-
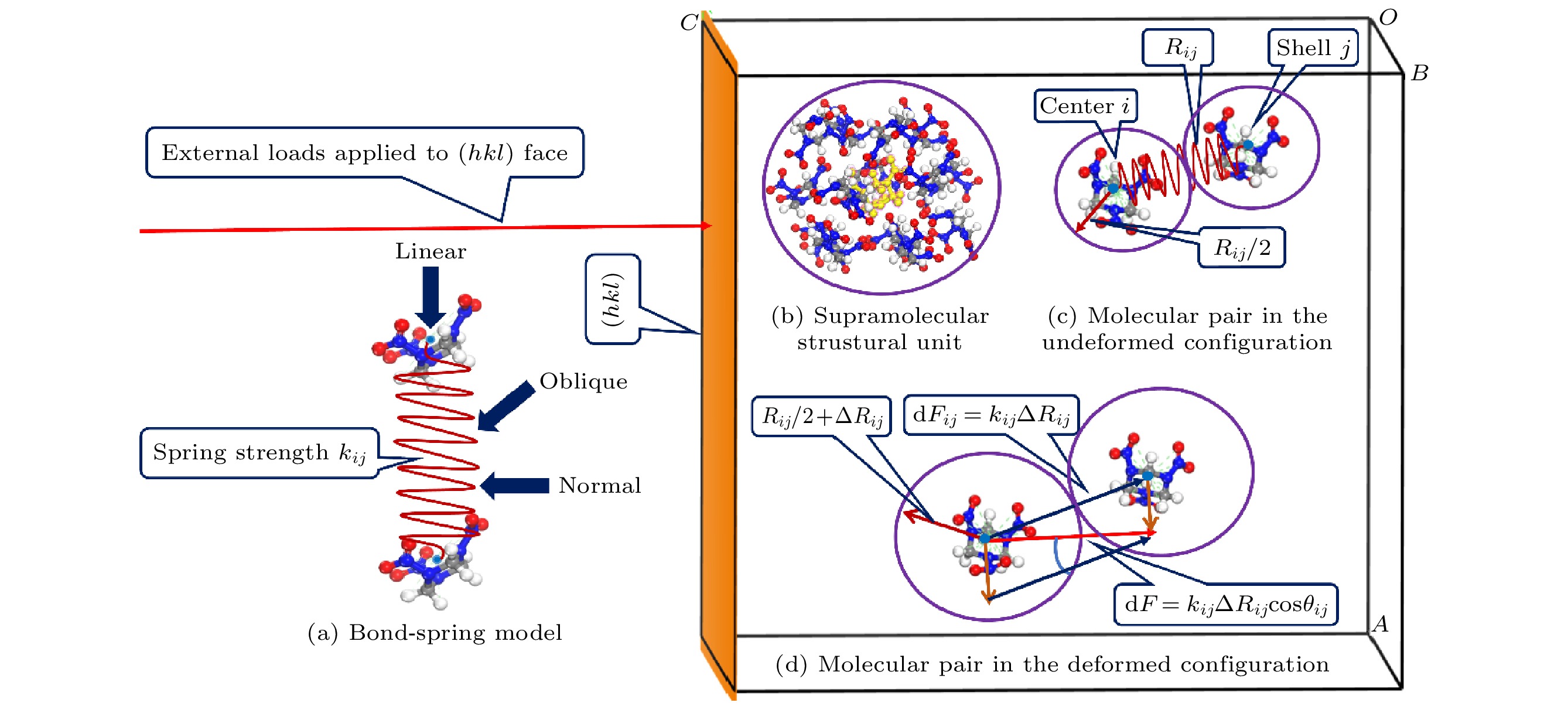
The relation between elastic property and crystal structure provides a foundation for designing new materials with desired properties and understanding the chemical decomposition and explosion of energetic materials. The supramolecular structural unit is proposed as the smallest chemical unit to quantitatively characterize the elastic anisotropy of 1, 3, 5-trinitro-1, 3, 5-triazacyclohexane (RDX). The supramolecular structural unit refers to the nearest-neighbor coordination polyhedron of one molecule. The supramolecular structural unit of RDX is composed of 15 molecules, and analyzed by the total molecular number density and the density of intermolecular interactions. The elastic modulus model is established on the assumption that 1) the RDX molecule is of sphere and rigid-body; 2) the intermolecular interaction is regarded as a linear spring, i.e. it is described by a bond-spring model; 3) the molecules are close-packed in the series mode. The elastic modulus model based on the supramolecular structural unit demonstrates that the elastic modulus is intrinsically determined by the total molecular number, the equilibrium distance of the molecular pair, the intermolecular force constant, and the angle between the intermolecular non-bonding interaction and the normal to crystal face. The intermolecular force constant is calculated as the second derivative of the intermolecular interaction with respect to the equilibrium centroid distance. The intermolecular interaction is expressed as the summation of van der Waals and electrostatic interactions calculated by COMPASS (condensed-phase optimized molecular potentials for atomistic simulation studies) II forcefield. The calculated elastic moduli are 21.7, 17.1, 20.1, 19.1, and 15.3 GPa for RDX (100), (010), (001), (210), and (021) crystal faces, respectively. The calculation results are consistent with the theoretical values computed by the density functional theory. Excluding RDX(001), the calculated elastic moduli accord with the experimental results measured by the resonant ultrasound spectroscopy (RUS), impulsive stimulated thermal scattering, Brillouin spectroscopy, and nanoindentation methods. The theoretical value (20.1 GPa) of RDX(001) overestimates the experimental values in a range of 15.9–16.6 GPa. The reason can be attributed to the rigid-body approximation for flexible molecules, in which are ignored the motion and deformation of the ring and NO2 groups when the external loads are applied to RDX(001). The results suggest that the supramolecular structural unit can be the smallest chemical unit to quantitatively characterize the elastic anisotropy of RDX and the elastic anisotropy is mainly due to the angle between the intermolecular interaction and the normal to crystal face.
-
Keywords:
- 1, 3, 5-trinitro-1, 3, 5-triazacyclohexane /
- supramolecular structural unit /
- elastic anisotropy
[1] Mishra M K, Sanphui P, Ramamurty U, Desiraju G 2014 Cryst. Growth. Des. 14 3054
 Google Scholar
Google Scholar
[2] Sunil S L, Kiran M, Ramamurty U, Varughese S 2018 Chem. Eur. J. 25 526
 Google Scholar
Google Scholar
[3] Armstrong R, Elban W L 2006 Mater. Sci. Technol. 22 381
 Google Scholar
Google Scholar
[4] 王鹏举, 范俊宇, 苏艳, 赵纪军 2020 69 238702
 Google Scholar
Google Scholar
Wang P J, Fan J Y, Su Y, Zhao J J 2020 Acta Phys. Sin. 69 238702
 Google Scholar
Google Scholar
[5] Ramos K J, Hooks D E, Bahr D F 2009 Philos. Mag. 89 2381
 Google Scholar
Google Scholar
[6] Haussühl S 2001 Z. Krist-Cryst. Mater. 216 339
 Google Scholar
Google Scholar
[7] Schwarz R, Hooks D, Dick J, Archuleta J, Martinez A 2005 J. Appl. Phys. 98 056106
 Google Scholar
Google Scholar
[8] Sun B, Winey J, Hemmi N, Dreger Z, Zimmerman K, Gupta Y, Torchinsky D H, Nelson K A 2008 J. Appl. Phys. 104 073517
 Google Scholar
Google Scholar
[9] Bolme C A, Ramos K J 2014 J. Appl. Phys. 116 77
 Google Scholar
Google Scholar
[10] Weingarten N S, Sausa R C 2015 J. Phys. Chem. A 119 9338
 Google Scholar
Google Scholar
[11] Taylor D E 2014 J. Appl. Phys. 116 053513
 Google Scholar
Google Scholar
[12] Liu J, Zeng Q, Zhang Y L, Zhang C Y 2016 J. Phys. Chem. C 120 15198
 Google Scholar
Google Scholar
[13] Shi Y B, Bai L F, Li J H, Sun G A, Gong J, Ju X 2019 J. Mol. Model. 25 299
 Google Scholar
Google Scholar
[14] Zhu S H, Qin H, Zeng W, Liu F S, Tang B, Liu Q J, Li R X, Gan Y D 2020 Philos Mag. 100 1015
 Google Scholar
Google Scholar
[15] Fan J Y, Su Y, Zhang Q Y, Zhao J J 2019 Comp. Mater. Sci. 161 379
 Google Scholar
Google Scholar
[16] Hang G Y, Yu W L, Wang T, Wang J T, Li Z 2017 J. Mol. Struct. 1141 577
 Google Scholar
Google Scholar
[17] Sun H J 1998 J. Phys. Chem. B 102 7338
 Google Scholar
Google Scholar
[18] Spackman P R, Grosjean A, Thomas S P, Karothu D P, Naumov P, Spackman M A 2022 Angew. Chem. Int. Ed. 61 e202110716
 Google Scholar
Google Scholar
[19] Day G M, Price S L, Leslie M 2001 Cryst. Growth. Des. 1 13
 Google Scholar
Google Scholar
[20] Zhang S, Wang Q, Dong C 2021 J. Mater. Inf. 1 8
 Google Scholar
Google Scholar
[21] Dong D D, Zhang S, Wang Z J, Dong C, Haeussler P 2016 Mater. Design. 96 115
 Google Scholar
Google Scholar
[22] Wang Z R, Qiang J B, Wang Y M, Wang Q, Dong D D, Dong C 2016 Acta Mater. 111 366
 Google Scholar
Google Scholar
[23] Ma Y, Wang Q, Jiang B B, Li C L, Hao J M, Li X N, Dong C, Nieh T G 2018 Acta Mater. 147 213
 Google Scholar
Google Scholar
[24] Dong D D, Cao Z M, Han G, Dong C 2021 AIP. Adv. 11 035140
 Google Scholar
Google Scholar
[25] Chen H, Luo L J, Qiang J B, Wang Y M, Dong C 2014 Philos. Mag. 94 1463
 Google Scholar
Google Scholar
[26] Friedel J 1958 II Nuovo. Cimento. 7 287
 Google Scholar
Google Scholar
[27] Dong C, Wang Z J, Zhang S, Wang Y M 2019 Int. Mater. Rev. 65 286
 Google Scholar
Google Scholar
[28] Li T, Morris K R, Park K 2000 J. Phys. Chem. B 104 2019
 Google Scholar
Google Scholar
[29] Bandyopadhya R, Grant D 2002 Pharm. Res. 19 491
 Google Scholar
Google Scholar
[30] Zaccone A, Lattuada M, Wu H, Morbidelli M 2007 J. Chem. Phys. 127 174512
 Google Scholar
Google Scholar
[31] Weiner J H 1984 J. Appl. Mech. 51 707
 Google Scholar
Google Scholar
[32] Gao C, Yang L, Zeng Y, Wang X, Zhang C, Dai R, Wang Z, Zheng X, Zhang Z 2017 J. Phys. Chem. C 121 17586
 Google Scholar
Google Scholar
[33] Accelrys. Materials Studio Release Notes, Release 5.5, Accelrys Software. Inc. San Diego 2010, https://www.3ds.com/products-services/biovia/ [2022-8-10]
[34] Desiraju G R 2013 J. Am. Chem. Soc. 135 9952
 Google Scholar
Google Scholar
[35] Bu R P, Xiong Y, Wei X F, Li H Z, Zhang C Y 2019 Cryst. Growth. Des. 19 5981
 Google Scholar
Google Scholar
[36] Konovalova I S, Shishkina S V, Bani-Khaled G, Muzyka E N, Boyko A N 2019 Cryst. Eng. Comm. 21 2908
 Google Scholar
Google Scholar
[37] Eckhardt C J, Gavezzotti A 2007 J. Phys. Chem. B 111 3430
 Google Scholar
Google Scholar
[38] Peng Q, Rahul, Wang G Y, Liu G R, Grimme S, De S 2015 J. Phys. Chem. B 119 5896
 Google Scholar
Google Scholar
-
图 1 弹性模量模型示意图 (a) 键-弹簧模型; (b) RDX的超分子结构单元; (c) 未变形 (即平衡状态)时的分子对构型; (d) 外界载荷作用下, 发生形变后的分子对构型. 其中, 黄色分子代表RDX超分子结构单元的中心分子
Figure 1. Schematic diagram of the elastic modulus model: (a) Bond-spring model; (b) the supramolecular structural unit of RDX; (c) molecular pair in the un-deformed (i.e., equilibrium position) configuration; (d) molecular pair in the deformed configuration under the external loads. The yellow molecule represents the central molecule of the supramolecular structural unit of RDX.
表 1 分子间非键能曲线确定的平衡距离R0[12]、分子间作用能Elow和分子间力常数k, 以及真实晶胞中的平衡距离r0和分子间非键能E0[37]
Table 1. Equilibrium distance R0[12], the lowest intermolecular interaction Elow, and intermolecular force constant k obtained by intermolecular non-bonded interaction curves, the equilibrium distance r0 and intermolecular non-bonded interaction E0[37] in the actual crystal lattice.
r0/nm R0/nm[12] R0/nm Elow/(kcal·mol–1) E0/(kcal·mol–1)[37] k/(N·m–1) 0.4415044 0.42603 0.428 –6.86 –6.25 19.985 0.6447523 0.63385 0.660 –1.57 –2.68 7.211 0.6550915 0.64700 0.640 –3.22 –2.68 6.729 0.6944433 0.68725 0.691 –3.94 –3.35 10.677 0.7291993 0.70889 0.737 –4.46 –5.58 9.546 0.7292055 — 0.710 –6.39 –5.80 18.522 0.8144825 0.76922 0.760 –2.28 — 5.045 0.8146958 — 0.754 –4.76 — 13.018 表 2 RDX超分子结构单元内分子对的平衡位置R0、分子间非键能与晶面法线(hkl )的夹角余弦值cosθ和分子间力常数k
Table 2. Equilibrium distance R0 of the molecular pair, the cosine value of the angle cosθ between the intermolecular non-bonded interactions and the normal to (hkl ), and the intermolecular force constants k within the RDX supramolecular structural unit.
R0/nm k/(N·m–1) cosθ (021) cosθ (210) cosθ (001) cosθ (100) cosθ (010) 0.428 19.985 0.0676 –0.1388 –0.7174 –0.9491 0 0.660 7.211 –0.0622 –0.3031 0.8305 0.9491 0 0.660 7.211 –0.8999 –0.3031 –0.8305 –0.2645 0.2695 0.640 6.729 –0.5944 –0.7566 0.3339 –0.4880 0.4972 0.640 6.729 0.9312 0.2048 0.3339 0.6084 –0.7936 0.691 10.677 0.1588 –0.7963 0.315 0.6084 0.7936 0.691 10.677 0.1588 0.7963 0.315 0 –0.5571 0.737 9.546 –0.6853 0.0786 0 0 –0.5571 0.737 9.546 0.6853 0.9423 0 0.6084 0.3011 0.710 18.522 0.6303 0.6743 0.7343 0.6084 0.3011 0.710 18.522 –0.1103 0.6743 –0.7343 –0.8090 –0.4409 0.760 5.045 0.6997 –0.0752 0.9260 0.8090 –0.4409 0.754 13.018 –0.5768 –0.9186 –0.3888 –0.3289 –0.8834 0.754 13.018 –0.5768 0.4389 –0.3888 –0.3289 0.8834 表 3 由不同实验方法和理论计算得到的RDX多个晶面的弹性模量. 其中, ERUS, EISTS, EBri和Enano分别代表由超声共振谱、脉冲激热散射法、布里渊散射法和纳米压痕法实验测定的弹性模量值; EDFT和Ecal为密度泛函理论和超分子结构单元法的计算值
Table 3. Elastic moduli of multiple crystal faces for RDX are obtained by experimental and theoretical calculations. ERUS, EISTS, EBri, and Enano refer to the elastic moduli experimentally measured by resonant ultrasound spectroscopy, impulsive stimulated thermal scattering, Brillouin spectroscopy, and nanoindentation approaches, respectively. EDFT and Ecal represent the elastic moduli theoretically calculated by the density functional theory and the supramolecular structural unit, respectively.
-
[1] Mishra M K, Sanphui P, Ramamurty U, Desiraju G 2014 Cryst. Growth. Des. 14 3054
 Google Scholar
Google Scholar
[2] Sunil S L, Kiran M, Ramamurty U, Varughese S 2018 Chem. Eur. J. 25 526
 Google Scholar
Google Scholar
[3] Armstrong R, Elban W L 2006 Mater. Sci. Technol. 22 381
 Google Scholar
Google Scholar
[4] 王鹏举, 范俊宇, 苏艳, 赵纪军 2020 69 238702
 Google Scholar
Google Scholar
Wang P J, Fan J Y, Su Y, Zhao J J 2020 Acta Phys. Sin. 69 238702
 Google Scholar
Google Scholar
[5] Ramos K J, Hooks D E, Bahr D F 2009 Philos. Mag. 89 2381
 Google Scholar
Google Scholar
[6] Haussühl S 2001 Z. Krist-Cryst. Mater. 216 339
 Google Scholar
Google Scholar
[7] Schwarz R, Hooks D, Dick J, Archuleta J, Martinez A 2005 J. Appl. Phys. 98 056106
 Google Scholar
Google Scholar
[8] Sun B, Winey J, Hemmi N, Dreger Z, Zimmerman K, Gupta Y, Torchinsky D H, Nelson K A 2008 J. Appl. Phys. 104 073517
 Google Scholar
Google Scholar
[9] Bolme C A, Ramos K J 2014 J. Appl. Phys. 116 77
 Google Scholar
Google Scholar
[10] Weingarten N S, Sausa R C 2015 J. Phys. Chem. A 119 9338
 Google Scholar
Google Scholar
[11] Taylor D E 2014 J. Appl. Phys. 116 053513
 Google Scholar
Google Scholar
[12] Liu J, Zeng Q, Zhang Y L, Zhang C Y 2016 J. Phys. Chem. C 120 15198
 Google Scholar
Google Scholar
[13] Shi Y B, Bai L F, Li J H, Sun G A, Gong J, Ju X 2019 J. Mol. Model. 25 299
 Google Scholar
Google Scholar
[14] Zhu S H, Qin H, Zeng W, Liu F S, Tang B, Liu Q J, Li R X, Gan Y D 2020 Philos Mag. 100 1015
 Google Scholar
Google Scholar
[15] Fan J Y, Su Y, Zhang Q Y, Zhao J J 2019 Comp. Mater. Sci. 161 379
 Google Scholar
Google Scholar
[16] Hang G Y, Yu W L, Wang T, Wang J T, Li Z 2017 J. Mol. Struct. 1141 577
 Google Scholar
Google Scholar
[17] Sun H J 1998 J. Phys. Chem. B 102 7338
 Google Scholar
Google Scholar
[18] Spackman P R, Grosjean A, Thomas S P, Karothu D P, Naumov P, Spackman M A 2022 Angew. Chem. Int. Ed. 61 e202110716
 Google Scholar
Google Scholar
[19] Day G M, Price S L, Leslie M 2001 Cryst. Growth. Des. 1 13
 Google Scholar
Google Scholar
[20] Zhang S, Wang Q, Dong C 2021 J. Mater. Inf. 1 8
 Google Scholar
Google Scholar
[21] Dong D D, Zhang S, Wang Z J, Dong C, Haeussler P 2016 Mater. Design. 96 115
 Google Scholar
Google Scholar
[22] Wang Z R, Qiang J B, Wang Y M, Wang Q, Dong D D, Dong C 2016 Acta Mater. 111 366
 Google Scholar
Google Scholar
[23] Ma Y, Wang Q, Jiang B B, Li C L, Hao J M, Li X N, Dong C, Nieh T G 2018 Acta Mater. 147 213
 Google Scholar
Google Scholar
[24] Dong D D, Cao Z M, Han G, Dong C 2021 AIP. Adv. 11 035140
 Google Scholar
Google Scholar
[25] Chen H, Luo L J, Qiang J B, Wang Y M, Dong C 2014 Philos. Mag. 94 1463
 Google Scholar
Google Scholar
[26] Friedel J 1958 II Nuovo. Cimento. 7 287
 Google Scholar
Google Scholar
[27] Dong C, Wang Z J, Zhang S, Wang Y M 2019 Int. Mater. Rev. 65 286
 Google Scholar
Google Scholar
[28] Li T, Morris K R, Park K 2000 J. Phys. Chem. B 104 2019
 Google Scholar
Google Scholar
[29] Bandyopadhya R, Grant D 2002 Pharm. Res. 19 491
 Google Scholar
Google Scholar
[30] Zaccone A, Lattuada M, Wu H, Morbidelli M 2007 J. Chem. Phys. 127 174512
 Google Scholar
Google Scholar
[31] Weiner J H 1984 J. Appl. Mech. 51 707
 Google Scholar
Google Scholar
[32] Gao C, Yang L, Zeng Y, Wang X, Zhang C, Dai R, Wang Z, Zheng X, Zhang Z 2017 J. Phys. Chem. C 121 17586
 Google Scholar
Google Scholar
[33] Accelrys. Materials Studio Release Notes, Release 5.5, Accelrys Software. Inc. San Diego 2010, https://www.3ds.com/products-services/biovia/ [2022-8-10]
[34] Desiraju G R 2013 J. Am. Chem. Soc. 135 9952
 Google Scholar
Google Scholar
[35] Bu R P, Xiong Y, Wei X F, Li H Z, Zhang C Y 2019 Cryst. Growth. Des. 19 5981
 Google Scholar
Google Scholar
[36] Konovalova I S, Shishkina S V, Bani-Khaled G, Muzyka E N, Boyko A N 2019 Cryst. Eng. Comm. 21 2908
 Google Scholar
Google Scholar
[37] Eckhardt C J, Gavezzotti A 2007 J. Phys. Chem. B 111 3430
 Google Scholar
Google Scholar
[38] Peng Q, Rahul, Wang G Y, Liu G R, Grimme S, De S 2015 J. Phys. Chem. B 119 5896
 Google Scholar
Google Scholar
Catalog
Metrics
- Abstract views: 7433
- PDF Downloads: 81
- Cited By: 0














 DownLoad:
DownLoad:


