bain M, Kellogg M, Bradforth S E, Lopez S A 2021 J. Am. Chem. Soc. 143 7002
 Google Scholar
Google Scholar
[33] Galvan I F, Delcey M G, Pedersen T B, Aquilante F, Lindh R 2016 J. Chem. Theory Comput. 12 3636
 Google Scholar
Google Scholar
[34] Boeije Y, Olivucci M 2023 Chem. Soc. Rev. 52 2643
 Google Scholar
Google Scholar
[35] Paulami G, Arpita G, Debshree G 2021 J. Phys. Chem. A 125 5556
 Google Scholar
Google Scholar
[36] Barbatti M, Aquino J A A, Lischka H 2005 J. Phys. Chem. A 109 5168
 Google Scholar
Google Scholar
[37] Li D, Zhang S 2022 Chin. Phys. B 31 083103
 Google Scholar
Google Scholar
[38] Suzuki T 2012 Int. Rev. Phys. Chem. 31 265
 Google Scholar
Google Scholar
[39] Palmer I J, Ragazos I N, Bernardi F, Olivucci M, Robb M A 1993 J. Am. Chem. Soc. 115 673
 Google Scholar
Google Scholar
[40] Suzuki Y, Horio T, Fuji T, Suzuki T 2011 J. Chem. Phys. 134 184313
 Google Scholar
Google Scholar
[41] Radloff W, Stert V, Freudenberg T, Hertel I V, Jouvet C, Dedonder-Lardeux C, Solgadi D 1997 Chem. Phys. Lett. 281 20
 Google Scholar
Google Scholar
[42] Radloff W, Freudenberg T, Ritze H H, Stert V, Noack F, Hertel I V 1996 Chem. Phys. Lett. 261 301
 Google Scholar
Google Scholar
[43] Enomoto K, LaVerne J A, Seki S, Tagawa S 2006 J. Phys. Chem. A 110 9874
 Google Scholar
Google Scholar
-
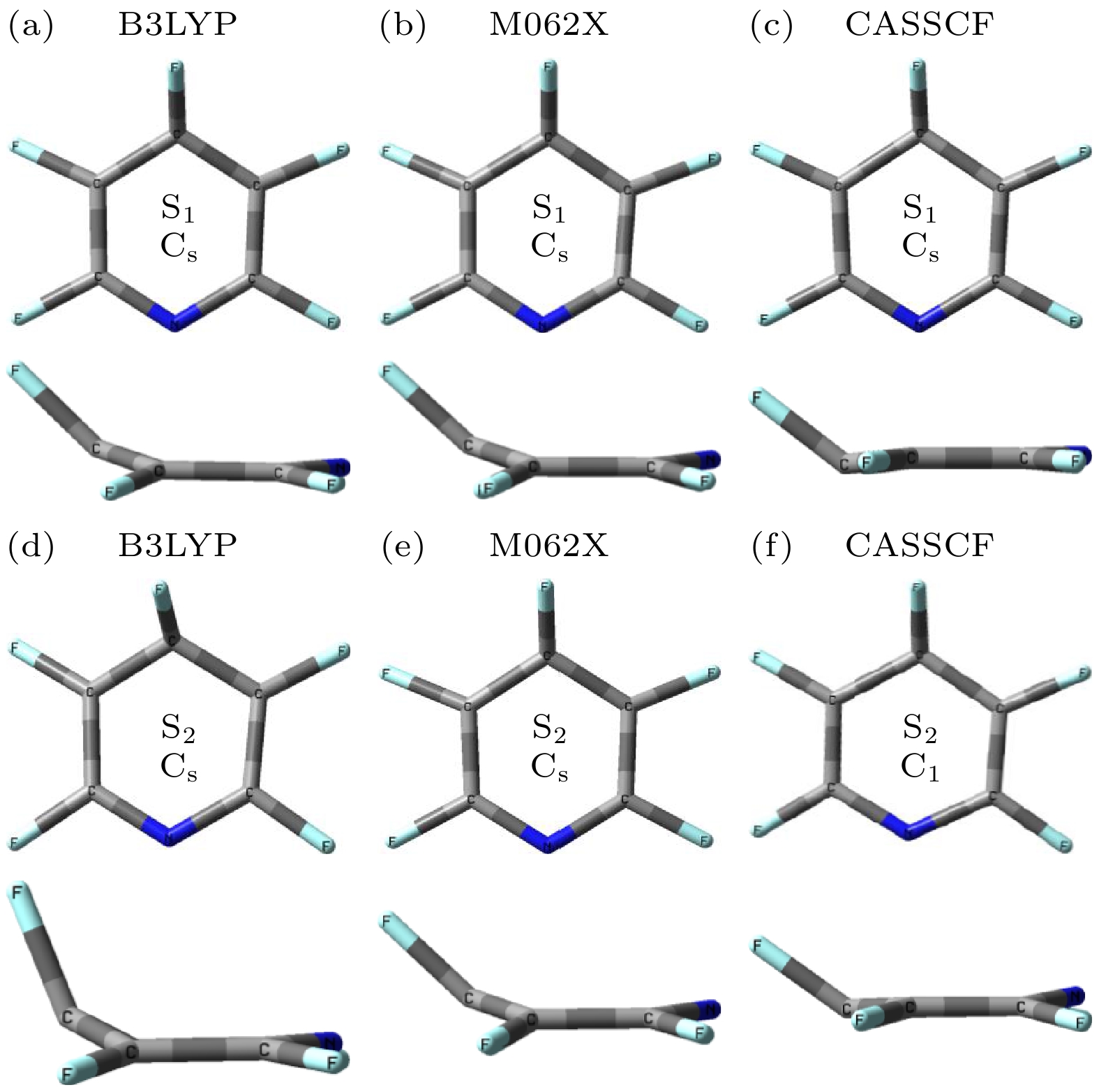
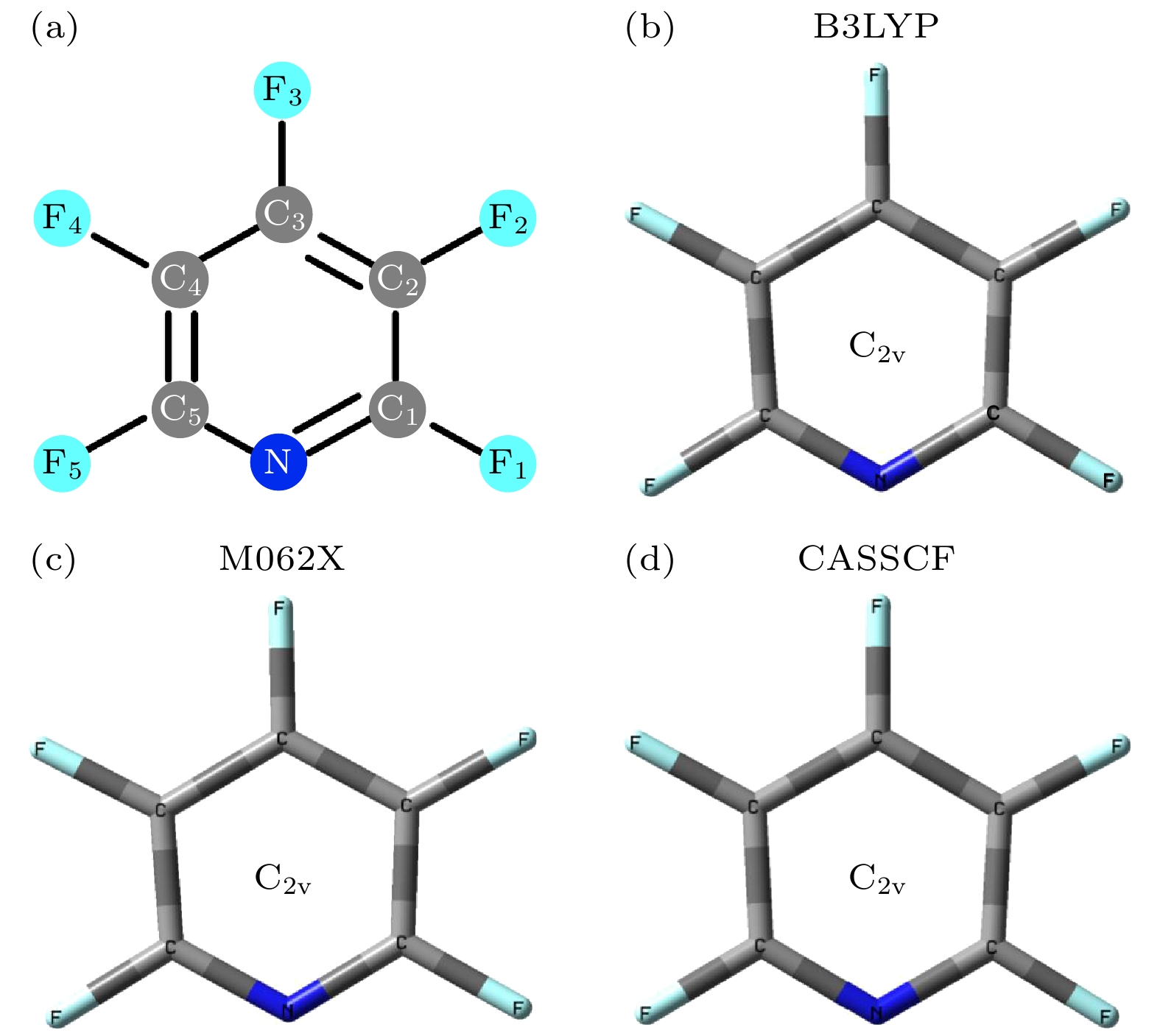
表 1 利用B3LYP, M062X, SA-CASSCF(8, 8)方法, 得到五氟吡啶分子的S1态和S2态的结构参数(键长单位Å, 二面角单位(°))
Table 1. Structural parameters of the S1 and S2 states were obtained by B3LYP, M062X and SA-CASSCF(8, 8) methods, respectively (Bond length and dihedral angle are Å, (°) in units, respectively).
结构参数 S1 S2 B3LYP/
6-311G*M062X/
6-311G*SA-CASSCF/
6-311G*B3LYP/
6-311G*M062X/
6-311G*SA-CASSCF/
6-311G*C1—F1 1.32 1.31 1.31 1.34 1.31 1.29 C2—F2 1.34 1.33 1.33 1.34 1.33 1.33 C3—F3 1.41 1.37 1.37 1.39 1.37 1.29 C4—F4 1.34 1.33 1.33 1.34 1.33 1.32 C5—F5 1.32 1.31 1.31 1.34 1.31 1.30 C1—N 1.32 1.32 1.32 1.33 1.32 1.44 C5—N 1.32 1.32 1.32 1.33 1.32 1.36 C1—C2 1.43 1.43 1.43 1.38 1.43 1.35 C2—C3 1.40 1.40 1.40 1.44 1.40 1.43 C3—C4 1.40 1.40 1.40 1.44 1.40 1.47 C4—C5 1.43 1.43 1.43 1.38 1.43 1.34 C1—C5 2.20 2.18 2.21 2.29 2.18 2.36 C2—C4 2.28 2.28 2.36 2.45 2.28 2.52 N—C1—C2—C5 3.83 5.31 1.13 3.07 5.42 20.69 C3—C2—C1—C4 13.26 13.33 0.30 16.25 13.16 20.64 F3—C3—C4—C1 54.38 52.09 45.11 75.49 51.89 55.04 F4—C4—C5—C1 13.68 14.05 3.13 12.19 14.58 32.02 F5—C5—C4—C2 6.96 9.10 2.96 7.32 9.66 18.20 表 2 B3LYP, SA-CASSCF(8, 8), M062X和CASPT2方法结合6-311G*基组计算得到五氟吡啶分子S1态和S2态的VEEs和AEEs (单位为eV)
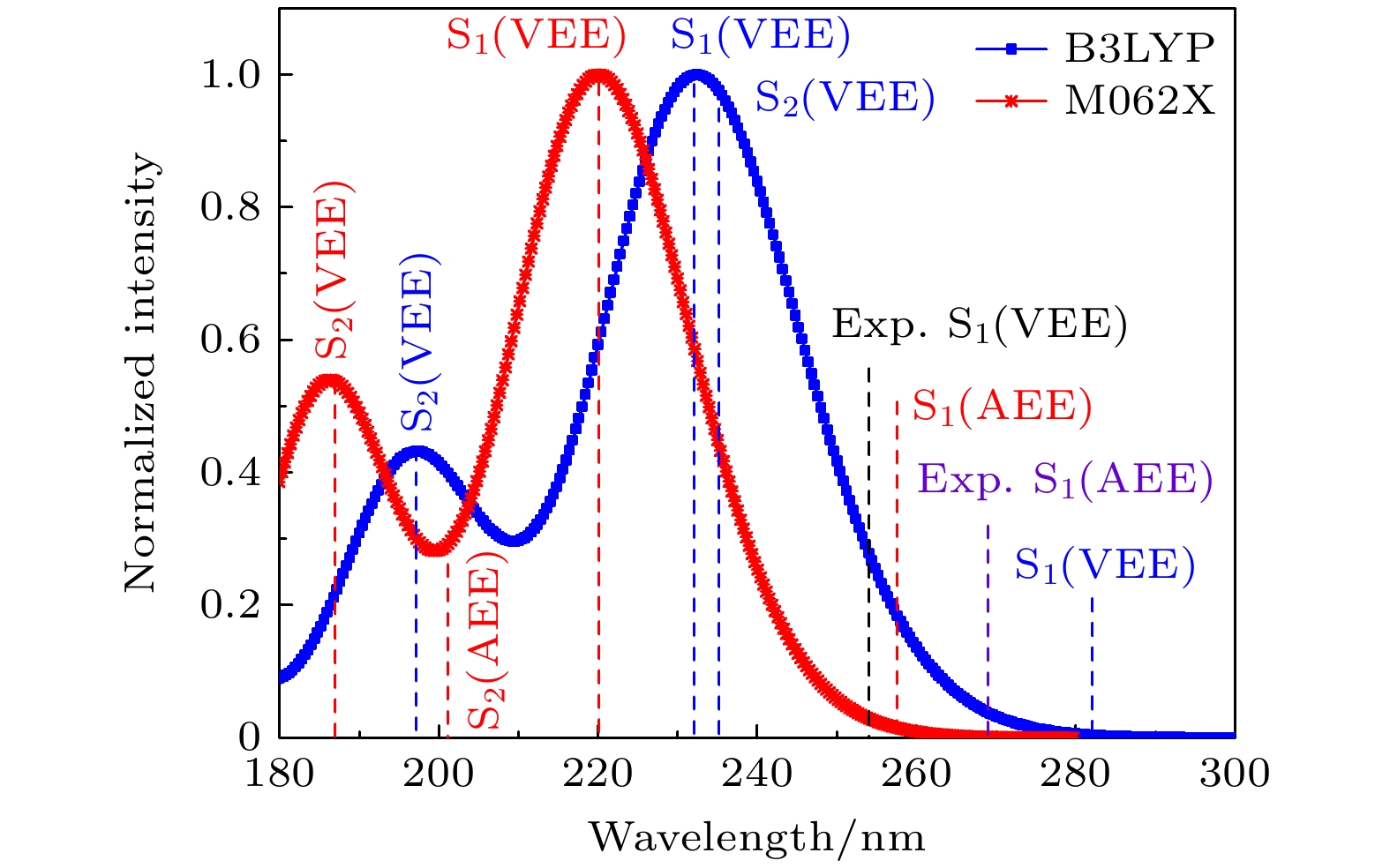
Table 2. VEEs and AEEs (in eV) of pentafluoropyridine in the S1 and S2 states calculated at B3LYP, SA-CASSCF(8, 8), M062X and CASPT2 levels with the 6-311G* basis set.
Methods S1 S2 VEEs Dev/% AEEs Dev/% VEEs AEEs Exp.a) 4.88 — 4.60 — — — RI-SCS-CC2a) 5.10 4.5 4.60 0 6.35 — XMCQDPT2a) 4.89 0.2 4.41 4.1 6.23 5.26 B3LYP 5.33 9.2 4.41 4.1 6.28 5.26 SA-CASSCF(8, 8) 5.47 12.1 4.84 5.2 6.92 6.69 M062X 5.63 9.8 4.80 4.3 6.50 6.15b) CASPT2 5.02 2.9 4.41 4.1 6.33 — 注: a) 来自参考文献[22]; b) 基于M062X/6-31G*的结果 表 3 SA-CASSCF水平下的锥形交叉的结构参数(键长单位Å, 二面角单位 (°))
Table 3. Structural parameters of conical intersections were obtained by SA-CASSCF(8, 8) methods (Bond length and dihedral angle are Å, (°) in units).
参数 S2/S1 S1/S0 S2/S0 C1—F1 1.30 1.30 1.31 C2—F2 1.32 1.31 1.30 C3—F3 1.30 1.32 1.32 C4—F4 1.32 1.31 1.30 C5—F5 1.30 1.30 1.31 C1—N 1.45 1.31 1.48 C5—N 1.29 1.33 1.42 C1—C2 1.47 1.46 1.49 C2—C3 1.39 1.46 1.48 C3—C4 1.49 1.47 1.47 C4—C5 1.45 1.45 1.49 N—C1—C2—C5 29.72 2.15 22.59 C3—C2—C1—C4 10.90 46.24 12.28 F3—C3—C4—C1 11.57 36.30 64.87 F4—C4—C5—C1 9.84 30.71 0.81 表 4 锥形交叉在分支空间中的拓扑参数
Table 4. Topological parameters of conical intersections in branching space.
参数 S1/S0 S2/S1 S2/S0 σx/(eV·Å–1) –0.0047 0.1413 0.4016 σy/(eV·Å–1) –0.0207 0.0757 –0.0001 ${\varDelta }_{\mathrm{gh}} $ –0.9904 –0.9796 –0.8647 dgh 1.5000 1.0212 0.6159 -
[1] Lim J S, Kim S K 2010 Nat. Chem. 2 627
 Google Scholar
Google Scholar
[2] Adachi S, Suzuki T 2020 Phys. Chem. Chem. Phys. 22 2814
 Google Scholar
Google Scholar
[3] Woo K C, Kang D H, Kim S K 2017 J. Am. Chem. Soc. 139 17152
 Google Scholar
Google Scholar
[4] Anand N, Isukapalli S V K, Vennapusa S R 2020 J. Comput. Chem. 41 1068
 Google Scholar
Google Scholar
[5] Zgrablic G, Novello A M, Parmigiani F 2012 J. Am. Chem. Soc. 134 955
 Google Scholar
Google Scholar
[6] Lee H, Kim S Y, Kim S K 2020 Chem. Sci. 11 6856
 Google Scholar
Google Scholar
[7] Adachi S, Schatteburg T, Humeniuk A, Mitric R, Suzuki T 2019 Phys. Chem. Chem. Phys. 21 13902
 Google Scholar
Google Scholar
[8] Chang K F, Reduzzi M, Wang H, Poullain S M, Kobayashi Y, Barreau L, Prendergast D, Neumark D M 2020 Nat. Commun. 11 4042
 Google Scholar
Google Scholar
[9] Pracht P, Bannwarth C 2022 J. Chem. Theory Comput. 18 6370
 Google Scholar
Google Scholar
[10] Benda Z, Jagau T C 2018 J. Chem. Theory Comput. 14 4216
 Google Scholar
Google Scholar
[11] De Sio A, Sommer E, Nguyen X T, Gross L, Popovic D, Nebgen B T, Fernandez-Alberti S, Pittalis S, Rozzi C A, Molinari E, Mena-Osteritz E, Bauerle P, Frauenheim T, Tretiak S, Lienau C 2021 Nat. Nanotechnol. 16 63
 Google Scholar
Google Scholar
[12] Bhebhe M N, De Eulate E A, Pei Y, Arrigan D W, Roth P J, Lowe A B 2017 Macromol. Rapid Comm. 38 1600450
 Google Scholar
Google Scholar
[13] Corley C A, Kobra K, Peloquin A J, Salmon K, Gumireddy L, Knoerzer T A, McMillen C D, Pennington W T, Schoffstall A M, Iacono S T 2019 J. Fluorine Chem. 228 109409
 Google Scholar
Google Scholar
[14] Houck M B, Fuhrer T J, Phelps C R, Brown L C, Iacono S T 2021 Macromolecules 54 5586
 Google Scholar
Google Scholar
[15] Iacono S T, Budy S M, Jin J, Smith D W 2007 J. Polym. Sci. Pol. Chem. 45 5705
 Google Scholar
Google Scholar
[16] Moore L M J, Greeson K T, Stewart K A, Kure D A, Corley C A, Jennings A R, Iacono S T, Ghiassi K B 2020 Macromol. Chem. Phys. 221 2000100
 Google Scholar
Google Scholar
[17] Seyb C, Kerres J 2013 Eur. Polym. J. 49 518
 Google Scholar
Google Scholar
[18] Miller W K, Samuel B, Roe A 1950 J. Am. Chem. Soc. 72 1629
 Google Scholar
Google Scholar
[19] Fuhrer T J, Houck M, Iacono S T 2021 ACS Omega. 48 32607
 Google Scholar
Google Scholar
[20] Hüter O, Sala M, Neumann H, Zhang S, Studzinski H, Egorova D, Temps F 2016 J. Chem. Phys. 145 014302
 Google Scholar
Google Scholar
[21] Studzinski H, Zhang S, Wang Y, Temps F 2008 J. Chem. Phys. 128 164314
 Google Scholar
Google Scholar
[22] Kus J A, Hüter O, Temps F 2017 J. Chem. Phys. 147 013938
 Google Scholar
Google Scholar
[23] Frisch M J, Trucks G W, Schlegel H B, et al. 2009 Gaussian Inc, Revision B.01, Wallingford CT
[24] Werner H J, Knowles P J, Knizia G, et al. 2010 MOLPRO
[25] Neese F 2022 Wires Comput. 12 1606
 Google Scholar
Google Scholar
[26] Dennington R, Keith T A, Millam J M 2016 Semichem Inc. Shawnee Mission, KS, GaussView, Version 6
[27] Lu T, Chen F 2012 J. Comput. Chem. 33 580
 Google Scholar
Google Scholar
[28] Schaftenaar G, Noordik J H 2000 J. Comput. Aid. Mol. Des. 14 123
 Google Scholar
Google Scholar
[29] Varras P C, Gritzapis P S, Fylaktakidou K C 2017 Mol. Phys. 116 154
 Google Scholar
Google Scholar
[30] Nagaoka S I, Nagashima U 1990 J. Chem. Phys. 94 4467
 Google Scholar
Google Scholar
[31] Chachisvilis M, Zewail A H 1999 J. Phys. Chem. A 103 7408
 Google Scholar
Google Scholar
[32] Cox J M, Bain M, Kellogg M, Bradforth S E, Lopez S A 2021 J. Am. Chem. Soc. 143 7002
 Google Scholar
Google Scholar
[33] Galvan I F, Delcey M G, Pedersen T B, Aquilante F, Lindh R 2016 J. Chem. Theory Comput. 12 3636
 Google Scholar
Google Scholar
[34] Boeije Y, Olivucci M 2023 Chem. Soc. Rev. 52 2643
 Google Scholar
Google Scholar
[35] Paulami G, Arpita G, Debshree G 2021 J. Phys. Chem. A 125 5556
 Google Scholar
Google Scholar
[36] Barbatti M, Aquino J A A, Lischka H 2005 J. Phys. Chem. A 109 5168
 Google Scholar
Google Scholar
[37] Li D, Zhang S 2022 Chin. Phys. B 31 083103
 Google Scholar
Google Scholar
[38] Suzuki T 2012 Int. Rev. Phys. Chem. 31 265
 Google Scholar
Google Scholar
[39] Palmer I J, Ragazos I N, Bernardi F, Olivucci M, Robb M A 1993 J. Am. Chem. Soc. 115 673
 Google Scholar
Google Scholar
[40] Suzuki Y, Horio T, Fuji T, Suzuki T 2011 J. Chem. Phys. 134 184313
 Google Scholar
Google Scholar
[41] Radloff W, Stert V, Freudenberg T, Hertel I V, Jouvet C, Dedonder-Lardeux C, Solgadi D 1997 Chem. Phys. Lett. 281 20
 Google Scholar
Google Scholar
[42] Radloff W, Freudenberg T, Ritze H H, Stert V, Noack F, Hertel I V 1996 Chem. Phys. Lett. 261 301
 Google Scholar
Google Scholar
[43] Enomoto K, LaVerne J A, Seki S, Tagawa S 2006 J. Phys. Chem. A 110 9874
 Google Scholar
Google Scholar
计量
- 文章访问数: 2989
- PDF下载量: 64
- 被引次数: 0













 下载:
下载: