-
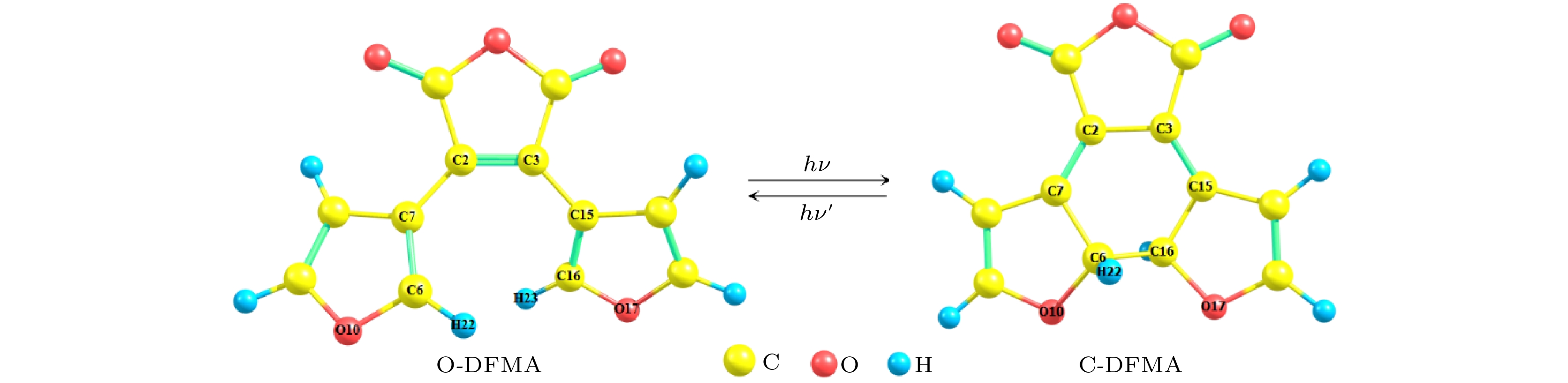
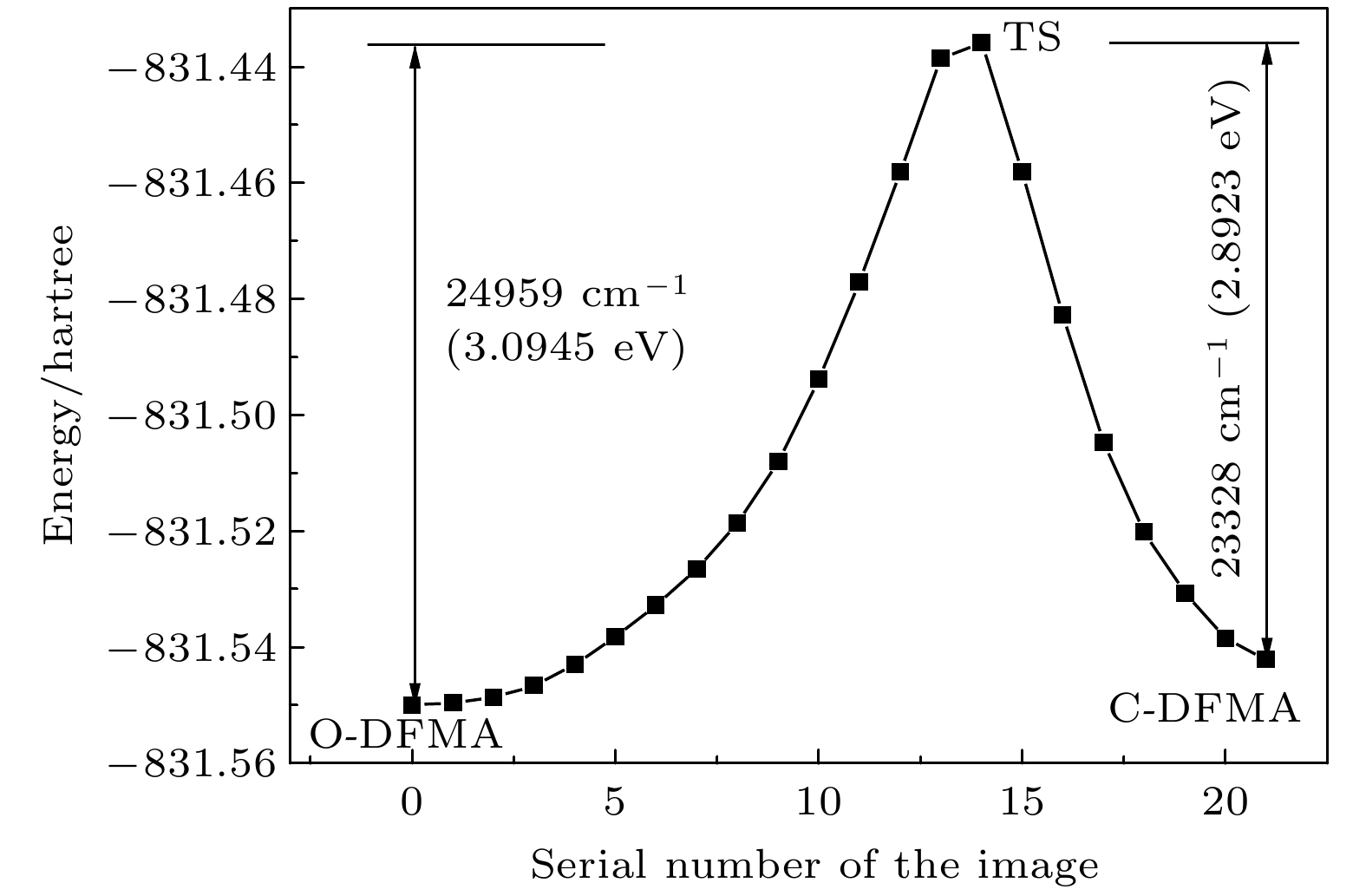
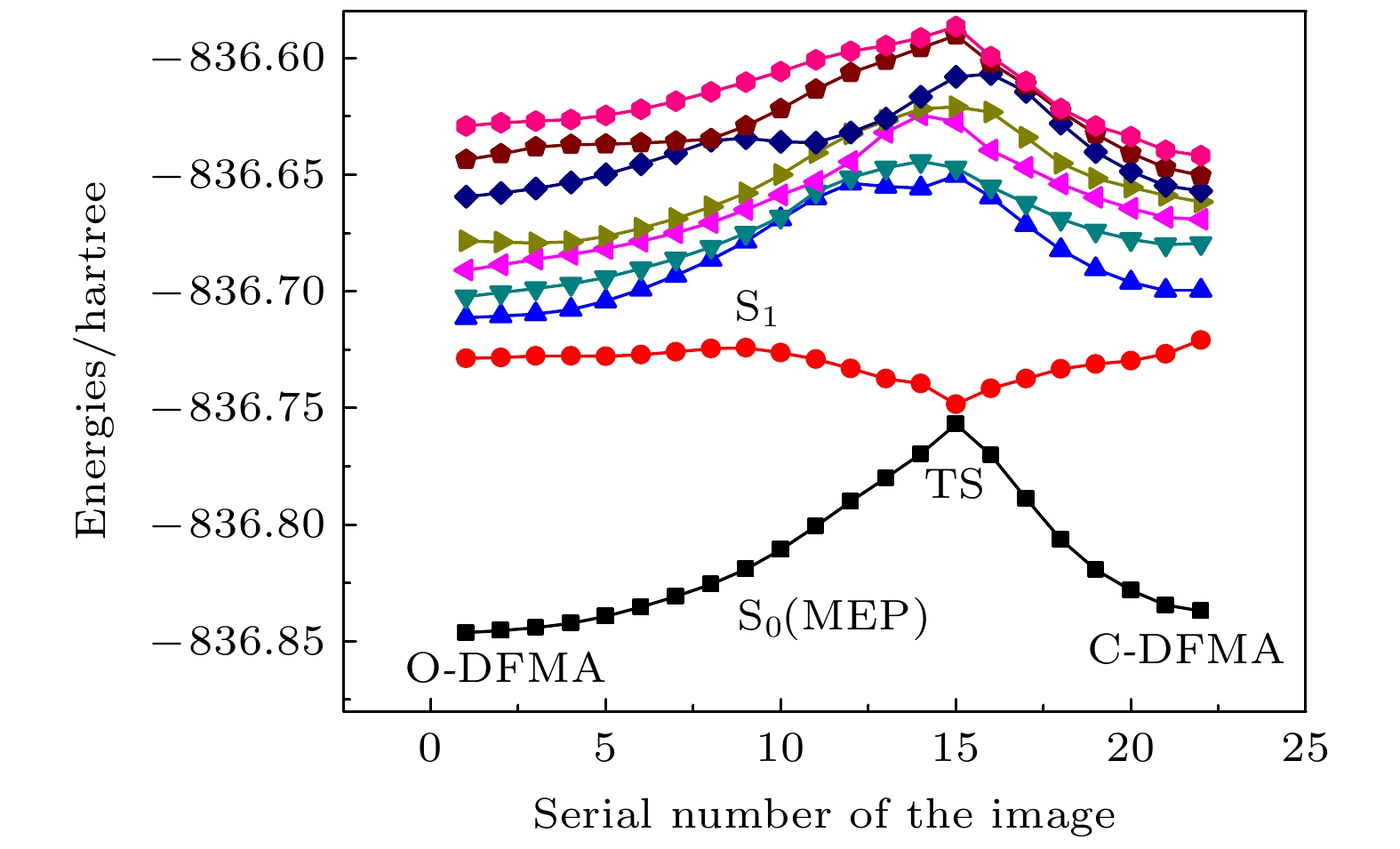
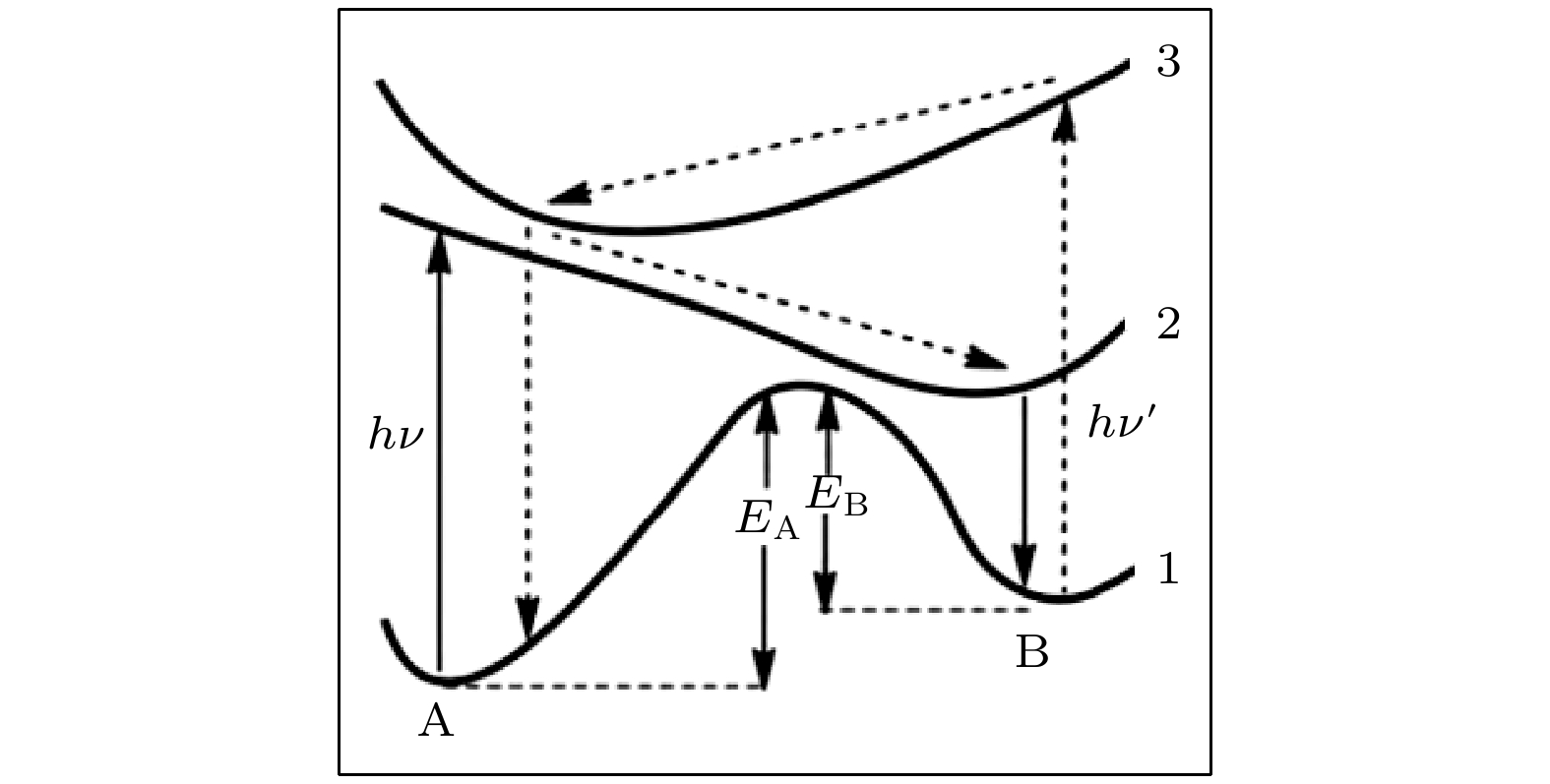
The photochromic switching mechanism of 2,3-difurylmaleic anhydride (DFMA) is investigated by first-principles calculations. Based on the stable structures of the open-ring (O-DFMA) and closed-ring (C-DFMA) of the DFMA, the minimum energy path (MEP) and the configuration of transition states (TS-DFMA) between the O-DFMA and C-DFMA are found by using the nudged elastic band (NEB) method, the potential barriers of O-DFMA and C-DFMA are 24959 cm–1(3.0945 eV) and 23328 cm–1(2.8923 eV), respectively, indicating that the DFMA molecule may be a thermally bistable molecule. Along the molecular configuration corresponding to the MEP curve (i.e. ground state S0), the potential energy curves of the lowest 8 singlet excited states of DFMA are calculated. Among these energy curves, only the first electronic excited state (i.e. S1 state) has a minimum value in the transition state (TS-DFMA) configuration. Combined with the molecular orbital transitions and orbital images, the photochromic mechanism of DFMA can be described as follows (1) From C-DFMA to O-DFMA process: under the action of the laser with S1–S0 resonance transition wavelength, the C-DFMA transits from S0 to S1 state, and then deactivates along the S1 potential energy curve, until a cross jumping transition occurs at the TS-DFMA structure from S1 to S0 and finally the molecule along the S0 potentioal energy curve returns to the O-DFMA configuration, then the switching action from closed-ring to open-ring is completed. The S1 state potential energy curve drops monotonically in this switching process, implying that there will be no fluorescent radiation in this process. (2) From O-DFMA to C-DFMA process: under the action of the laser with S1–S0 resonance transition wavelength, O-DFMA transits from S0 to S1 state. From the O-DFMA to TS-DFMA structure, there is a relatively “flat” area in the potential energy curve of the S1 state, and it decreases significantly only when it is close to the TS-DFMA. This means that O-DFMA needs to be excited with some vibrational modes to pass through the “flat” region of S1 and approaching to the TS-DFMA configuration, and then DFMA de-excites from the S1 state potential energy curve along a monotonic decline and a cross jumping transition from S1 to S0 occurs in the TS-DFMA configuration, completing the switching action from open-ring to closed-ring. It is also precisely because of the flat region of the potential energy curve of the initial S1 state that this excitation and switching process is accompanied by fluorescent radiations. The photochromic mechanism of DFMA indicates that it is suitable for making fluorescent molecular switches.
-
Keywords:
- 2, 3-difurylmaleic anhydride /
- photochromic mechanism /
- molecular switch /
- density functional theory /
- Nudged Elastic Band method /
- bistable states
[1] Irie M, Mohri M 1988 J. Org. Chem. 53 803
 Google Scholar
Google Scholar
[2] Nakamura S, Irie M 1988 J. Org. Chem. 53 6136
 Google Scholar
Google Scholar
[3] 徐海兵, 何亭, 唐明静, 彭雪峰, 邓建国 2014 科学通报 59 2900
 Google Scholar
Google Scholar
Hu H B, He T, Tang M J, Peng X F, Deng J G 2014 Chin. Sci. Bull. 59 2900
 Google Scholar
Google Scholar
[4] Irie M, Uchida K 1998 Bull. Chem. Soc. Jpn. 71 985
 Google Scholar
Google Scholar
[5] Tian H, Yang S 2004 Chem. Soc. Rev. 33 85
 Google Scholar
Google Scholar
[6] 齐国生, 肖家曦, 刘嵘, 蒋培军, 佘鹏, 徐端颐 2005 53 1076
Qi G S, Xiao J X, Liu R, Jiang P J, She P, Xu D Y 2005 Acta Phys. Sin. 53 1076
[7] 孙峰, 刘然, 索雨晴, 牛乐乐, 傅焕俨, 季文芳, 李宗良 2019 68 178502
 Google Scholar
Google Scholar
Sun F, Liu R, Suo Y Q, Niu L L, Fu H Y, Ji W F, Li Z L 2019 Acta Phys. Sin. 68 178502
 Google Scholar
Google Scholar
[8] Irie M 2000 Chem. Rev. 100 1685
 Google Scholar
Google Scholar
[9] Feringa B L 2001 Molecular Switches (Germany: Wiley-VCH Verlag GmbH, Weinheim)
[10] Tian H, Wang S 2007 Chem. Commun. 8 781
[11] Zhang J, Zou Q, Tian H 2013 Adv. Mater. 25 378
 Google Scholar
Google Scholar
[12] Patel P D, Mikhailov I A, Belfield K D 2009 Int. J. Quantum Chem. 109 3711
 Google Scholar
Google Scholar
[13] Shoji H, Kobatake S 2013 Chem.Commun. 49 2362
 Google Scholar
Google Scholar
[14] Mei X, Wang J, Zhou Z, Wu S, Huang L, Lin Z, Ling Q 2017 J. Mater. Chem. C 5 2135
 Google Scholar
Google Scholar
[15] Uchida K, Nakayama Y, Irie M 1990 Bull. Chem. Soc. Jpn. 63 1311
 Google Scholar
Google Scholar
[16] Nakayama Y, Hayashi K, Irie M 1990 J. Org. Chem. 55 2592
 Google Scholar
Google Scholar
[17] Nakayama Y, Hayashi K, Irie M 1991 Bull. Chem. Soc. Jpn. 64 789
 Google Scholar
Google Scholar
[18] 郭妮, 李苑莹, 李亚珍, 刘峰毅 2019 中国科学化学 49 1289
 Google Scholar
Google Scholar
Guo N, Li Y Y, Li Y Z, Liu F Y 2019 Sci. Sin. Chim. 49 1289
 Google Scholar
Google Scholar
[19] Zakharov A V, Gaeva E B, Lvov A G, Metelitsa A V, Shirinian V Z 2017 J. Org. Chem. 82 8651
 Google Scholar
Google Scholar
[20] Liu Y, Wang Q, Liu Y, Yang X Z 2003 Chem. Phys. Lett. 373 338
 Google Scholar
Google Scholar
[21] Jacquemin D, Perpète E A 2006 Chem. Phys. Lett. 429 147
 Google Scholar
Google Scholar
[22] Isegawa M, Morokuma K 2015 J. Phys. Chem. A 119 4191
 Google Scholar
Google Scholar
[23] 陈利菊, 姚保利, 韩俊鹤, 郜鹏, 陈懿, 王英利, 雷铭 2008 57 5571
 Google Scholar
Google Scholar
Chen L J, Yao B L, Han J H, Gao P, Chen Y, Wang Y L, Lei M 2008 Acta Phys. Sin. 57 5571
 Google Scholar
Google Scholar
[24] Liu R, Bi J J, Xie Z, Yin K K, Wang D Y, Zhang G P, Xiang D, Wang C K, Li Z L 2018 Phys. Rev. Appl. 9 054023
 Google Scholar
Google Scholar
[25] Li Z L, Bi J J, Liu R, Yi X H, Fu H Y, Sun F, Wei M Z, Wang C K 2017 Chin. Phys. B 26 098508
 Google Scholar
Google Scholar
[26] Weigend F, Ahlrichs R 2005 Phys. Chem. Chem. Phys. 7 3297
 Google Scholar
Google Scholar
[27] Mills G, J′onsson H, Schenter G 1995 Surf. Sci. 324 305
 Google Scholar
Google Scholar
[28] Henkelman G, J′onsson H 2000 J. Chem. Phys. 113 9978
 Google Scholar
Google Scholar
[29] de Souza B, Neese F, Izsák R 2018 J. Chem. Phys. 148 034104
 Google Scholar
Google Scholar
[30] Neese F 2017 Comput. Mol. Sci. 8 e1327
[31] Neese F, Wennmohs F, Becker U 2020 J. Chem. Phys. 152 224108
 Google Scholar
Google Scholar
-
表 1 O-DFMA和C-DFMA分子稳态构型参数
Table 1. Stable state configuration parameters of O-DFMA and C-DFMA.
Methods Structure RC2-C3/nm RO10-O17/nm DO10-C2-C3-O17/(°) Total thermal energy (hartree) Energy difference/cm–1 BP/SVP O-DFMA 0.1382 0.5552 20.24 –836.23050 1232 C-DFMA 0.1450 0.3156 0.92 –836.22488 B3LYP/TZVP O-DFMA 0.1359 0.5482 21.33 –836.63680 1815 C-DFMA 0.1447 0.3153 0.98 –836.62853 B3LYP/def2-TZVP O-DFMA 0.1359 0.5480 19.82 –836.68342 2055 C-DFMA 0.1445 0.3150 0.75 –836.67406 NEB/def2-SVP TS-DFMA 0.1380 0.3365 8.45 -
[1] Irie M, Mohri M 1988 J. Org. Chem. 53 803
 Google Scholar
Google Scholar
[2] Nakamura S, Irie M 1988 J. Org. Chem. 53 6136
 Google Scholar
Google Scholar
[3] 徐海兵, 何亭, 唐明静, 彭雪峰, 邓建国 2014 科学通报 59 2900
 Google Scholar
Google Scholar
Hu H B, He T, Tang M J, Peng X F, Deng J G 2014 Chin. Sci. Bull. 59 2900
 Google Scholar
Google Scholar
[4] Irie M, Uchida K 1998 Bull. Chem. Soc. Jpn. 71 985
 Google Scholar
Google Scholar
[5] Tian H, Yang S 2004 Chem. Soc. Rev. 33 85
 Google Scholar
Google Scholar
[6] 齐国生, 肖家曦, 刘嵘, 蒋培军, 佘鹏, 徐端颐 2005 53 1076
Qi G S, Xiao J X, Liu R, Jiang P J, She P, Xu D Y 2005 Acta Phys. Sin. 53 1076
[7] 孙峰, 刘然, 索雨晴, 牛乐乐, 傅焕俨, 季文芳, 李宗良 2019 68 178502
 Google Scholar
Google Scholar
Sun F, Liu R, Suo Y Q, Niu L L, Fu H Y, Ji W F, Li Z L 2019 Acta Phys. Sin. 68 178502
 Google Scholar
Google Scholar
[8] Irie M 2000 Chem. Rev. 100 1685
 Google Scholar
Google Scholar
[9] Feringa B L 2001 Molecular Switches (Germany: Wiley-VCH Verlag GmbH, Weinheim)
[10] Tian H, Wang S 2007 Chem. Commun. 8 781
[11] Zhang J, Zou Q, Tian H 2013 Adv. Mater. 25 378
 Google Scholar
Google Scholar
[12] Patel P D, Mikhailov I A, Belfield K D 2009 Int. J. Quantum Chem. 109 3711
 Google Scholar
Google Scholar
[13] Shoji H, Kobatake S 2013 Chem.Commun. 49 2362
 Google Scholar
Google Scholar
[14] Mei X, Wang J, Zhou Z, Wu S, Huang L, Lin Z, Ling Q 2017 J. Mater. Chem. C 5 2135
 Google Scholar
Google Scholar
[15] Uchida K, Nakayama Y, Irie M 1990 Bull. Chem. Soc. Jpn. 63 1311
 Google Scholar
Google Scholar
[16] Nakayama Y, Hayashi K, Irie M 1990 J. Org. Chem. 55 2592
 Google Scholar
Google Scholar
[17] Nakayama Y, Hayashi K, Irie M 1991 Bull. Chem. Soc. Jpn. 64 789
 Google Scholar
Google Scholar
[18] 郭妮, 李苑莹, 李亚珍, 刘峰毅 2019 中国科学化学 49 1289
 Google Scholar
Google Scholar
Guo N, Li Y Y, Li Y Z, Liu F Y 2019 Sci. Sin. Chim. 49 1289
 Google Scholar
Google Scholar
[19] Zakharov A V, Gaeva E B, Lvov A G, Metelitsa A V, Shirinian V Z 2017 J. Org. Chem. 82 8651
 Google Scholar
Google Scholar
[20] Liu Y, Wang Q, Liu Y, Yang X Z 2003 Chem. Phys. Lett. 373 338
 Google Scholar
Google Scholar
[21] Jacquemin D, Perpète E A 2006 Chem. Phys. Lett. 429 147
 Google Scholar
Google Scholar
[22] Isegawa M, Morokuma K 2015 J. Phys. Chem. A 119 4191
 Google Scholar
Google Scholar
[23] 陈利菊, 姚保利, 韩俊鹤, 郜鹏, 陈懿, 王英利, 雷铭 2008 57 5571
 Google Scholar
Google Scholar
Chen L J, Yao B L, Han J H, Gao P, Chen Y, Wang Y L, Lei M 2008 Acta Phys. Sin. 57 5571
 Google Scholar
Google Scholar
[24] Liu R, Bi J J, Xie Z, Yin K K, Wang D Y, Zhang G P, Xiang D, Wang C K, Li Z L 2018 Phys. Rev. Appl. 9 054023
 Google Scholar
Google Scholar
[25] Li Z L, Bi J J, Liu R, Yi X H, Fu H Y, Sun F, Wei M Z, Wang C K 2017 Chin. Phys. B 26 098508
 Google Scholar
Google Scholar
[26] Weigend F, Ahlrichs R 2005 Phys. Chem. Chem. Phys. 7 3297
 Google Scholar
Google Scholar
[27] Mills G, J′onsson H, Schenter G 1995 Surf. Sci. 324 305
 Google Scholar
Google Scholar
[28] Henkelman G, J′onsson H 2000 J. Chem. Phys. 113 9978
 Google Scholar
Google Scholar
[29] de Souza B, Neese F, Izsák R 2018 J. Chem. Phys. 148 034104
 Google Scholar
Google Scholar
[30] Neese F 2017 Comput. Mol. Sci. 8 e1327
[31] Neese F, Wennmohs F, Becker U 2020 J. Chem. Phys. 152 224108
 Google Scholar
Google Scholar
Catalog
Metrics
- Abstract views: 7940
- PDF Downloads: 95
- Cited By: 0














 DownLoad:
DownLoad: