-
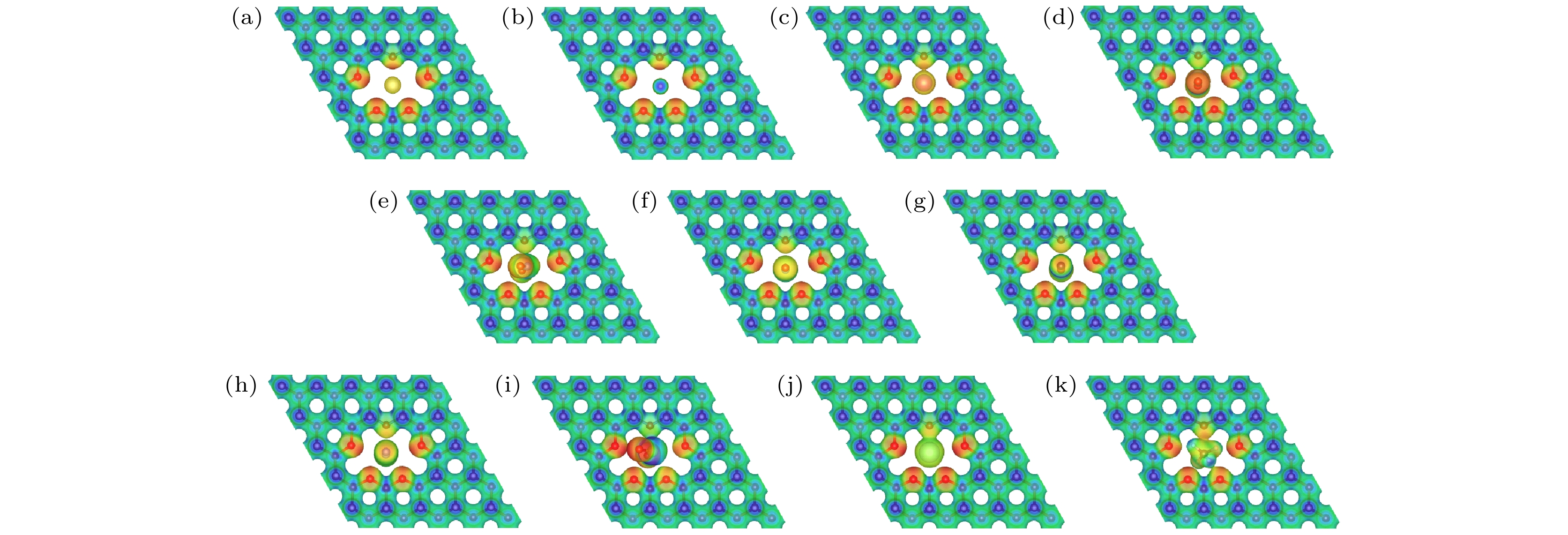
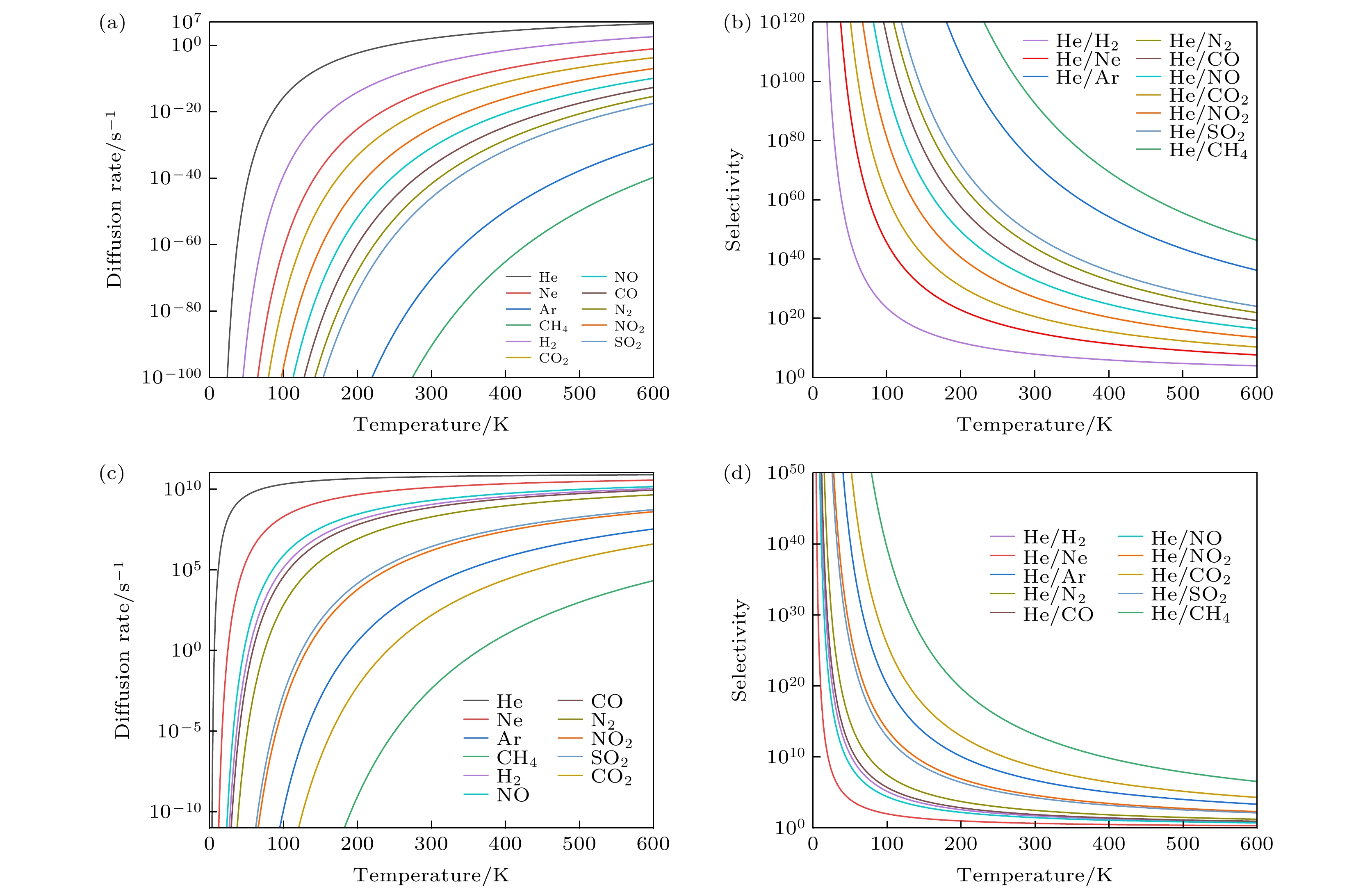
Helium (He) is widely used in many scientific and industrial fields, and the shortage of He resources and the growing demand make He separation extremely important. In this work, the He separation performances of a series of graphanes containing crown ether nanopores (crown ether graphane, CG-n, n = 3, 4, 5, 6) are studied by first-principles calculations. At first, the minimum energy paths of He and other 10 gas molecules (Ne, Ar, H2, CO, NO, NO2, N2, CO2, SO2 and CH4) passing through CG-n membranes are calculated, and the factors affecting the energy barriers are also investigated. The calculated results show that He is the easiest to pass through all the four CG-n membranes with energy barriers of 4.55, 1.05, 0.53 and 0.01 eV, respectively. He can be separated by CG-5 and CG-6 with very low energy barriers, and the energy barrier of He passing through CG-6 is the lowest, so far as we know. Moreover, all gas molecules can pass through CG-6 with low energy barriers, including many molecules with large kinetic diameters, such as CO (0.13 eV) and N2 (0.16 eV). Therefore, CG-6 is also expected to be used in the screening field of other gas molecules. In addition, it is found that the energy barriers of gas molecules passing through CG-n are synergistically affected by the size of the crown ether nanopore, the kinetic diameter and the type of the gas molecules. Secondly, the diffusion rates of gas molecules passing through CG-5 and CG-6 and the He selectivity towards other 10 gases of CG-5 and CG-6 at different temperatures are calculated. It is found that CG-5 exhibits extremely high He selectivity in a wide temperature range (0–600 K). In summary, the crown ether graphanes CG-5 and CG-6 can serve as excellent He separation membranes with high He selectivity. This work is expected to inspire one to develop other graphene-based two-dimensional separation membranes for separating He and other gas molecules.
-
Keywords:
- crown ether /
- hydrogenated graphene /
- membrane separation /
- density functional theory calculation /
- helium
[1] Cho A 2009 Science 326 778
 Google Scholar
Google Scholar
[2] 杨初平, 耿屹南, 王捷, 刘兴南, 时振刚 2021 70 135102
 Google Scholar
Google Scholar
Yang C P, Geng Y N, Wang J, Liu X N, Shi Z G 2021 Acta Phys. Sin. 70 135102
 Google Scholar
Google Scholar
[3] Fatemi S M, Abbasi Z, Rajabzadeh H, Hashemizadeh S A, Deldar A N 2017 Eur. Phys. J. D 71 194
 Google Scholar
Google Scholar
[4] Dai Z, Deng J, He X, Scholes C A, Jiang X, Wang B, Guo H, Ma Y, Deng L 2021 Sep. Purif. Technol. 274 119044
 Google Scholar
Google Scholar
[5] 王倩, 赵江山, 范元媛, 郭馨, 周翊 2020 69 174207
 Google Scholar
Google Scholar
Wang Q, Zhao J S, Fan Y Y, Guo X, Zhou Y 2020 Acta Phys. Sin. 69 174207
 Google Scholar
Google Scholar
[6] Wei S, Zhou S, Wu Z, Wang M, Wang Z, Guo W, Lu X 2018 Appl. Surf. Sci. 441 631
 Google Scholar
Google Scholar
[7] Rufford T E, Chan K I, Huang S H, May E F 2014 Adsorpt. Sci. Technol. 32 49
 Google Scholar
Google Scholar
[8] Stern S A, Sinclair T F, Gareis P J, Vahldieck N P, Mohr P H 1965 Ind. Eng. Chem. 57 49
[9] Yao B, Mandrà S, Curry J O, Shaikhutdinov S, Freund H J, Schrier J 2017 ACS Appl. Mater. Interfaces 9 43061
 Google Scholar
Google Scholar
[10] Pakdel S, Erfan-Niya H, Azamat J 2022 J. Mol. Graphics Modell. 115 108211
 Google Scholar
Google Scholar
[11] Mirzaei M, Karimi-Sabet J, Nikkho S, Towfighi-Darian J 2022 ACS Appl. Nano Mater. 5 1745
 Google Scholar
Google Scholar
[12] Schrier J 2010 J. Phys. Chem. Lett. 1 2284
 Google Scholar
Google Scholar
[13] Andrews N L P, Fan J Z, Forward R L, Chen M C, Loock H P 2017 Phys. Chem. Chem. Phys. 19 73
 Google Scholar
Google Scholar
[14] Malekian F, Ghafourian H, Zare K, Sharif A A, Zamani Y 2019 Eur. Phys. J. Plus 134 212
 Google Scholar
Google Scholar
[15] Liu M, Gurr P A, Fu Q, Webley P A, Qiao G G 2018 J. Mater. Chem. A 6 23169
 Google Scholar
Google Scholar
[16] Koenig S P, Wang L, Pellegrino J, Bunch J S 2012 Nat. Nanotechnol. 7 728
 Google Scholar
Google Scholar
[17] Peng Y, Li Y, Ban Y, Jin H, Jiao W, Liu X, Yang W 2014 Science 346 1356
 Google Scholar
Google Scholar
[18] Oyama S, Lee D, Hacarlioglu P, Saraf R 2004 J. Membr. Sci. 244 45
 Google Scholar
Google Scholar
[19] Kim H W, Yoon H W, Yoon S M, Yoo B M, Ahn B K, Cho Y H, Shin H J, Yang H, Paik U, Kwon S, Choi J Y, Park H B 2013 Science 342 91
 Google Scholar
Google Scholar
[20] Sun W 2021 Nat. Nanotechnol. 16 1054
 Google Scholar
Google Scholar
[21] Liu X, Chang X, Zhu L, Li X 2019 Comput. Mater. Sci. 157 1
 Google Scholar
Google Scholar
[22] Chen X, Zhang S, Hou D, Duan H, Deng B, Zeng Z, Liu B, Sun L, Song R, Du J, Gao P, Peng H, Liu Z, Wang L 2021 ACS Appl. Mater. Interfaces 13 29926
 Google Scholar
Google Scholar
[23] Wang Y, Li J, Yang Q, Zhong C 2016 ACS Appl. Mater. Interfaces 8 8694
 Google Scholar
Google Scholar
[24] Boutilier M S H, Sun C, O’Hern S C, Au H, Hadjiconstantinou N G, Karnik R 2014 ACS Nano 8 841
 Google Scholar
Google Scholar
[25] Hu W, Wu X, Li Z, Yang J 2013 Nanoscale 5 9062
 Google Scholar
Google Scholar
[26] Sluiter M H F, Kawazoe Y 2003 Phys. Rev. B 68 085410
 Google Scholar
Google Scholar
[27] Elias D C, Nair R R, Mohiuddin T M G, Morozov S V, Blake P, Halsall M P, Ferrari A C, Boukhvalov D W, Katsnelson M I, Geim A K, Novoselov K S 2009 Science 323 610
 Google Scholar
Google Scholar
[28] Pumera M, Wong C H A 2013 Chem. Soc. Rev. 42 5987
 Google Scholar
Google Scholar
[29] Guo K, Liu S, Tu H, Wang Z, Chen L, Lin H, Miao M, Xu J, Liu W 2021 Phys. Chem. Chem. Phys. 23 18983
 Google Scholar
Google Scholar
[30] Kresse G, Furthmüller J 1996 Comput. Mater. Sci. 6 15
 Google Scholar
Google Scholar
[31] Blöchl P E 1994 Phys. Rev. B 50 17953
 Google Scholar
Google Scholar
[32] Perdew J P, Burke K, Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[33] Grimme S, Antony J, Ehrlich S, Krieg H 2010 J. Chem. Phys. 132 154104
 Google Scholar
Google Scholar
[34] Chadi D J 1977 Phys. Rev. B 16 1746
 Google Scholar
Google Scholar
[35] Henkelman G, Uberuaga B P, Jónsson H 2000 J. Chem. Phys. 113 9901
 Google Scholar
Google Scholar
[36] Li X, Guo T, Zhu L, Ling C, Xue Q, Xing W 2018 Chem. Eng. J. 338 92
 Google Scholar
Google Scholar
[37] Li J R, Kuppler R J, Zhou H C 2009 Chem. Soc. Rev. 38 1477
 Google Scholar
Google Scholar
[38] Li F, Qu Y, Zhao M 2015 Carbon 95 51
 Google Scholar
Google Scholar
[39] Zhu L, Jin Y, Xue Q, Li X, Zheng H, Wu T, Ling C 2016 J. Mater. Chem. A 4 15015
 Google Scholar
Google Scholar
[40] Blankenburg S, Bieri M, Fasel R, Müllen K, Pignedoli C A, Passerone D 2010 Small 6 2266
 Google Scholar
Google Scholar
[41] Zhu L, Xue Q, Li X, Wu T, Jin Y, Xing W 2015 J. Mater. Chem. A 3 21351
 Google Scholar
Google Scholar
[42] Zhu Z 2006 J. Membr. Sci. 281 754
 Google Scholar
Google Scholar
-
表 1 气体分子在CG-n上稳定吸附时的吸附能Ead 和吸附高度H
Table 1. Adsorption energies Ead and the adsorption heights H of gas molecules adsorbed stably on CG-n.
CG-3 CG-4 CG-5 CG-6 Ead/eV H/Å Ead/eV H/Å Ead/eV H/Å Ead/eV H/Å He –0.15 2.89 –0.10 2.00 –0.12 2.40 –0.11 2.00 Ne –0.22 3.11 –0.17 2.82 –0.11 2.00 –0.14 2.00 Ar –0.18 4.00 –0.16 4.00 –0.15 4.00 –0.22 2.00 CH4 — — –0.37 2.40 –0.36 2.30 –0.29 1.79 H2 –0.24 2.70 –0.23 2.50 –0.14 2.00 –0.18 2.00 CO2 — — –0.15 3.70 –0.15 3.70 –0.53 0.00 NO — — –0.49 2.90 –0.22 3.10 –0.27 1.80 CO — — –0.25 3.00 –0.23 2.90 –0.22 2.00 N2 –0.33 3.10 –0.24 3.20 –0.22 3.10 –0.24 1.90 NO2 — — –0.40 3.10 –0.11 3.60 –0.29 0.00 SO2 — — — — –0.20 3.60 –0.27 0.00 表 2 气体分子的动力学直径 (D) 和通过CG-n膜时的能垒 Ebarrier. D值来自文献[37]
Table 2. Kinetic diameters (D) of the gas molecules, and energy barriers Ebarrier for gas molecules passing through each CG-n membrane. D values from literature [37].
D/Å Ebarrier/eV CG-3 CG-4 CG-5 CG-6 He 2.60 4.55 1.05 0.53 0.01 Ne 2.82 12.07 2.80 1.44 0.05 Ar 3.54 22.80 8.90 4.86 0.42 CH4 3.80 — 10.81 6.07 0.80 H2 2.89 6.23 1.91 1.00 0.12 CO2 3.30 — 3.45 1.76 0.53 NO 3.17 — 5.12 2.50 0.10 CO 3.69 — 5.48 2.83 0.13 N2 3.64 15.56 5.95 3.15 0.16 NO2 — — 5.42 2.15 0.29 SO2 4.12 — — 3.40 0.27 表 3 室温(300 K) 下, 多孔膜材料对He (相对于其他气体)的选择性 (S)
Table 3. Selectivity (S) of porous membrane materials for He (over other gases) at room temperature (300 K).
Type CG-5a CG-6a IGPb CTF-0c C2Nd g-C3N4e g-C2Of PGg S(He/Ne) 1.63×1015 4.66 1×106 4×106 3×103 1×1010 30 2×107 S(He/CH4) 4.03×1092 1.32×1013 7×1031 6×1038 7×1031 1×1065 1.15×1028 8×1037 S(He/Ar) 2.39×1072 5.24×106 6×1021 5×1035 4×1018 1×1051 1.68×1014 6×1036 S(He/N2) 6.24×1043 3.09×102 1×1012 2×1027 3×1012 1×1034 1.54×106 6×1027 S(He/CO) 2.79×1038 80.5 1×1011 5×1024 — 1×1030 6.72×104 6×1024 S(He/CO2) 3.63×1020 4.22×108 3×108 4×1016 8×1018 — 5.82×102 — S(He/H2) 7.18×107 52.7 — — — — — — S(He/NO) 8.51×1032 29.6 — — — — — — S(He/NO2) 1.20×1027 4.11×104 — — — — — — S(He/SO2) 9.42×1047 1.90×104 — — — — — — 注: a本工作, b文献[13], c文献[23], d文献[41], e文献[38], f文献[21], g文献[6]. -
[1] Cho A 2009 Science 326 778
 Google Scholar
Google Scholar
[2] 杨初平, 耿屹南, 王捷, 刘兴南, 时振刚 2021 70 135102
 Google Scholar
Google Scholar
Yang C P, Geng Y N, Wang J, Liu X N, Shi Z G 2021 Acta Phys. Sin. 70 135102
 Google Scholar
Google Scholar
[3] Fatemi S M, Abbasi Z, Rajabzadeh H, Hashemizadeh S A, Deldar A N 2017 Eur. Phys. J. D 71 194
 Google Scholar
Google Scholar
[4] Dai Z, Deng J, He X, Scholes C A, Jiang X, Wang B, Guo H, Ma Y, Deng L 2021 Sep. Purif. Technol. 274 119044
 Google Scholar
Google Scholar
[5] 王倩, 赵江山, 范元媛, 郭馨, 周翊 2020 69 174207
 Google Scholar
Google Scholar
Wang Q, Zhao J S, Fan Y Y, Guo X, Zhou Y 2020 Acta Phys. Sin. 69 174207
 Google Scholar
Google Scholar
[6] Wei S, Zhou S, Wu Z, Wang M, Wang Z, Guo W, Lu X 2018 Appl. Surf. Sci. 441 631
 Google Scholar
Google Scholar
[7] Rufford T E, Chan K I, Huang S H, May E F 2014 Adsorpt. Sci. Technol. 32 49
 Google Scholar
Google Scholar
[8] Stern S A, Sinclair T F, Gareis P J, Vahldieck N P, Mohr P H 1965 Ind. Eng. Chem. 57 49
[9] Yao B, Mandrà S, Curry J O, Shaikhutdinov S, Freund H J, Schrier J 2017 ACS Appl. Mater. Interfaces 9 43061
 Google Scholar
Google Scholar
[10] Pakdel S, Erfan-Niya H, Azamat J 2022 J. Mol. Graphics Modell. 115 108211
 Google Scholar
Google Scholar
[11] Mirzaei M, Karimi-Sabet J, Nikkho S, Towfighi-Darian J 2022 ACS Appl. Nano Mater. 5 1745
 Google Scholar
Google Scholar
[12] Schrier J 2010 J. Phys. Chem. Lett. 1 2284
 Google Scholar
Google Scholar
[13] Andrews N L P, Fan J Z, Forward R L, Chen M C, Loock H P 2017 Phys. Chem. Chem. Phys. 19 73
 Google Scholar
Google Scholar
[14] Malekian F, Ghafourian H, Zare K, Sharif A A, Zamani Y 2019 Eur. Phys. J. Plus 134 212
 Google Scholar
Google Scholar
[15] Liu M, Gurr P A, Fu Q, Webley P A, Qiao G G 2018 J. Mater. Chem. A 6 23169
 Google Scholar
Google Scholar
[16] Koenig S P, Wang L, Pellegrino J, Bunch J S 2012 Nat. Nanotechnol. 7 728
 Google Scholar
Google Scholar
[17] Peng Y, Li Y, Ban Y, Jin H, Jiao W, Liu X, Yang W 2014 Science 346 1356
 Google Scholar
Google Scholar
[18] Oyama S, Lee D, Hacarlioglu P, Saraf R 2004 J. Membr. Sci. 244 45
 Google Scholar
Google Scholar
[19] Kim H W, Yoon H W, Yoon S M, Yoo B M, Ahn B K, Cho Y H, Shin H J, Yang H, Paik U, Kwon S, Choi J Y, Park H B 2013 Science 342 91
 Google Scholar
Google Scholar
[20] Sun W 2021 Nat. Nanotechnol. 16 1054
 Google Scholar
Google Scholar
[21] Liu X, Chang X, Zhu L, Li X 2019 Comput. Mater. Sci. 157 1
 Google Scholar
Google Scholar
[22] Chen X, Zhang S, Hou D, Duan H, Deng B, Zeng Z, Liu B, Sun L, Song R, Du J, Gao P, Peng H, Liu Z, Wang L 2021 ACS Appl. Mater. Interfaces 13 29926
 Google Scholar
Google Scholar
[23] Wang Y, Li J, Yang Q, Zhong C 2016 ACS Appl. Mater. Interfaces 8 8694
 Google Scholar
Google Scholar
[24] Boutilier M S H, Sun C, O’Hern S C, Au H, Hadjiconstantinou N G, Karnik R 2014 ACS Nano 8 841
 Google Scholar
Google Scholar
[25] Hu W, Wu X, Li Z, Yang J 2013 Nanoscale 5 9062
 Google Scholar
Google Scholar
[26] Sluiter M H F, Kawazoe Y 2003 Phys. Rev. B 68 085410
 Google Scholar
Google Scholar
[27] Elias D C, Nair R R, Mohiuddin T M G, Morozov S V, Blake P, Halsall M P, Ferrari A C, Boukhvalov D W, Katsnelson M I, Geim A K, Novoselov K S 2009 Science 323 610
 Google Scholar
Google Scholar
[28] Pumera M, Wong C H A 2013 Chem. Soc. Rev. 42 5987
 Google Scholar
Google Scholar
[29] Guo K, Liu S, Tu H, Wang Z, Chen L, Lin H, Miao M, Xu J, Liu W 2021 Phys. Chem. Chem. Phys. 23 18983
 Google Scholar
Google Scholar
[30] Kresse G, Furthmüller J 1996 Comput. Mater. Sci. 6 15
 Google Scholar
Google Scholar
[31] Blöchl P E 1994 Phys. Rev. B 50 17953
 Google Scholar
Google Scholar
[32] Perdew J P, Burke K, Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[33] Grimme S, Antony J, Ehrlich S, Krieg H 2010 J. Chem. Phys. 132 154104
 Google Scholar
Google Scholar
[34] Chadi D J 1977 Phys. Rev. B 16 1746
 Google Scholar
Google Scholar
[35] Henkelman G, Uberuaga B P, Jónsson H 2000 J. Chem. Phys. 113 9901
 Google Scholar
Google Scholar
[36] Li X, Guo T, Zhu L, Ling C, Xue Q, Xing W 2018 Chem. Eng. J. 338 92
 Google Scholar
Google Scholar
[37] Li J R, Kuppler R J, Zhou H C 2009 Chem. Soc. Rev. 38 1477
 Google Scholar
Google Scholar
[38] Li F, Qu Y, Zhao M 2015 Carbon 95 51
 Google Scholar
Google Scholar
[39] Zhu L, Jin Y, Xue Q, Li X, Zheng H, Wu T, Ling C 2016 J. Mater. Chem. A 4 15015
 Google Scholar
Google Scholar
[40] Blankenburg S, Bieri M, Fasel R, Müllen K, Pignedoli C A, Passerone D 2010 Small 6 2266
 Google Scholar
Google Scholar
[41] Zhu L, Xue Q, Li X, Wu T, Jin Y, Xing W 2015 J. Mater. Chem. A 3 21351
 Google Scholar
Google Scholar
[42] Zhu Z 2006 J. Membr. Sci. 281 754
 Google Scholar
Google Scholar
-
 068201-20222183-补充材料.pdf
068201-20222183-补充材料.pdf

Catalog
Metrics
- Abstract views: 4651
- PDF Downloads: 68
- Cited By: 0

















 DownLoad:
DownLoad: