-
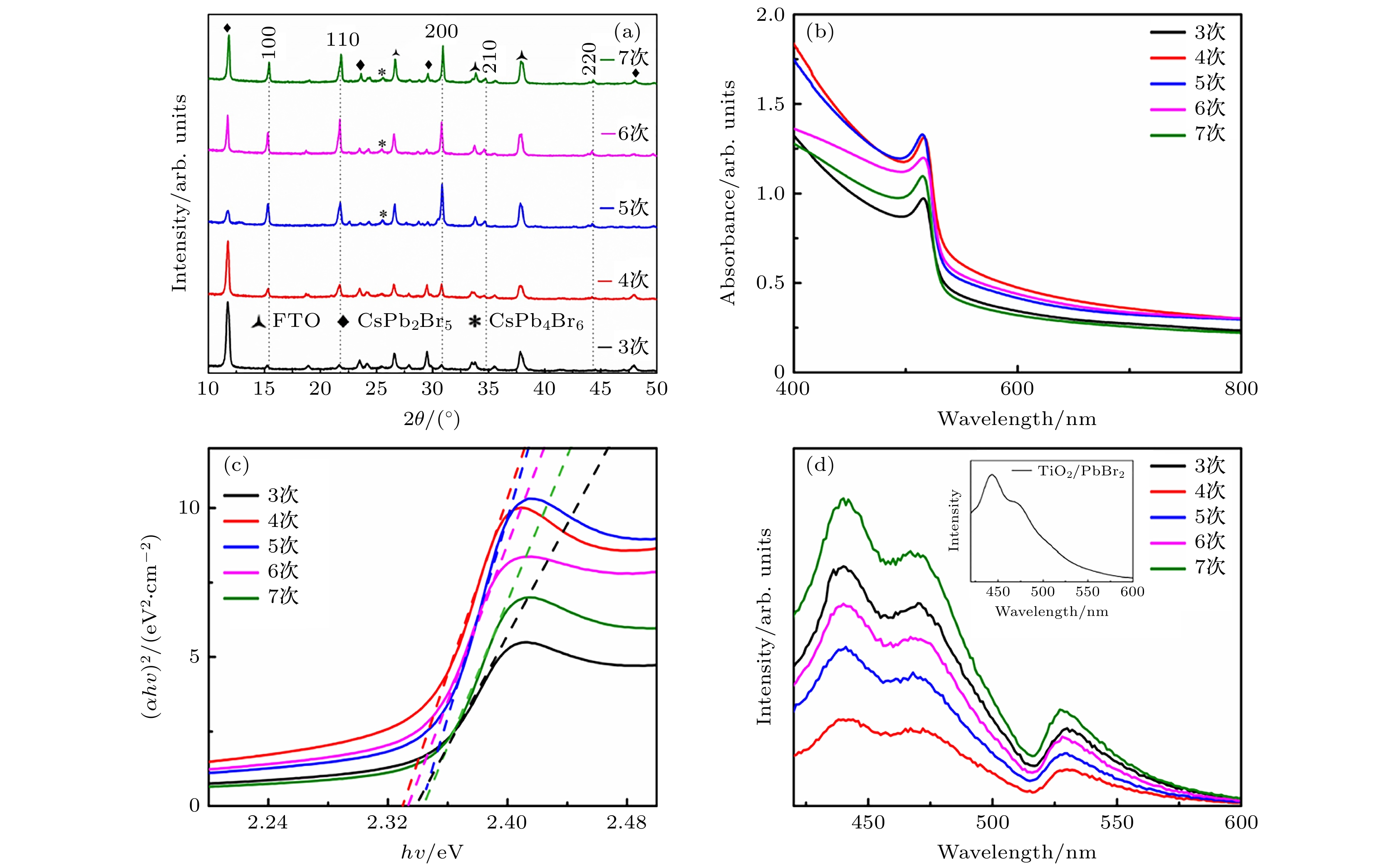
The quality of perovskite films plays a crucial role in solar cell, which can affect the stability and power conversion efficiency (PCE). As one of inorganic perovskites with excellent stability, CsPbBr3 perovskite is usually prepared by multi-step method due to the large difference in solubility between its precursor salts (PbBr2 and CsBr). The main reason is that the formation mechanism of CsPbBr3 film is not thoroughly studied. The incomplete reaction of PbBr2 and emergence of Cs4PbBr6 when the CsBr is excessive become problems that need to be solved urgently. In this paper, the phase transition of films during spin coating is observed in detail. In the process of film formation, the CsBr diffuses into the predeposited PbBr2 film to complete the reaction. The short reaction time results in insufficient reactions inside the film but overreaction on the surface of film. The CsPb2Br5 and Cs4PbBr6 appear with CsPbBr3 perovskite, and the film formed by repetitively annealing blocks the diffusion of CsBr. Methanol has an etching effect on the perovskite film which can eliminate the blocking effect. By extending the reaction time of CsBr solution on the film surface, the PbBr2 in the bottom layer is fully reacted, and after being annealed, the perovskite film will recrystallize to form a compact film. With the reaction time controlled appropriately, the CsPb2Br5 in the film can be effectively reduced and Cs4PbBr6 will not appear. The film grain size increases, grain boundary decreases, and the recombination is effectively inhibited, which ensures the improvement of the photoelectric performance of the solar cell. Under the condition of spin-coating four times and reaction time of 30 s, the solar cell has 6.30% PCE, Voc = 1.28 V, Jsc = 8.40 mA/cm2, FF = 0.59 . Comparing with the solar cells with no extended reaction time, the PCE improves more than 18%. This work will provide an important insight into the growth mechanism of perovskite film toward high crystallinity and less defects.
-
Keywords:
- inorganic perovskite /
- CsPbBr3 /
- multi-step spin-coating method /
- formation mechanism /
- reaction time
[1] National Renewable Energy Laboratory. Best Research-Cell Efficiencies https://www.nrel.gov/pv/cell-efficiency.html [2022-01-24]
[2] Min H, Lee D Y, Kim J, et al. 2021 Nature 598 444
 Google Scholar
Google Scholar
[3] Colsmann A, RöhmA H 2020 Energy Technol. 8 2000912
 Google Scholar
Google Scholar
[4] Abdulrahim S M, Ahmad Z, Bhadra J, Al-Thani N J 2020 Molecules. 25 5794
 Google Scholar
Google Scholar
[5] Wei J W, Huang F R, Wang S N, et al. 2018 Mater. Res. Bull. 106 35
 Google Scholar
Google Scholar
[6] Yu S S, Liu H L, Wang S R, Zhu H W, Dong X F, Li X G 2021 Chem. Eng. J. 403 125724
 Google Scholar
Google Scholar
[7] Zhu C, Yang Y, Lin F, Luo Y, Ma S, Zhu L, Guo X 2021 Rare Met. 40 2402
 Google Scholar
Google Scholar
[8] Yang Y, Chen T, Pan D, Gao J, Zhu C, Lin F, Zhou C, Tai Q, Xiao S, Yuan Y, Dai Q, Han Y, Xie H, Guo X 2020 Nano Energy 67 104246
 Google Scholar
Google Scholar
[9] Cheng N, Li W, Zhang M, Wu H, Sun S, Zhao Z, Xiao Z, Sun Z, Zi W, Fang L 2019 Curr. Appl. Phys. 19 25
 Google Scholar
Google Scholar
[10] Hu Y, Bai F, Liu X, Ji Q, Miao X, Qiu T, Zhang S 2017 ACS Energy Lett. 2 2219
 Google Scholar
Google Scholar
[11] Zhang J, Bai D, Jin Z, Bian H, Wang K, Sun J, Wang Q, Liu S 2018 Adv. Energy Mater. 8 1703246
 Google Scholar
Google Scholar
[12] Bai D, Bian H, Jin Z, Wang H, Meng L, Wang Q, Liu S 2018 Nano Energy 52 408
 Google Scholar
Google Scholar
[13] Lin F, Yang Y, Zhu C, Chen T, Ma S, Luo Y, Zhu L, Guo X 2020 Acta Phys. Chim. Sin. 37 2005007
 Google Scholar
Google Scholar
[14] Kulbak M, Cahen D, Hodes G 2015 J. Phys. Chem. Lett. 6 2452
 Google Scholar
Google Scholar
[15] Duan J, Zhao Y, He B, Tang Q 2018 Angew. Chem. 57 3787
 Google Scholar
Google Scholar
[16] Liu X, Tan X, Liu Z, Ye H, Sun B, Shi T, Tang Z, Liao G 2019 Nano Energy 56 184
 Google Scholar
Google Scholar
[17] Teng P, Han X, Li J, Xu Y, Kang L, Wang Y, Yang Y, Yu T 2018 ACS Appl. Mater. Interfaces 10 9541
 Google Scholar
Google Scholar
[18] Lan H, Xiao H, Zhao J, Chen X, Fan P, Liang G 2021 Mater. Sci. Semicond. Process. 132 105869
 Google Scholar
Google Scholar
[19] Lei J, Gao F, Wang H, Li J, Jiang J, Wu X, Gao R, Yang Z, Liu S 2018 Sol. Energy Mater. Sol. Cells 187 1
 Google Scholar
Google Scholar
[20] Wang H, Wu Y, Ma M, Dong S, Li Q, Du J, Zhang H, Xu Q 2019 ACS Appl. Energy Mater. 2 2305
 Google Scholar
Google Scholar
[21] Yang X, Li M, Jiang J, Ma L, Tang W, Xu C, Cai H L, Zhang F M, Wu X S 2021 J. Phys. D 54 154001
 Google Scholar
Google Scholar
[22] Li H, Tong G, Chen T, Zhu H, Li G, Chang Y, Wang L, Jiang Y 2018 J. Mater. Chem. A 6 14255
 Google Scholar
Google Scholar
[23] Saidaminov M I, Almutlaq J, Sarmah S, Dursun I, Zhumekenov A A, Begum R, Pan J, Cho N, Mohammed O F Bakr O M 2016 ACS Energy Lett. 1 840
 Google Scholar
Google Scholar
[24] Ryu J, Yoon S, Lee S, Lee D, Parida B, Kwak H W, Kang D W 2021 Electrochim. Acta 368 137539
 Google Scholar
Google Scholar
[25] Zhang X, Jin Z, Zhang J, Bai D, Bian H, Wang K, Sun J, Wang Q, Liu S F 2018 ACS Appl. Mater. Interfaces. 10 7145
 Google Scholar
Google Scholar
[26] Jiang Y, Juarez-Perez E J, Ge Q, Wang S, Leyden M R, Ono L K, Raga S R, Hu J, Qi Y 2016 Mater. Horiz. 3 548
 Google Scholar
Google Scholar
[27] Ding Y, He B, Zhu J, Zhang W, Su G, Duan J, Zhao Y, Chen H, Tang Q 2019 ACS Sustainable Chem. Eng. 7 19286
 Google Scholar
Google Scholar
[28] Zhou F, Liu H, Wang X, Shen W 2017 Adv. Funct. Mater. 27 1606156
 Google Scholar
Google Scholar
[29] Li H, Guo L, Li C N, Wang C, Wang G, Wen S, Wu J, Dong W, Li Z J, Ruan S 2019 ACS Sustainable Chem. Eng. 7 8579
 Google Scholar
Google Scholar
[30] Saidaminov M I, Haque M A, Almutlaq J, Sarmah S, Miao X H, Begum R, Zhumekenov A A, Dursun I, Cho N, Murali B 2017 Adv. Opt. Mater. 5 1600704
 Google Scholar
Google Scholar
-
图 7 (a) 不同反应时间下的CsPbBr3钙钛矿薄膜器件J-V曲线; (b) CsPbBr3电池Nyquist 图, 插图为等效电路图及相关参数; (c) 暗态下结构为FTO/TiO2/CsPbBr3/PC61BM/Ag的器件的J-V曲线
Figure 7. (a) J-V curves of CsPbBr3 perovskite solar cell based on different reaction time; (b) Nyquist plots of CsPbBr3 PSCs under 1 sun illumination, the inset provides the equivalent circuit and relevant parameter; (c) J-V curves of the device with an architecture of FTO/TiO2/CsPbBr3/PC61BM/Ag under dark conditions.
表 1 不同反应时间下CsPbBr3钙钛矿薄膜的电池器件J-V参数
Table 1. J-V parameters of CsPbBr3 perovskite for solar cell with different reaction time.
反应时间/s Jsc/(mA·cm–2) Voc/V FF PCE/% 0 8.78 1.17 0.52 5.32 10 8.51 1.22 0.56 5.86 20 7.95 1.20 0.58 5.55 30 8.40 1.28 0.59 6.30 40 7.94 1.03 0.52 4.22 50 6.28 1.08 0.59 4.02 60 4.19 1.03 0.48 2.09 -
[1] National Renewable Energy Laboratory. Best Research-Cell Efficiencies https://www.nrel.gov/pv/cell-efficiency.html [2022-01-24]
[2] Min H, Lee D Y, Kim J, et al. 2021 Nature 598 444
 Google Scholar
Google Scholar
[3] Colsmann A, RöhmA H 2020 Energy Technol. 8 2000912
 Google Scholar
Google Scholar
[4] Abdulrahim S M, Ahmad Z, Bhadra J, Al-Thani N J 2020 Molecules. 25 5794
 Google Scholar
Google Scholar
[5] Wei J W, Huang F R, Wang S N, et al. 2018 Mater. Res. Bull. 106 35
 Google Scholar
Google Scholar
[6] Yu S S, Liu H L, Wang S R, Zhu H W, Dong X F, Li X G 2021 Chem. Eng. J. 403 125724
 Google Scholar
Google Scholar
[7] Zhu C, Yang Y, Lin F, Luo Y, Ma S, Zhu L, Guo X 2021 Rare Met. 40 2402
 Google Scholar
Google Scholar
[8] Yang Y, Chen T, Pan D, Gao J, Zhu C, Lin F, Zhou C, Tai Q, Xiao S, Yuan Y, Dai Q, Han Y, Xie H, Guo X 2020 Nano Energy 67 104246
 Google Scholar
Google Scholar
[9] Cheng N, Li W, Zhang M, Wu H, Sun S, Zhao Z, Xiao Z, Sun Z, Zi W, Fang L 2019 Curr. Appl. Phys. 19 25
 Google Scholar
Google Scholar
[10] Hu Y, Bai F, Liu X, Ji Q, Miao X, Qiu T, Zhang S 2017 ACS Energy Lett. 2 2219
 Google Scholar
Google Scholar
[11] Zhang J, Bai D, Jin Z, Bian H, Wang K, Sun J, Wang Q, Liu S 2018 Adv. Energy Mater. 8 1703246
 Google Scholar
Google Scholar
[12] Bai D, Bian H, Jin Z, Wang H, Meng L, Wang Q, Liu S 2018 Nano Energy 52 408
 Google Scholar
Google Scholar
[13] Lin F, Yang Y, Zhu C, Chen T, Ma S, Luo Y, Zhu L, Guo X 2020 Acta Phys. Chim. Sin. 37 2005007
 Google Scholar
Google Scholar
[14] Kulbak M, Cahen D, Hodes G 2015 J. Phys. Chem. Lett. 6 2452
 Google Scholar
Google Scholar
[15] Duan J, Zhao Y, He B, Tang Q 2018 Angew. Chem. 57 3787
 Google Scholar
Google Scholar
[16] Liu X, Tan X, Liu Z, Ye H, Sun B, Shi T, Tang Z, Liao G 2019 Nano Energy 56 184
 Google Scholar
Google Scholar
[17] Teng P, Han X, Li J, Xu Y, Kang L, Wang Y, Yang Y, Yu T 2018 ACS Appl. Mater. Interfaces 10 9541
 Google Scholar
Google Scholar
[18] Lan H, Xiao H, Zhao J, Chen X, Fan P, Liang G 2021 Mater. Sci. Semicond. Process. 132 105869
 Google Scholar
Google Scholar
[19] Lei J, Gao F, Wang H, Li J, Jiang J, Wu X, Gao R, Yang Z, Liu S 2018 Sol. Energy Mater. Sol. Cells 187 1
 Google Scholar
Google Scholar
[20] Wang H, Wu Y, Ma M, Dong S, Li Q, Du J, Zhang H, Xu Q 2019 ACS Appl. Energy Mater. 2 2305
 Google Scholar
Google Scholar
[21] Yang X, Li M, Jiang J, Ma L, Tang W, Xu C, Cai H L, Zhang F M, Wu X S 2021 J. Phys. D 54 154001
 Google Scholar
Google Scholar
[22] Li H, Tong G, Chen T, Zhu H, Li G, Chang Y, Wang L, Jiang Y 2018 J. Mater. Chem. A 6 14255
 Google Scholar
Google Scholar
[23] Saidaminov M I, Almutlaq J, Sarmah S, Dursun I, Zhumekenov A A, Begum R, Pan J, Cho N, Mohammed O F Bakr O M 2016 ACS Energy Lett. 1 840
 Google Scholar
Google Scholar
[24] Ryu J, Yoon S, Lee S, Lee D, Parida B, Kwak H W, Kang D W 2021 Electrochim. Acta 368 137539
 Google Scholar
Google Scholar
[25] Zhang X, Jin Z, Zhang J, Bai D, Bian H, Wang K, Sun J, Wang Q, Liu S F 2018 ACS Appl. Mater. Interfaces. 10 7145
 Google Scholar
Google Scholar
[26] Jiang Y, Juarez-Perez E J, Ge Q, Wang S, Leyden M R, Ono L K, Raga S R, Hu J, Qi Y 2016 Mater. Horiz. 3 548
 Google Scholar
Google Scholar
[27] Ding Y, He B, Zhu J, Zhang W, Su G, Duan J, Zhao Y, Chen H, Tang Q 2019 ACS Sustainable Chem. Eng. 7 19286
 Google Scholar
Google Scholar
[28] Zhou F, Liu H, Wang X, Shen W 2017 Adv. Funct. Mater. 27 1606156
 Google Scholar
Google Scholar
[29] Li H, Guo L, Li C N, Wang C, Wang G, Wen S, Wu J, Dong W, Li Z J, Ruan S 2019 ACS Sustainable Chem. Eng. 7 8579
 Google Scholar
Google Scholar
[30] Saidaminov M I, Haque M A, Almutlaq J, Sarmah S, Miao X H, Begum R, Zhumekenov A A, Dursun I, Cho N, Murali B 2017 Adv. Opt. Mater. 5 1600704
 Google Scholar
Google Scholar
Catalog
Metrics
- Abstract views: 9431
- PDF Downloads: 269
- Cited By: 0















 DownLoad:
DownLoad: