-
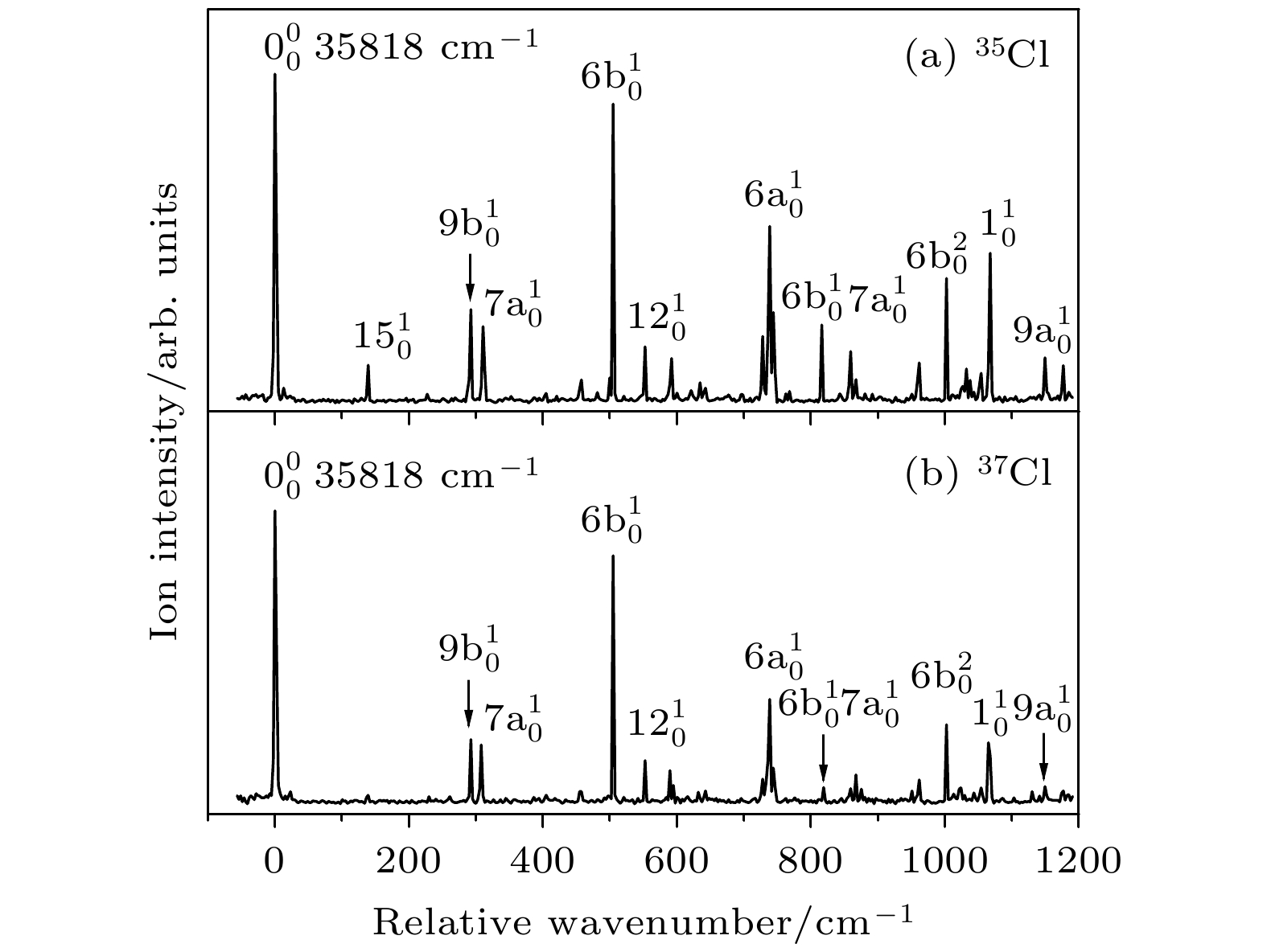
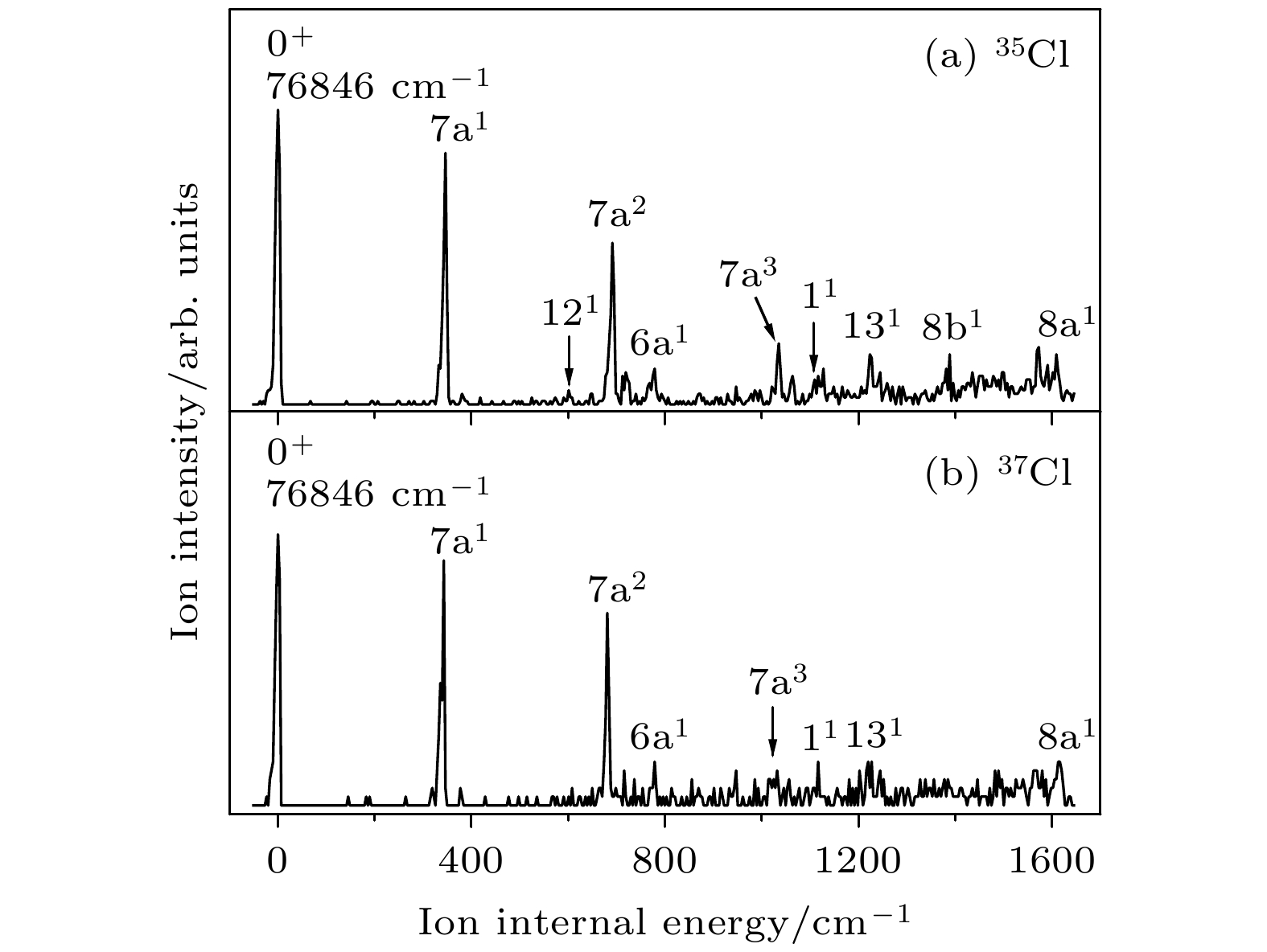
The vibrational features of p-chlorobenzonitrile in its first electronically excited state S1 and cationic ground state D0 have been investigated by two-color resonance enhanced two-photon ionization and mass analyzed threshold ionization spectroscopy. The excitation energy of S1 ← S0 and the ionization energy of 35Cl and 37Cl isotopomers of p-chlorobenzonitrile are determined to be 35818 ± 2, and 76846 ± 5 cm–1, respectively. These two isotopomers have similar vibrational features. Most of the active vibrations in the S1 and D0 states are related to the motions of the in-plane ring deformation. The stable structures and vibrational frequencies of p-chlorobenzonitrile are also calculated by the B3LYP/aug-cc-pVDZ method for the S0 and D0 states, and TD-B3LYP/aug-cc-pVDZ method for the S1 state. The changes in the molecular geometry are discussed in the S1 ← S0 photoexcitation process and the D0 ← S1 photoionization process. The comparisons between the transition energy of p-chlorophenol, p-chloroaniline, p-chloroanisole, and p-chlorobenzonitrile with those of phenol, anisole, aniline, and benzonitrile provide an insight into the substitution effect of Cl atom.
-
Keywords:
- p-chlorobenzonitrile /
- first electronically excited state /
- cationic ground state /
- vibrational spectrum
[1] Lee J K, Fujiwara T, Kofron W G, Zgierski M Z, Lim E C 2008 J. Chem. Phys. 128 164512
 Google Scholar
Google Scholar
[2] Perveaux A, Castro P J, Lauvergnat D, Reguero M, Lasorne B 2015 J. Phys. Chem. Lett. 6 1316
 Google Scholar
Google Scholar
[3] Livingstone R A, Thompson J O, Iljina M, Donaldson R J, Sussman B J, Paterson M J, Townsend D 2012 J. Chem. Phys. 137 184304
 Google Scholar
Google Scholar
[4] King G A, Devine A L, Nix M G, Kelly D E, Ashfold M N 2008 Phys. Chem. Chem. Phys. 10 6417
 Google Scholar
Google Scholar
[5] Miyazaki M, Sakata Y, Schutz M, Dopfer O, Fujii M 2016 Phys. Chem. Chem. Phys. 18 24746
 Google Scholar
Google Scholar
[6] Aschaffenburg D J, Moog R S 2009 J. Phys. Chem. B 113 12736
 Google Scholar
Google Scholar
[7] Chang C, Lu Y, Wang T, Diau E W 2004 J. Am. Chem. Soc. 126 10109
 Google Scholar
Google Scholar
[8] Hertel I V, Radloff W 2006 Rep. Prog. Phys. 69 1897
 Google Scholar
Google Scholar
[9] Schneider M, Wilke M, Hebestreit M L, Ruiz-Santoyo J A, Alvarez-Valtierra L, Yi J T, Meerts W L, Pratt D W, Schmitt M 2017 Phys. Chem. Chem. Phys. 19 21364
 Google Scholar
Google Scholar
[10] 李鑫, 赵岩, 靳颖辉, 王晓锐, 余谢秋, 武媚, 韩昱行, 杨勇刚, 李昌勇, 贾锁堂 2017 66 093301
 Google Scholar
Google Scholar
Li X, Zhao Y, Jin Y H, Wang X R, Yu X Q, Wu M, Han Y X, Yang Y G, Li C Y, Jia S T 2017 Acta Phys. Sin. 66 093301
 Google Scholar
Google Scholar
[11] Zhao Y, Jin Y H, Li C Y, Jia S T 2019 J. Mol. Spectrosc. 363 111182
 Google Scholar
Google Scholar
[12] Corrales M E, Shternin P S, Rubio L L, De N R, Vasyutinskii O S, Bañares L 2016 J. Phys. Chem. Lett. 7 4458
 Google Scholar
Google Scholar
[13] Tzeng S Y, Shivatare V S, Tzeng W B 2019 J. Phys. Chem. A 123 5969
 Google Scholar
Google Scholar
[14] Findley A M, Bernstorff S, Köhler A M, Saile V, Findley G L 1987 Phys. Scr. 35 633
 Google Scholar
Google Scholar
[15] Onda M, Saegusa N, Yamaguchi I 1986 J. Mol. Struct. 145 185
 Google Scholar
Google Scholar
[16] Rocha I M, Galvão T L, Ribeiro da Silva M D, Ribeiro da Silva M A 2014 J. Phys. Chem. A 118 1502
 Google Scholar
Google Scholar
[17] Trivedi M K, Branton A, Trivedi D, Nayak G, Singh R, Jana S 2015 J. Chem. Sci. 3 84
 Google Scholar
Google Scholar
[18] Zhao Y, Jin Y H, Hao J Y, Yang Y G, Wang L R, Li C Y, Jia S T 2019 Spectrochim. Acta, Part A 207 328
 Google Scholar
Google Scholar
[19] 段春泱, 李娜, 赵岩, 李昌勇 2021 70 053301
 Google Scholar
Google Scholar
Duan C Y, Li N, Zhao Y, Li C Y 2021 Acta Phys. Sin. 70 053301
 Google Scholar
Google Scholar
[20] Frisch M J, Trucks G W, Schlegel H B, Scuseria G E, Robb M A, Cheeseman J R, Scalmani G, Barone V, Mennucci B, Petersson G A, Nakatsuji H, Caricato M, Li X, Hratchian H P, Izmaylov A F, Bloino J, Zheng G, Sonnenberg J L, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery J A, Peralta J E, Ogliaro F, Bearpark M, Heyd J J, Brothers E, Kudin K N, Staroverov V N, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant J C, Iyengar S S, Tomasi J, Cossi M, Rega N, Millam J M, Klene M, Knox J E, Cross J B, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann R E, Yazyev O, Austin A J, Cammi R, Pomelli C, Ochterski J W, Martin R L, Morokuma K, Zakrzewski V G, Voth G A, Salvador P, Dannenberg J J, Dapprich S, Daniels A D, Farkas Ö, Foresman J B, Ortiz J V, Cioslowski J, Fox D J 2009 Gaussian 09 (Wallingford CT: Gaussian Inc.)
[21] Merrick J P, Moran D, Radom L 2007 J. Phys. Chem. A 111 11683
 Google Scholar
Google Scholar
[22] Maiti A K, Sarkar S K, Kastha G S 1985 Proc. Indian Acad. Sci. (Chem. Sci.) 95 409
 Google Scholar
Google Scholar
[23] Lin J L, Tzeng W B 2000 J. Chem. Phys. 113 4109
 Google Scholar
Google Scholar
[24] Huang J H, Huang K L, Liu S Q, Luo Q, Tzeng W B 2008 J. Photochem. Photobiol. , A 193 245
 Google Scholar
Google Scholar
[25] Lin J L, Li C Y, Tzeng W B 2004 J. Chem. Phys. 120 10513
 Google Scholar
Google Scholar
[26] Yu D, Dong C W, Cheng M, Hu L L, Du Y K, Zhu Q H, Zhang C H 2011 J. Mol. Spectrosc. 265 86
 Google Scholar
Google Scholar
[27] Wilson E B 1934 Phys. Rev. 45 706
 Google Scholar
Google Scholar
[28] Varsanyi G 1974 Assignments of Vibrational Spectra of Seven Hundred Benzene Derivatives (New York: Wiley) pp185–190
[29] Tzeng S Y, Takahashi K, Tzeng W B 2019 Chem. Phys. Lett. 731 136626
 Google Scholar
Google Scholar
[30] Huang J, Lin J L, Tzeng W B 2006 Chem. Phys. Lett. 422 271
 Google Scholar
Google Scholar
[31] Desiraju G R, Harlow R L 1989 J. Am. Chem. Soc. 111 6757
 Google Scholar
Google Scholar
[32] Zhao Y, Jin Y H, Hao J Y, Yang Y, Li C Y, Jia S T 2018 Chem. Phys. Lett. 711 127
 Google Scholar
Google Scholar
[33] Dopfer O, Müller-Dethlefs K 1994 J. Chem. Phys. 101 8508
 Google Scholar
Google Scholar
[34] Pradhan M, Li C Y, Lin J L, Tzeng W B 2005 Chem. Phys. Lett. 407 100
 Google Scholar
Google Scholar
[35] Lin J L, Tzeng W B 2001 J. Chem. Phys. 115 743
 Google Scholar
Google Scholar
[36] Araki M, Sato S, Kimura K 1996 J. Phys. Chem. 100 10542
 Google Scholar
Google Scholar
[37] Suzuki K, Ishiuchi S, Sakai M, Fujii M 2005 J. Electron Spectrosc. Relat. Phenom. 142 215
 Google Scholar
Google Scholar
[38] Huang L C, Lin J L, Tzeng W B 2000 Chem. Phys. 261 449
 Google Scholar
Google Scholar
[39] Zhang B, Li C, Su H, Lin J L, Tzeng W B 2004 Chem. Phys. Lett. 390 65
 Google Scholar
Google Scholar
-
表 1 对氯苯腈分子35Cl和37Cl 同位素激发态S1的振动频率及光谱归属(单位: cm–1)
Table 1. The measured vibrational frequencies and assignments for the S1 state of 35Cl and 37Cl isotopomers of p-chlorobenzonitrile (unit: cm–1).
35Cl 37Cl 光谱归属b 实验值a 理论值a 实验值a 理论值a 0 0 $ 0_0^0 $ 139 141 139 141 $ 15_0^1 $, β(C—CN) 292 291 292 291 $ 9{\text{b}}_0^1 $, β(C—Cl) 310 300 309 300 $ 7{\text{a}}_0^1 $, β(CCC),
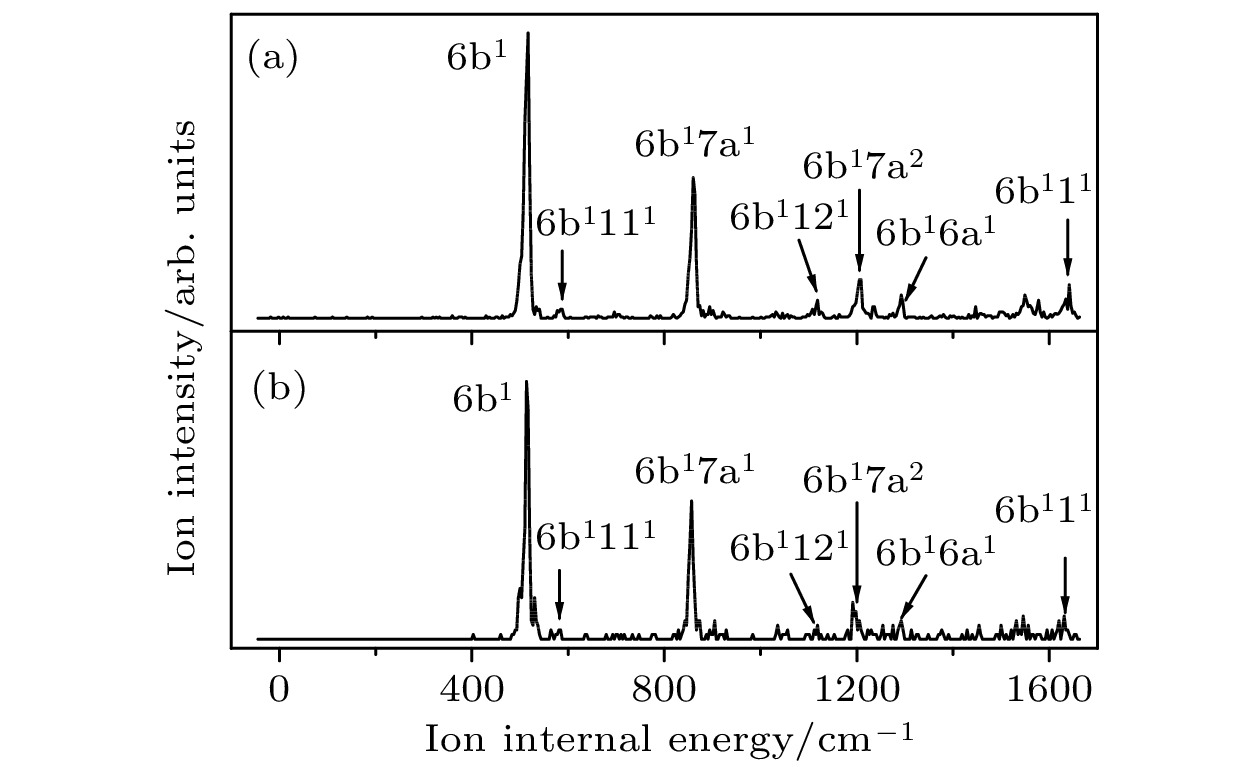
ν (C—Cl)457 454 457 454 $ 16{\text{b}}_0^1 $, γ(CCC) 504 495 504 495 $ 6{\text{b}}_0^1 $, β(CCC) 552 567 552 567 $ 12_0^1 $, β(CCC) 592 597 590 597 β(C—CN) 739 747 739 746 $ 6{\text{a}}_0^1 $, β(CCC) 816 818 $ 6{\text{b}}_0^17{\text{a}}_0^1 $ 859 859 $ 12_0^17{\text{a}}_0^1 $ 867 867 $ 6{\text{a}}_0^115_0^1 $ 962 970 962 970 $ 18{\text{a}}_0^1 $, β(CH) 1002 1002 $ 6{\text{b}}_0^2 $ 1054 1054 $ 6{\text{a}}_0^17{\text{a}}_0^1 $ 1067 1055 1066 1055 $ 1_0^1 $, breathing 1149 1147 1149 1147 $ 9{\text{a}}_0^1 $, β(CH) 1176 1187 1176 1187 $ 13_0^1 $, ν(C—CN) a 实验值是相对于对氯苯腈分子激发能(35818 cm–1)的偏移, 理论值是TD-B3LYP/aug-cc-pVDZ方法计算的振动频率(校正因子0.984) b ν, 伸缩振动; β, 苯环平面内的弯曲振动; γ, 垂直于苯环平面的弯曲振动 表 2 对氯苯腈分子35Cl和37Cl 同位素离子基态D0的振动频率及光谱归属a(单位: cm–1)
Table 2. The measured vibrational frequencies and assignments in the MATI spectra for the D0 state of 35Cl and 37Cl isotopomers of p-chlorobenzonitrilea (unit: cm–1).
35Cl 37Cl 光谱归属b S1中间态 理论值a S1中间态 理论值a S100 S16b1 S100 S16b1 0 346 347 343 344 7a1, β(CCC) , ν (C—Cl) 517 528 514 529 6b1, β(CCC) 587 584 6b1111 601 598 121, β(CCC) 691 693 681 687 7a2 719 731 715 734 41, γ(C—CN) 778 772 778 775 6a1, β(CCC) 860 857 6b17a1 947 947 7a1121 1032 6b2 1035 1040 1031 1031 7a3 1063 7a141 1110 1093 1116 1096 11, breathing 1127 1118 1118 6b1121 1205 1191 6b17a2 1223 1223 1227 1228 131, ν(C—CN) 1236 6b141 1292 1292 6b16a1 1388 1390 8b1, ν(CC) 1549 1545 6b17a3 1572 1577 1569 1570 7a1131 1608 1614 1612 1621 8a1, ν(CC) 1641 1631 6b111 a实验值是相对于对氯苯腈分子电离能(76846 cm–1)的偏移, 理论值是B3LYP/aug-cc-pVDZ方法计算的振动频率(校正因子0.981) b ν, 伸缩振动; β, 苯环平面内的弯曲振动; γ, 垂直于苯环平面的弯曲振动 表 3 对氯苯腈分子在电子基态、激发态和离子基态的基本结构参数
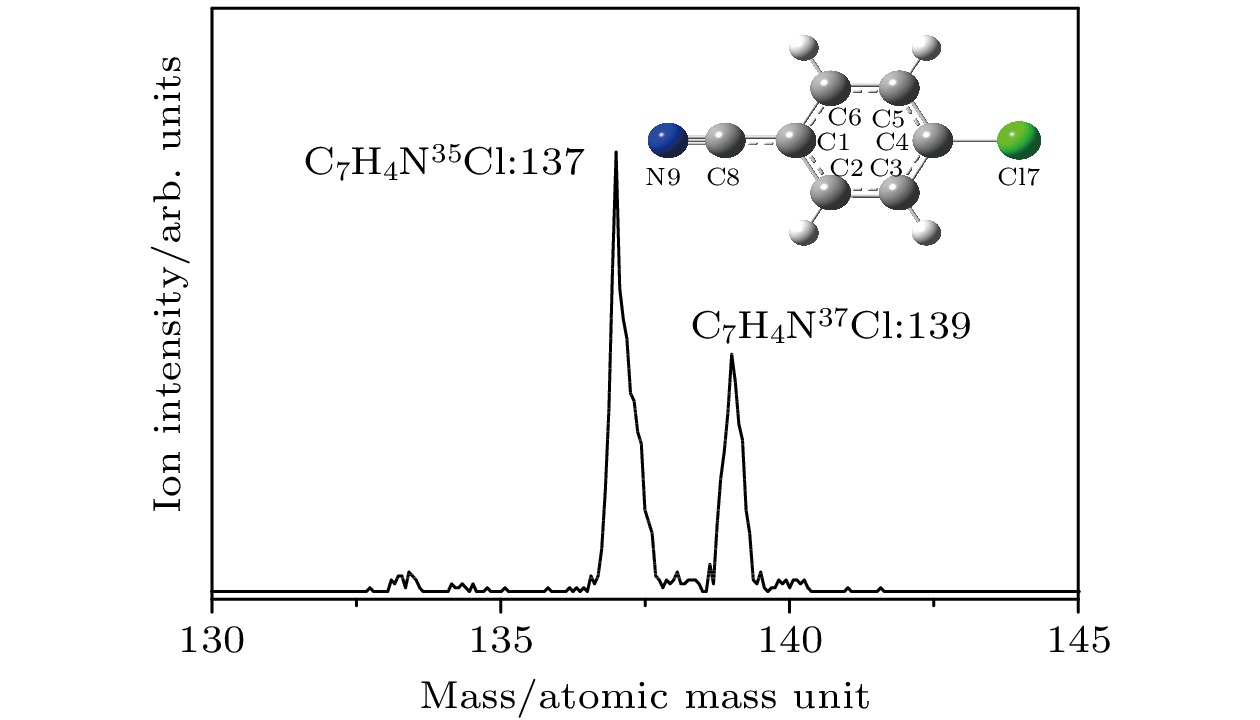
Table 3. Geometrical parameters of p-chlorobenzonitrile in its electronic ground, first excited and cationic ground states.
结构
参数Exp.a S0b S1c D0b Δ(S1-S0) Δ(D0-S1) 键长/Å C1-C2 1.397 1.406 1.432 1.433 0.026 0.008 C2-C3 1.384 1.393 1.429 1.373 0.036 –0.056 C3-C4 1.387 1.397 1.418 1.429 0.021 0.011 C4-C5 1.380 1.397 1.418 1.429 0.021 0.011 C5-C6 1.382 1.393 1.429 1.373 0.036 –0.056 C6-C1 1.386 1.406 1.432 1.433 0.026 0.001 C4-Cl7 1.745 1.755 1.733 1.695 –0.022 –0.038 C1-C8 1.454 1.436 1.414 1.413 –0.023 –0.001 C8-N9 1.110 1.163 1.171 1.170 0.008 –0.001 键角/° C1C2C3 119.1 120.2 119.5 119.6 –0.7 0.1 C2C3C4 119.4 119.2 118.7 118.9 –0.5 0.2 C3C4C5 121.7 121.4 122.7 121.9 1.3 –0.8 C4C5C6 118.8 119.2 118.7 118.9 –0.5 0.2 C5C6C1 120.2 120.2 119.5 119.6 –0.7 0.1 C6C1C2 120.6 119.8 120.8 121.0 1.0 0.2 a对氯苯腈分子的晶体结构参数[31] bB3LYP/aug-cc-pVDZ方法理论计算的结构参数 cTD-B3LYP/aug-cc-pVDZ方法理论计算的结构参数 表 4 苯酚、苯甲醚、苯胺、苯腈及其衍生物分子的跃迁能(单位: cm–1) a
Table 4. The transition energies (cm–1) of phenol, anisole, aniline, benzonitrile and their derivatives.a
Molecule E1(S1 ← S0) ΔE1 E2(D0 ← S1) ΔE2 IE ΔIE 苯酚[33] 36, 349 0 32, 276 0 68, 625 0 对氯苯酚[30] 34, 813 –1537 33, 291 1015 68, 104 –521 苯甲醚[34] 36, 383 0 30, 016 0 66, 399 0 对氯苯甲醚[29] 34, 859 –1524 31, 253 1237 66, 112 –287 苯胺[35] 34, 029 0 28, 242 0 62, 271 0 对氯苯胺[23] 32, 573 –1456 29, 837 1593 62, 410 139 苯腈[36] 36, 518 0 41, 972 0 78, 490 0 对氯苯腈 35, 818 –700 41, 028 –944 76, 846 –1644 对甲基苯腈[37] 36, 222 –296 38, 933 –3039 75, 155 –2845 对氨基苯腈[38] 33, 481 –3037 33, 012 –8960 66, 493 –11997 aΔE1, ΔE2, 和ΔIE 是衍生物分子E1, E2跃迁能和IE电离能相对苯酚、苯甲醚、苯胺、苯腈E1, E2, IE能量的差值 -
[1] Lee J K, Fujiwara T, Kofron W G, Zgierski M Z, Lim E C 2008 J. Chem. Phys. 128 164512
 Google Scholar
Google Scholar
[2] Perveaux A, Castro P J, Lauvergnat D, Reguero M, Lasorne B 2015 J. Phys. Chem. Lett. 6 1316
 Google Scholar
Google Scholar
[3] Livingstone R A, Thompson J O, Iljina M, Donaldson R J, Sussman B J, Paterson M J, Townsend D 2012 J. Chem. Phys. 137 184304
 Google Scholar
Google Scholar
[4] King G A, Devine A L, Nix M G, Kelly D E, Ashfold M N 2008 Phys. Chem. Chem. Phys. 10 6417
 Google Scholar
Google Scholar
[5] Miyazaki M, Sakata Y, Schutz M, Dopfer O, Fujii M 2016 Phys. Chem. Chem. Phys. 18 24746
 Google Scholar
Google Scholar
[6] Aschaffenburg D J, Moog R S 2009 J. Phys. Chem. B 113 12736
 Google Scholar
Google Scholar
[7] Chang C, Lu Y, Wang T, Diau E W 2004 J. Am. Chem. Soc. 126 10109
 Google Scholar
Google Scholar
[8] Hertel I V, Radloff W 2006 Rep. Prog. Phys. 69 1897
 Google Scholar
Google Scholar
[9] Schneider M, Wilke M, Hebestreit M L, Ruiz-Santoyo J A, Alvarez-Valtierra L, Yi J T, Meerts W L, Pratt D W, Schmitt M 2017 Phys. Chem. Chem. Phys. 19 21364
 Google Scholar
Google Scholar
[10] 李鑫, 赵岩, 靳颖辉, 王晓锐, 余谢秋, 武媚, 韩昱行, 杨勇刚, 李昌勇, 贾锁堂 2017 66 093301
 Google Scholar
Google Scholar
Li X, Zhao Y, Jin Y H, Wang X R, Yu X Q, Wu M, Han Y X, Yang Y G, Li C Y, Jia S T 2017 Acta Phys. Sin. 66 093301
 Google Scholar
Google Scholar
[11] Zhao Y, Jin Y H, Li C Y, Jia S T 2019 J. Mol. Spectrosc. 363 111182
 Google Scholar
Google Scholar
[12] Corrales M E, Shternin P S, Rubio L L, De N R, Vasyutinskii O S, Bañares L 2016 J. Phys. Chem. Lett. 7 4458
 Google Scholar
Google Scholar
[13] Tzeng S Y, Shivatare V S, Tzeng W B 2019 J. Phys. Chem. A 123 5969
 Google Scholar
Google Scholar
[14] Findley A M, Bernstorff S, Köhler A M, Saile V, Findley G L 1987 Phys. Scr. 35 633
 Google Scholar
Google Scholar
[15] Onda M, Saegusa N, Yamaguchi I 1986 J. Mol. Struct. 145 185
 Google Scholar
Google Scholar
[16] Rocha I M, Galvão T L, Ribeiro da Silva M D, Ribeiro da Silva M A 2014 J. Phys. Chem. A 118 1502
 Google Scholar
Google Scholar
[17] Trivedi M K, Branton A, Trivedi D, Nayak G, Singh R, Jana S 2015 J. Chem. Sci. 3 84
 Google Scholar
Google Scholar
[18] Zhao Y, Jin Y H, Hao J Y, Yang Y G, Wang L R, Li C Y, Jia S T 2019 Spectrochim. Acta, Part A 207 328
 Google Scholar
Google Scholar
[19] 段春泱, 李娜, 赵岩, 李昌勇 2021 70 053301
 Google Scholar
Google Scholar
Duan C Y, Li N, Zhao Y, Li C Y 2021 Acta Phys. Sin. 70 053301
 Google Scholar
Google Scholar
[20] Frisch M J, Trucks G W, Schlegel H B, Scuseria G E, Robb M A, Cheeseman J R, Scalmani G, Barone V, Mennucci B, Petersson G A, Nakatsuji H, Caricato M, Li X, Hratchian H P, Izmaylov A F, Bloino J, Zheng G, Sonnenberg J L, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery J A, Peralta J E, Ogliaro F, Bearpark M, Heyd J J, Brothers E, Kudin K N, Staroverov V N, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant J C, Iyengar S S, Tomasi J, Cossi M, Rega N, Millam J M, Klene M, Knox J E, Cross J B, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann R E, Yazyev O, Austin A J, Cammi R, Pomelli C, Ochterski J W, Martin R L, Morokuma K, Zakrzewski V G, Voth G A, Salvador P, Dannenberg J J, Dapprich S, Daniels A D, Farkas Ö, Foresman J B, Ortiz J V, Cioslowski J, Fox D J 2009 Gaussian 09 (Wallingford CT: Gaussian Inc.)
[21] Merrick J P, Moran D, Radom L 2007 J. Phys. Chem. A 111 11683
 Google Scholar
Google Scholar
[22] Maiti A K, Sarkar S K, Kastha G S 1985 Proc. Indian Acad. Sci. (Chem. Sci.) 95 409
 Google Scholar
Google Scholar
[23] Lin J L, Tzeng W B 2000 J. Chem. Phys. 113 4109
 Google Scholar
Google Scholar
[24] Huang J H, Huang K L, Liu S Q, Luo Q, Tzeng W B 2008 J. Photochem. Photobiol. , A 193 245
 Google Scholar
Google Scholar
[25] Lin J L, Li C Y, Tzeng W B 2004 J. Chem. Phys. 120 10513
 Google Scholar
Google Scholar
[26] Yu D, Dong C W, Cheng M, Hu L L, Du Y K, Zhu Q H, Zhang C H 2011 J. Mol. Spectrosc. 265 86
 Google Scholar
Google Scholar
[27] Wilson E B 1934 Phys. Rev. 45 706
 Google Scholar
Google Scholar
[28] Varsanyi G 1974 Assignments of Vibrational Spectra of Seven Hundred Benzene Derivatives (New York: Wiley) pp185–190
[29] Tzeng S Y, Takahashi K, Tzeng W B 2019 Chem. Phys. Lett. 731 136626
 Google Scholar
Google Scholar
[30] Huang J, Lin J L, Tzeng W B 2006 Chem. Phys. Lett. 422 271
 Google Scholar
Google Scholar
[31] Desiraju G R, Harlow R L 1989 J. Am. Chem. Soc. 111 6757
 Google Scholar
Google Scholar
[32] Zhao Y, Jin Y H, Hao J Y, Yang Y, Li C Y, Jia S T 2018 Chem. Phys. Lett. 711 127
 Google Scholar
Google Scholar
[33] Dopfer O, Müller-Dethlefs K 1994 J. Chem. Phys. 101 8508
 Google Scholar
Google Scholar
[34] Pradhan M, Li C Y, Lin J L, Tzeng W B 2005 Chem. Phys. Lett. 407 100
 Google Scholar
Google Scholar
[35] Lin J L, Tzeng W B 2001 J. Chem. Phys. 115 743
 Google Scholar
Google Scholar
[36] Araki M, Sato S, Kimura K 1996 J. Phys. Chem. 100 10542
 Google Scholar
Google Scholar
[37] Suzuki K, Ishiuchi S, Sakai M, Fujii M 2005 J. Electron Spectrosc. Relat. Phenom. 142 215
 Google Scholar
Google Scholar
[38] Huang L C, Lin J L, Tzeng W B 2000 Chem. Phys. 261 449
 Google Scholar
Google Scholar
[39] Zhang B, Li C, Su H, Lin J L, Tzeng W B 2004 Chem. Phys. Lett. 390 65
 Google Scholar
Google Scholar
Catalog
Metrics
- Abstract views: 7226
- PDF Downloads: 77
- Cited By: 0














 DownLoad:
DownLoad: