-
大气压非平衡等离子体由于其独特的非平衡特性, 可为甲烷和二氧化碳稳定温室气体分子活化和重整提供非热平衡和活化环境. 本文采用了零维等离子体化学反应动力学模型, 考虑了详细的CH4/CO2等离子体化学反应集, 重点研究了反应气体CH4/CO2摩尔分数(5%—95%)对大气压非平衡等离子体甲烷干法重整制合成气和重要含氧化合物的影响. 首先, 给出了进料气体不同体积比时电子密度和温度随时间的演化规律, 结果表明初始甲烷摩尔分数的提高有利于获得较高的电子密度和电子温度. 随后, 讨论了主要自由基和离子数密度在不同的甲烷摩尔分数下随着时间的变化规律, 并给出了反应气体的转化率、合成气体和重要含氧化合物的选择性. 此外, 还明确了合成气和含氧化合物主要生成和损耗的化学反应路径, 发现甲基和羟基是合成含氧化合物的关键中间体. 最后, 归纳总结给出了主要等离子体粒子之间的总体等离子体化学反应流程图.
-
关键词:
- 大气压非平衡等离子体 /
- 甲烷干法重整 /
- 零维等离子体化学反应动力学模型 /
- 反应机理
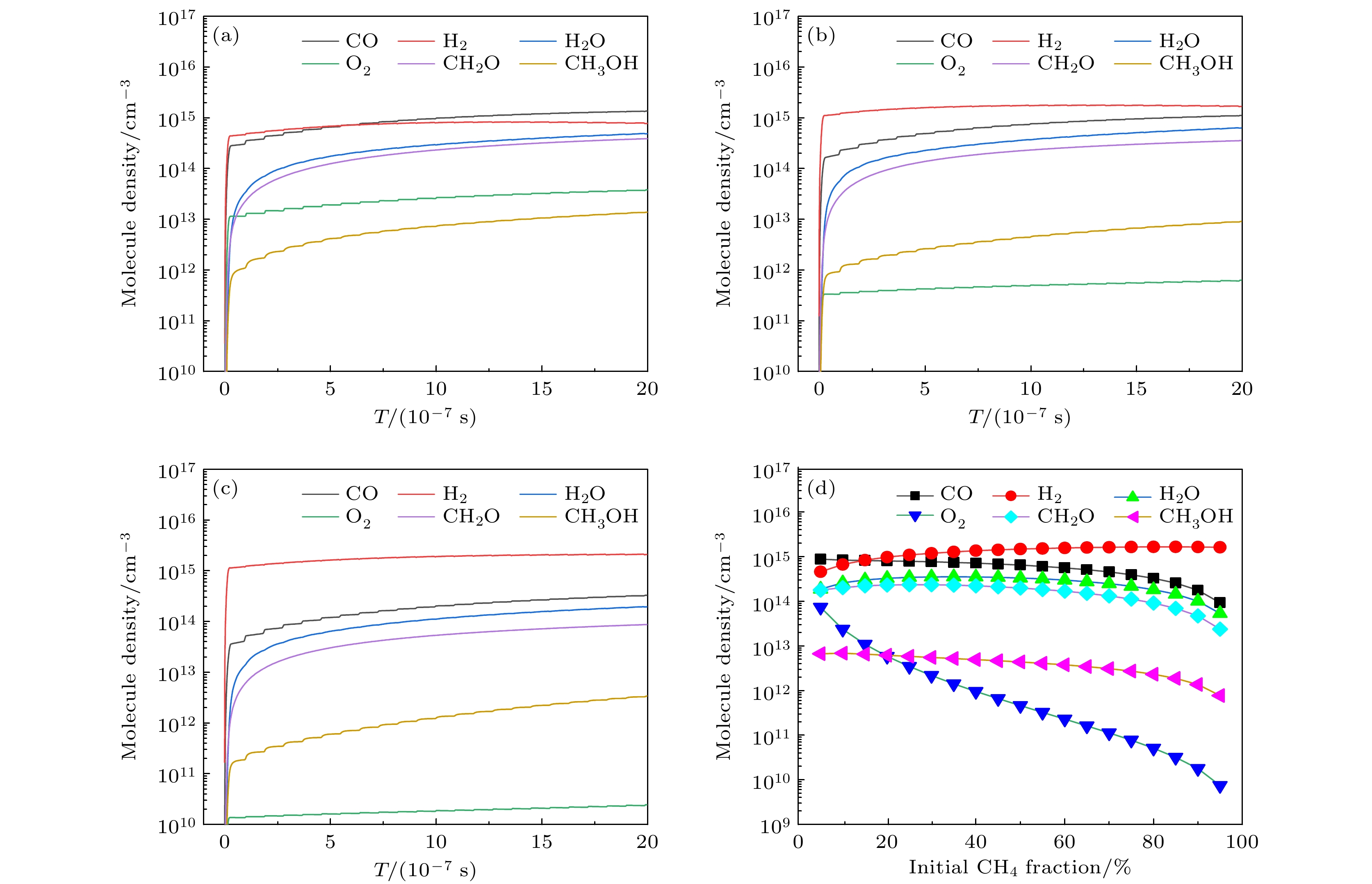
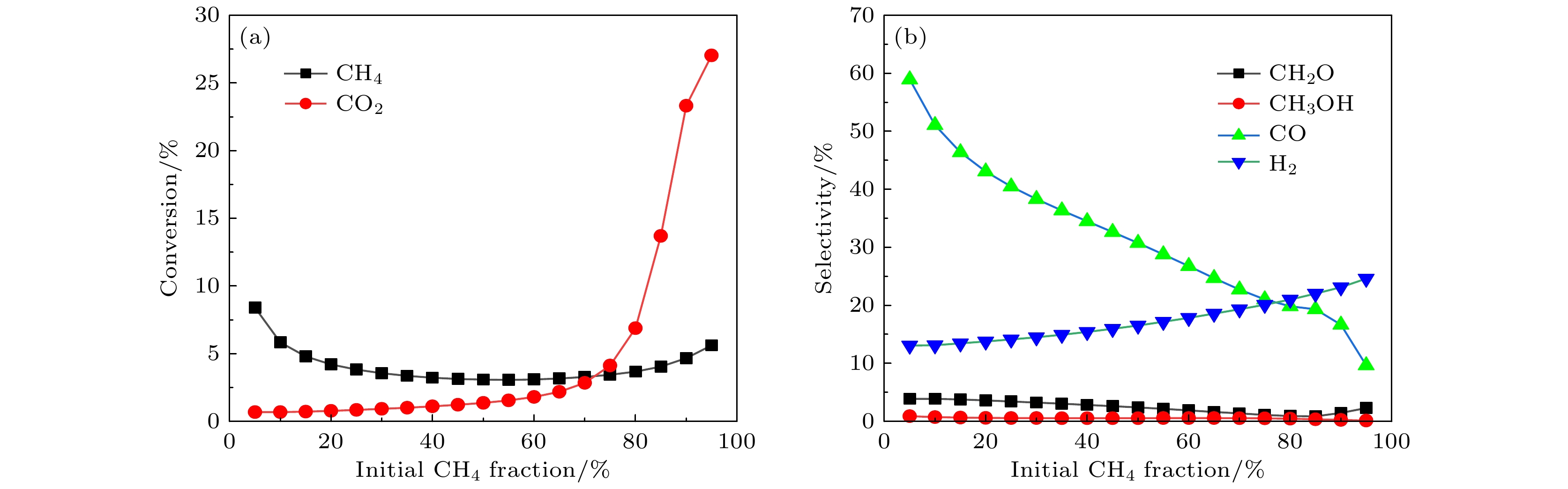
Recently, atmospheric non-equilibrium plasma has been proposed as a potential and novel type of “reaction carrier” for the activation and conversion of greenhouse gases (methane and carbon dioxide) into value-added chemicals, due to its unique non-equilibrium characteristics. In this paper, a zero-dimensional plasma chemical reaction kinetic model in CH4/CO2 gas mixture is constructed, with an emphasis on reaction mechanism for plasma dry reforming of methane to syngas and oxygenates. Especially, the effect of the CH4 molar fraction (5%–95%) on plasma dry reforming of methane is investigated. First, the time evolution of electron temperature and density with initial methane content is presented, and the results show that both the electron temperature and electron density vary periodically with the applied triangular power density pulse, and the higher initial methane content in gas mixture is favored for a larger electron temperature and density. Subsequently, the time evolution of number densities of free radicals, ions and molecules at different CH4/CO2 molar fraction are given. The higher the initial methane content, the greater the number densities of H, H–, H2, and CH3, leading to insufficient oxygen atoms to participate in the reaction for oxygenates synthesis. The conversions of inlet gases, the selectivities of syngas and important oxygenates are also calculated. The conversion rate of carbon dioxide increases with the increasing methane content, but the conversion rate of methane is insensitive to the variation of methane content. As methane mole fraction is increased from 5% to 95%, the selectivities of important oxygenates (CH3OH and CH2O) are relatively low (<5%), and the selectivity of H2 gradually increases from 13.0% to 24.6%, while the selectivity of CO significantly decreases from 58.9% to 9.7%. Moreover, the dominant reaction pathways governing production and destruction of H2, CO, CH2O and CH3OH are determined, and CH3 and OH radicals are found to be the key intermediate for the production of valuable oxygenates. Finally, a schematic overview of the transformation relationship between dominant plasma species is summarized and shown to clearly reveal intrinsic reaction mechanism of dry reforming of methane in atmospheric non-equilibrium plasma.-
Keywords:
- atmospheric pressure non-equilibrium plasma /
- dry reforming of methane /
- zero-dimensional chemical reaction kinetic model /
- reaction mechanism
[1] Gangadharan P, Kanchi K C, Lou H H 2012 Chem. Eng. Res. Des. 90 1956
 Google Scholar
Google Scholar
[2] Asinger F 1986 Methanol-Chemie und Energierohstoff (Heidelberg: Springer) pp1−9
[3] Fakley M E, Jennings J R, Spencer M S 1989 J. Catal. 118 483
 Google Scholar
Google Scholar
[4] Abdulrasheed A, Jalil A A, Gambo Y, Ibrahim M, Hambali H U, Hamid M Y S 2019 Renewable Sustainable Energy Rev. 108 175
 Google Scholar
Google Scholar
[5] Jang W J, Shim J O, Kim H M, Yoo S Y, Roh H S 2019 Catal. Today 324 15
 Google Scholar
Google Scholar
[6] Aramouni N A K, Touma J G, Tarboush B A, Zeaiter J, Ahmad M N 2018 Renewable Sustainable Energy Rev. 82 2570
 Google Scholar
Google Scholar
[7] Wang Y, Yao L, Wang S, Mao D, Hu C 2018 Fuel Process. Technol. 169 199
 Google Scholar
Google Scholar
[8] Abdullah B, Ghani N A A, Vo D V N 2017 J. Cleaner Prod. 162 170
 Google Scholar
Google Scholar
[9] Luo Y R 2007 Comprehensive Handbook of Chemical Bond Energies (Boca Raton: CRC) pp19−342
[10] Zhang X, Wenren Y, Zhou W, Han J, Lu H, Zhu Z, Wu Z, Cha M S 2020 J. Phys. D: Appl. Phys. 53 194002
 Google Scholar
Google Scholar
[11] Brune L, Ozkan A, Genty E, Bocarmé T V, Reniers F 2018 J. Phys. D: Appl. Phys. 51 234002
 Google Scholar
Google Scholar
[12] Maqueo P D G, Coulombe S, Bergthorson J M 2019 J. Phys. D: Appl. Phys. 52 274002
 Google Scholar
Google Scholar
[13] Alawi N M, Sunarso J, Pham G H, Barifcani A, Nguyen M H, Liu S 2020 J. Ind. Eng. Chem. 85 118
 Google Scholar
Google Scholar
[14] 王晓玲, 高远, 张帅, 孙昊, 李杰, 邵涛 2019 电工技术学报 34 1329
Wang X L, Gao Y, Zhang S, Sun H, Li J, Shao T 2019 Trans. Chin. Electrotechn. Soc. 34 1329
[15] Wu A, Yan J, Zhang H, Zhang M, Du C, Li X 2014 Int. J. Hydrogen Energy 39 17656
 Google Scholar
Google Scholar
[16] Khoja A H, Tahir M, Amin N A S 2019 Energy Convers. Manage. 183 529
 Google Scholar
Google Scholar
[17] 王建龙, 丁芳, 朱晓东 2015 64 045206
 Google Scholar
Google Scholar
Wang J L, Ding F, Zhu X D 2015 Acta Phys. Sin. 64 045206
 Google Scholar
Google Scholar
[18] 赵曰峰, 王超, 王伟宗, 李莉, 孙昊, 邵涛, 潘杰 2018 67 085202
 Google Scholar
Google Scholar
Zhao Y F, Wang C, Wang W Z, Li L, Sun H, Shao T, Pan J 2018 Acta Phys. Sin. 67 085202
 Google Scholar
Google Scholar
[19] Slaets J, Aghaei M, Ceulemans S, Van Alphen S, Bogaerts A 2020 Green Chem. 22 1366
 Google Scholar
Google Scholar
[20] Wang W, Snoeckx R, Zhang X, Cha M S, Bogaerts A 2018 J. Phys. Chem. C 122 8704
 Google Scholar
Google Scholar
[21] Snoeckx R, Aerts R, Tu X, Bogaerts A 2013 J. Phys. Chem. C 117 4957
 Google Scholar
Google Scholar
[22] Liu S, Winter L R, Chen J G 2020 ACS Catal. 10 2855
 Google Scholar
Google Scholar
[23] Bogaerts A, De Bie C, Snoecks R, Kozak T 2017 Plasma Processes Polym. 14 1600070
 Google Scholar
Google Scholar
[24] De Bie C, van Dijk J, Bogaerts A 2015 J. Phys. Chem. C 119 22331
 Google Scholar
Google Scholar
[25] Lietz A M, Kushner M J 2016 J. Phys. D: Appl. Phys. 49 425204
 Google Scholar
Google Scholar
[26] Aerts R, Martens T, Bogaerts A 2012 J. Phys. Chem. C 116 23257
 Google Scholar
Google Scholar
[27] Aerts R, Somers W, Bogaerts A 2015 ChemSusChem 8 702
 Google Scholar
Google Scholar
[28] Luo Y C, Lietz A M, Yatom S, Kushner M J, Bruggeman P J 2019 J. Phys. D: Appl. Phys. 52 044003
 Google Scholar
Google Scholar
[29] Qian M Y, Zhong W S, Kang J S, Liu S Q, Ren C S, Zhang J L, Wang D Z 2020 Jpn. J. Appl. Phys. 59 066003
 Google Scholar
Google Scholar
[30] Brown P N, Byrne G D, Hindmarsh A C 1989 SIAM J. Sci. Stat. Comput. 10 1038
 Google Scholar
Google Scholar
[31] Zhang S, Gao Y, Sun H, Bai H, Wang R X, Shao T 2018 J. Phys. D: Appl. Phys. 51 274005
 Google Scholar
Google Scholar
[32] Bai C J, Wang L J, Li L, Dong X, Xiao Q H, Liu Z Q, Sun J H, Pan J 2019 AIP Adv. 9 035023
 Google Scholar
Google Scholar
-
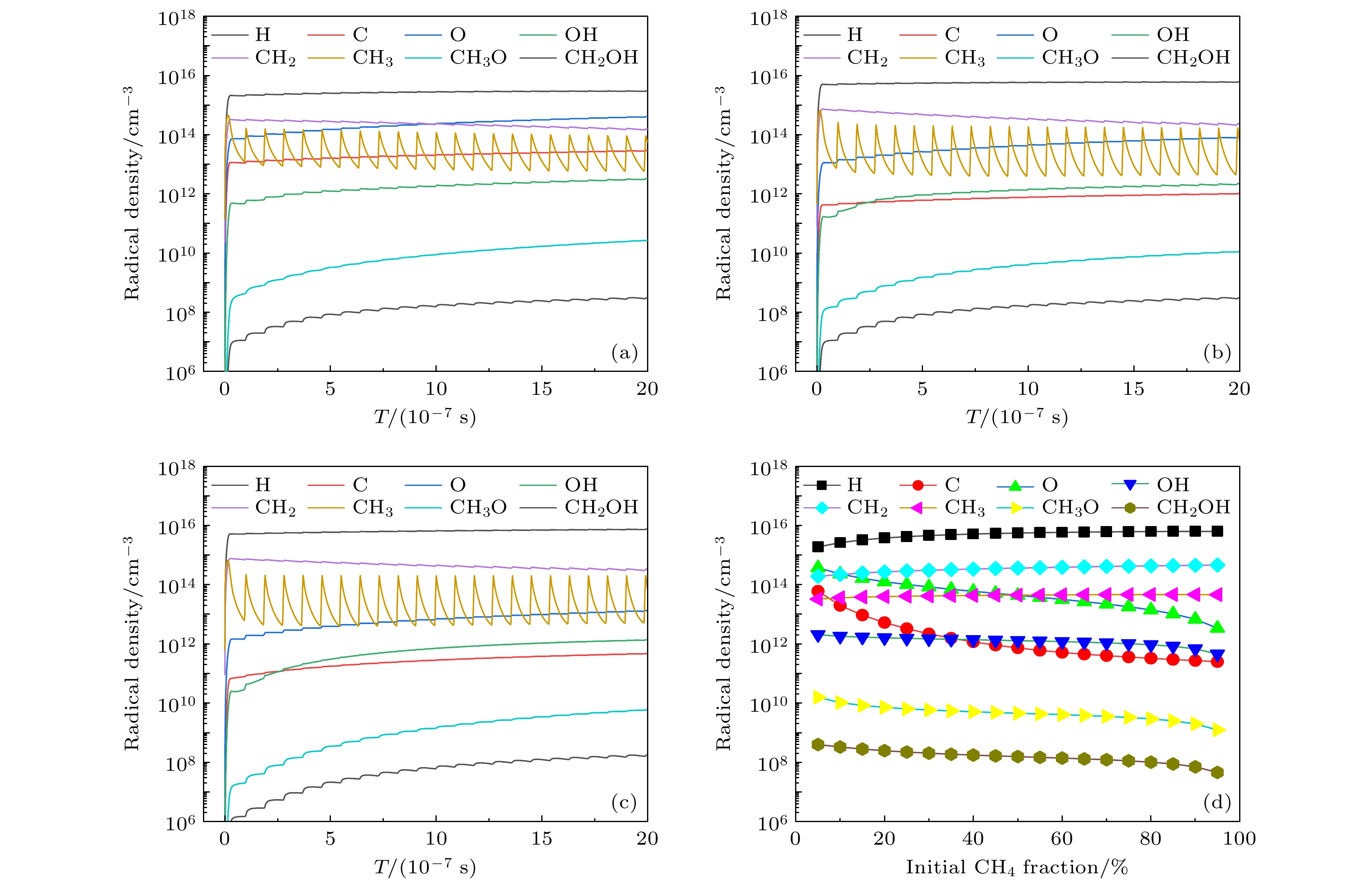
图 3 (a) 10%, (b) 50%和(c) 90%甲烷摩尔分数时主要自由基的数密度随时间变化趋势, 以及(d) 主要自由基的周期平均值随甲烷摩尔分数的变化
Fig. 3. The number densities of main radicals as a function of time for methane mole fractions of (a) 10%, (b) 50%, (c) 90%, and (d) time averaged number densities of main radicals as a function of initial CH4 fraction.
图 11 CH4/CO2摩尔分数比为1:1的大气压非平衡等离子体DRM反应总体流程图. 箭头的粗细与时间平均的净反应速率成线性正比
Fig. 11. Schematic overview of the dominant reaction pathways for the conversion of CH4 and CO2 into representative higher oxygenates and syngas in atmospheric non-equilibrium plasma for a 1:1 CH4/CO2 gas mixture. The thickness of the arrows is linearly proportional to time-averaged rate of net reaction.
-
[1] Gangadharan P, Kanchi K C, Lou H H 2012 Chem. Eng. Res. Des. 90 1956
 Google Scholar
Google Scholar
[2] Asinger F 1986 Methanol-Chemie und Energierohstoff (Heidelberg: Springer) pp1−9
[3] Fakley M E, Jennings J R, Spencer M S 1989 J. Catal. 118 483
 Google Scholar
Google Scholar
[4] Abdulrasheed A, Jalil A A, Gambo Y, Ibrahim M, Hambali H U, Hamid M Y S 2019 Renewable Sustainable Energy Rev. 108 175
 Google Scholar
Google Scholar
[5] Jang W J, Shim J O, Kim H M, Yoo S Y, Roh H S 2019 Catal. Today 324 15
 Google Scholar
Google Scholar
[6] Aramouni N A K, Touma J G, Tarboush B A, Zeaiter J, Ahmad M N 2018 Renewable Sustainable Energy Rev. 82 2570
 Google Scholar
Google Scholar
[7] Wang Y, Yao L, Wang S, Mao D, Hu C 2018 Fuel Process. Technol. 169 199
 Google Scholar
Google Scholar
[8] Abdullah B, Ghani N A A, Vo D V N 2017 J. Cleaner Prod. 162 170
 Google Scholar
Google Scholar
[9] Luo Y R 2007 Comprehensive Handbook of Chemical Bond Energies (Boca Raton: CRC) pp19−342
[10] Zhang X, Wenren Y, Zhou W, Han J, Lu H, Zhu Z, Wu Z, Cha M S 2020 J. Phys. D: Appl. Phys. 53 194002
 Google Scholar
Google Scholar
[11] Brune L, Ozkan A, Genty E, Bocarmé T V, Reniers F 2018 J. Phys. D: Appl. Phys. 51 234002
 Google Scholar
Google Scholar
[12] Maqueo P D G, Coulombe S, Bergthorson J M 2019 J. Phys. D: Appl. Phys. 52 274002
 Google Scholar
Google Scholar
[13] Alawi N M, Sunarso J, Pham G H, Barifcani A, Nguyen M H, Liu S 2020 J. Ind. Eng. Chem. 85 118
 Google Scholar
Google Scholar
[14] 王晓玲, 高远, 张帅, 孙昊, 李杰, 邵涛 2019 电工技术学报 34 1329
Wang X L, Gao Y, Zhang S, Sun H, Li J, Shao T 2019 Trans. Chin. Electrotechn. Soc. 34 1329
[15] Wu A, Yan J, Zhang H, Zhang M, Du C, Li X 2014 Int. J. Hydrogen Energy 39 17656
 Google Scholar
Google Scholar
[16] Khoja A H, Tahir M, Amin N A S 2019 Energy Convers. Manage. 183 529
 Google Scholar
Google Scholar
[17] 王建龙, 丁芳, 朱晓东 2015 64 045206
 Google Scholar
Google Scholar
Wang J L, Ding F, Zhu X D 2015 Acta Phys. Sin. 64 045206
 Google Scholar
Google Scholar
[18] 赵曰峰, 王超, 王伟宗, 李莉, 孙昊, 邵涛, 潘杰 2018 67 085202
 Google Scholar
Google Scholar
Zhao Y F, Wang C, Wang W Z, Li L, Sun H, Shao T, Pan J 2018 Acta Phys. Sin. 67 085202
 Google Scholar
Google Scholar
[19] Slaets J, Aghaei M, Ceulemans S, Van Alphen S, Bogaerts A 2020 Green Chem. 22 1366
 Google Scholar
Google Scholar
[20] Wang W, Snoeckx R, Zhang X, Cha M S, Bogaerts A 2018 J. Phys. Chem. C 122 8704
 Google Scholar
Google Scholar
[21] Snoeckx R, Aerts R, Tu X, Bogaerts A 2013 J. Phys. Chem. C 117 4957
 Google Scholar
Google Scholar
[22] Liu S, Winter L R, Chen J G 2020 ACS Catal. 10 2855
 Google Scholar
Google Scholar
[23] Bogaerts A, De Bie C, Snoecks R, Kozak T 2017 Plasma Processes Polym. 14 1600070
 Google Scholar
Google Scholar
[24] De Bie C, van Dijk J, Bogaerts A 2015 J. Phys. Chem. C 119 22331
 Google Scholar
Google Scholar
[25] Lietz A M, Kushner M J 2016 J. Phys. D: Appl. Phys. 49 425204
 Google Scholar
Google Scholar
[26] Aerts R, Martens T, Bogaerts A 2012 J. Phys. Chem. C 116 23257
 Google Scholar
Google Scholar
[27] Aerts R, Somers W, Bogaerts A 2015 ChemSusChem 8 702
 Google Scholar
Google Scholar
[28] Luo Y C, Lietz A M, Yatom S, Kushner M J, Bruggeman P J 2019 J. Phys. D: Appl. Phys. 52 044003
 Google Scholar
Google Scholar
[29] Qian M Y, Zhong W S, Kang J S, Liu S Q, Ren C S, Zhang J L, Wang D Z 2020 Jpn. J. Appl. Phys. 59 066003
 Google Scholar
Google Scholar
[30] Brown P N, Byrne G D, Hindmarsh A C 1989 SIAM J. Sci. Stat. Comput. 10 1038
 Google Scholar
Google Scholar
[31] Zhang S, Gao Y, Sun H, Bai H, Wang R X, Shao T 2018 J. Phys. D: Appl. Phys. 51 274005
 Google Scholar
Google Scholar
[32] Bai C J, Wang L J, Li L, Dong X, Xiao Q H, Liu Z Q, Sun J H, Pan J 2019 AIP Adv. 9 035023
 Google Scholar
Google Scholar
计量
- 文章访问数: 9323
- PDF下载量: 172
- 被引次数: 0














 下载:
下载: