-
As their characteristic dimensions are reduced to the nanoscale regime, such as single layer and single atom, the materials exhibit novel physical and chemical properties. Both the two-dimensional materials and the ordered array of single atoms or molecules have become cutting-edge research topics in the area of modern quantum devices and catalytic science. Silicene prepared on the Ag(111) substrate exhibits abundant superstructures at different substrate temperatures and coverages. These superstructures can be reliable templates for fabricating the ordered array of single atoms or molecules. Using in-situ silicene preparation, molecular deposition, ultra-high vacuum scanning tunneling microscope (STM), and scanning tunneling spectroscopy (STS), the electronic structures, surface work functions and adsorption behaviors of CoPc molecules on three silicene superstructures ((4 × 4), (
$\sqrt {13} \times \sqrt {13} $ ), and ($2\sqrt 3 \times 2\sqrt 3 $ )) are studied. Firstly, the three silicene superstructures have similar electronic structures according to the characterization from the dI/dV curve at 77 K. The electronic structure varies on an atomic scale. With the disordering increasing, the full width at half maximum of the +0.6 V states broadens from (4 × 4) to ($\sqrt {13} \times \sqrt {13} $ ) to ($2\sqrt 3 \times 2\sqrt 3 $ ). Secondly, the average surface work functions of the three superstructures of silicene also vary on an atomic scale and are all higher than those on the Silver surface. So, electrons are probably transferred from the Ag substrate to the single-layer silicene. The number of the transferred electrons increases from (4 × 4) structure, ($\sqrt {13} \times \sqrt {13} $ ) structure, to ($2\sqrt 3 \times 2\sqrt 3 $ ) structure. Thirdly, the change of the surface work function on an atomic scale plays an important role in selectively adsorbing the CoPc molecules, which causes the symmetry of CoPc electronic structure to break. It indicates that none of the three silicene superstructures belongs to a complete π-bond system. Especially, on the (4 × 4) superstructure, all CoPc molecules are divided into two halves. One half is similar to the free standing ones, in which there are HOMO (–0.45 V) and LUMO (+0.7 V) state. The other half has strong interaction with the silicene. The HOMO state is suppressed and there is a hybrid state at 1.0 V according to the dI/dV characterization.-
Keywords:
- silicene /
- electronic structure /
- molecular adsorption /
- symmetry broken
[1] Ternes M, Heinrich A J, Schneider W D 2008 J. Phys.: Condens. Matter 21 053001
[2] He Y, Gorman S K, Keith D, Kranz L, Keizer J G, Simmons M Y 2019 Nature 571 371
 Google Scholar
Google Scholar
[3] Fricke L, Hile S J, Kranz L, Chung Y, He Y, Pakkiam P, House M G, Keizer J G, Simmons MY 2021 Nat. Commun. 12 3323
 Google Scholar
Google Scholar
[4] Wang A, Li J, Zhang T 2018 Nat. Rev. Chem. 2 65
 Google Scholar
Google Scholar
[5] Samantaray M K, D'Elia V, Pump E, Falivene L, Harb M, Chikh S O, Cavallo L, Basset J M 2020 Chem. Rev. 120 734
 Google Scholar
Google Scholar
[6] Kharadi M A, Malik G F A, Khanday F A, Shah K A, Mittal S, KaushikB K 2020 ECS J. Solid State Sci. Technol. 9 115031
 Google Scholar
Google Scholar
[7] Gao N, Lu G Y, Jiang Q 2017 J. Mater. Chem. C 5 627
 Google Scholar
Google Scholar
[8] Guo Z X, Furuya S, Iwata J, Oshiyama A 2013 Phys. Rev. B 87 235435
 Google Scholar
Google Scholar
[9] Lin C L, Kim B K, Ahn Y H, Kim J J, Choi M S, Bae M H, Kang K, Lim J S, López R, KimN 2013 Phys. Rev. Lett. 110 076803
 Google Scholar
Google Scholar
[10] Stephan R, Hanf M C, Sonnet P 2015 J. Phys.:Condens. Matter 27 015002
 Google Scholar
Google Scholar
[11] Vogt P, Padova P D, Quaresima C, Avila J, Frantzeskakis E, Asensio M C, Resta A, Ealet B, LayG L 2012 Phys. Rev. Lett. 108 155501
 Google Scholar
Google Scholar
[12] Feng B J, Ding Z J, Meng S, Yao Y G, He X Y, Cheng P, Chen L, Wu K H 2012 Nano Lett. 12 3507
 Google Scholar
Google Scholar
[13] Enriquez H, Vizzini S, Kara A, Lalmi B, Oughaddou H 2012 J. Phys.:Condens. Matter 24 314211
 Google Scholar
Google Scholar
[14] Resta A, Leoni T, Barth C, Ranguis A, Becker C, Bruhn T, Vogt P, Lay GL 2013 Sci. Rep. 3 2399
 Google Scholar
Google Scholar
[15] Kawahara K, Shirasawa T, Arafune R, Lin C L, Takahashi T, Kawai M, Takagi N 2014 Surf. Sci. 623 25
 Google Scholar
Google Scholar
[16] Ryuichi A, Liang L C, Kazuaki K, Noriyuki T, Emi M, Kim Y, Noriaki T, Maki K 2013 Surf. Sci. 608 297
 Google Scholar
Google Scholar
[17] Liu ZL, Wang MX, Liu C H, Jia JF, Vogt P, Quaresima C, Ottaviani C, Olivieri B, DePadova P, Lay G L 2014 APL Mat. 2 092513
 Google Scholar
Google Scholar
[18] RahmanMS, Nakagawa T, MizunoS 2015 Jpn. J. Appl. Phys. 54 015502
 Google Scholar
Google Scholar
[19] Jamgotchian H, Ealet B, Colignon Y, Maradj H, Hoarau JY, Biberian JP, AufrayB 2015 J. Phys. Condens. Matter 27 395002
 Google Scholar
Google Scholar
[20] Curcella A, Bernard R, Borensztein Y, Resta A, Lazzeri M, Prévot G 2016 Phys. Rev. B 94 165438
 Google Scholar
Google Scholar
[21] Curcella A, Bernard R, Borensztein Y, Resta A, Lazzeri M, Prévot G 2019 Phys. Rev. B 99 205411
 Google Scholar
Google Scholar
[22] Pawlak R, Drechsel C, D’Astolfo P, Kisiel M, Meyer E, Cerda J I 2020 PNAS 117 228
 Google Scholar
Google Scholar
[23] Krull C, Robles R, Mugarza A, GambardellaP 2013 Nat. Mater. 12 337
 Google Scholar
Google Scholar
[24] Liu L W, Yang K, Jiang Y H, Song B Q, Xiao W D, Song S R, Du S X, Ouyang M, Hofer WA, Neto AHC, Gao HJ 2015 Phys. Rev. Lett. 114 126601
 Google Scholar
Google Scholar
[25] Wintterlin J, BocquetM L 2009 Surf. Sci. 603 1841
 Google Scholar
Google Scholar
[26] Li Z Y, Li B, Yang J L, Hou J G 2010 Acc. Chem. Res. 43 954
 Google Scholar
Google Scholar
[27] Mugarza A, Robles R, Krull C, Korytr R, Lorente N, GambardellaP 2012 Phys. Rev. B 85 155437
 Google Scholar
Google Scholar
-
图 1 硅烯的STM形貌图和STS谱图 (a)(4 × 4)和(
$ \sqrt {{\text{13}}} $ ×$ \sqrt {{\text{13}}} $ )超结构及Ag衬底; (b) ($ 2\sqrt {\text{3}} $ ×$ 2\sqrt {\text{3}} $ )超结构及Ag衬底; (c)不同重构结构的dI/dV谱图Figure 1. STM topography and STS curves: (a) (4 × 4) and (
$ \sqrt {{\text{13}}} $ ×$ \sqrt {{\text{13}}} $ ) superstructures and Ag substrate; (b) ($2\sqrt 3 \times $ $ 2\sqrt 3$ ) superstructure and Ag substrate; (c) dI/dV spectral curves of different reconstructed structures.图 2 硅烯的表面功函数(a), (c)和(e)分别为(4 × 4), (
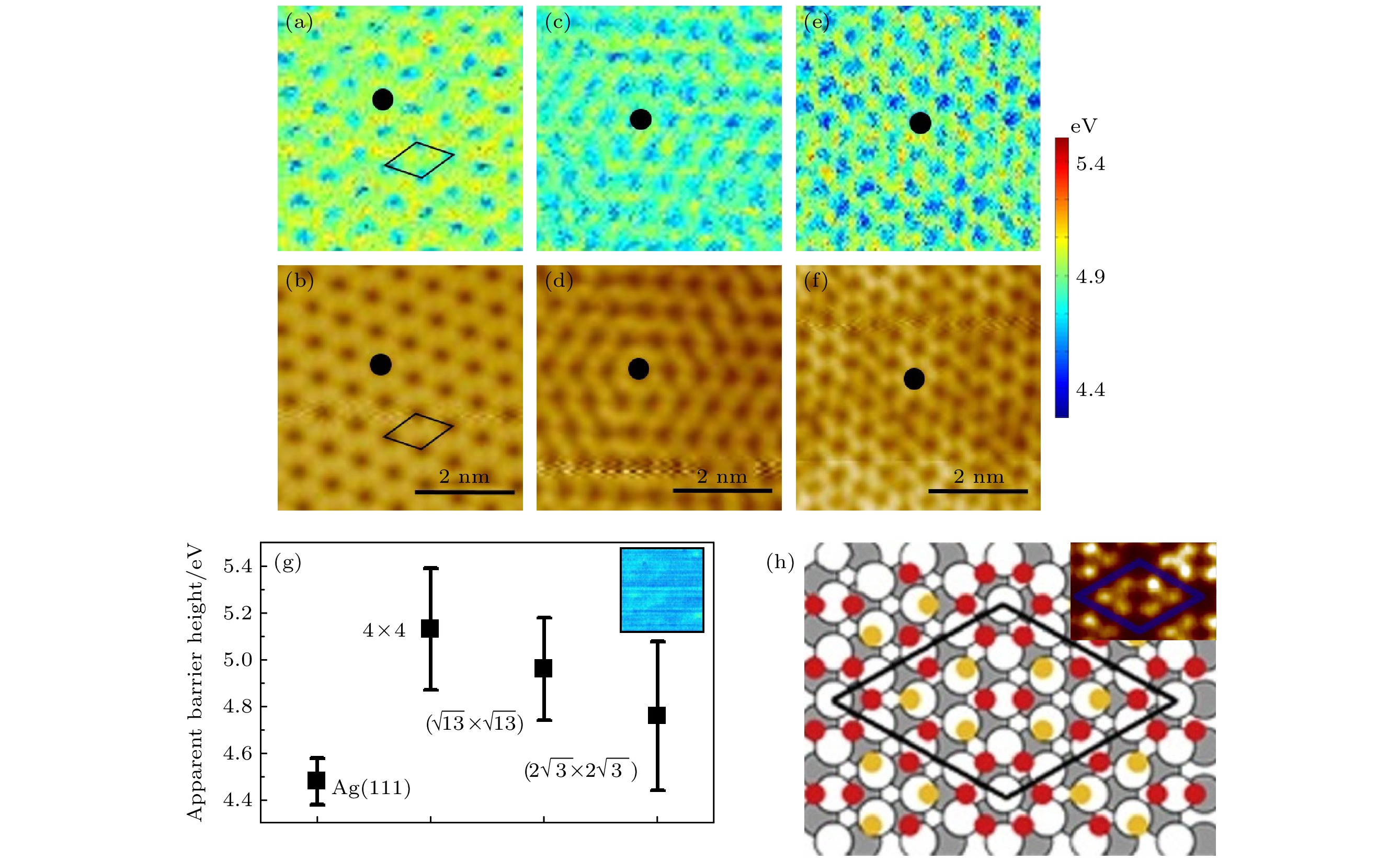
$ \sqrt {{\text{13}}} $ ×$ \sqrt {{\text{13}}} $ ), ($ 2\sqrt {\text{3}} $ ×$ 2\sqrt {\text{3}} $ )超结构的表面功函数图; (b), (d)和(f)对应获得的STM形貌图; (g)3种超结构和Ag衬底的平均表面功函数分布, 插图是Ag(111)表面功函数图; (h)(4 × 4)超结构的原子模型, 插图是高分辨率的(4 × 4)超结构STM图像Figure 2. Surface work function of silicene: (a), (c), (e) (4 × 4), (
$ \sqrt {{\text{13}}} $ ×$ \sqrt {{\text{13}}} $ ), ($ 2\sqrt {\text{3}} $ ×$ 2\sqrt {\text{3}} $ ) super structures of the surface work function maps, respectively; (b), (d), (f) the corresponding STM topography images obtained at the same time; (g) the average surface work function distribution of the three superstructures and Ag substrates, the inset is a diagram of the height of the Ag(111) surface barrier; (h) atomic model of (4 × 4) super structure, the inset is a high resolution STM image of (4 × 4) superstructure.图 3 CoPc分子吸附在 (a) (4 × 4), (c) (
$ \sqrt {{\text{13}}} $ ×$ \sqrt {{\text{13}}} $ ), (e) ($ 2\sqrt {\text{3}} $ ×$ 2\sqrt {\text{3}} $ )超结构上的STM形貌图, 扫描条件见实验条件部分; (b), (d)和(f)是其相应的偏压为–0.4 V的dI/dV谱图Figure 3. STM topography images of CoPc molecules adsorbed on superstructures of (a) (4 × 4), (c) (
$ \sqrt {{\text{13}}} $ ×$ \sqrt {{\text{13}}} $ ) and (e) ($ 2\sqrt {\text{3}} $ ×$ 2\sqrt {\text{3}} $ ), the scanning parameters are described in the experiment condition section; (b), (d) and (f) show their corresponding dI/dV spectrum map sat -0.4 V.图 4 (a), (b)偏压分别为–1.00 V和+1.00 V时, 单个CoPc分子在(4 × 4)重构表面上的STM形貌图; (c), (d)和(f)偏压分别为–0.40 V、+0.70 V和+1.00 V时, 单个CoPc分子的dI/dV谱图; (f), (g)分别为在重构表面及单个CoPc分子上获得的dI/dV曲线
Figure 4. (a), (b) The STM images of a single CoPc molecule adsorbed on (4 × 4) reconstructed surface when the sample bias is –1.00 V and +1.00 V, respectively; (c), (d), (f) the dI/dV spectra on a single CoPc molecule when the sample bias voltage is –0.40 V, +0.70 V and +1.00 V, respectively; (f), (g) the dI/dV curves obtained on (4 × 4) reconstructed surface and a single CoPc molecule, respectively.
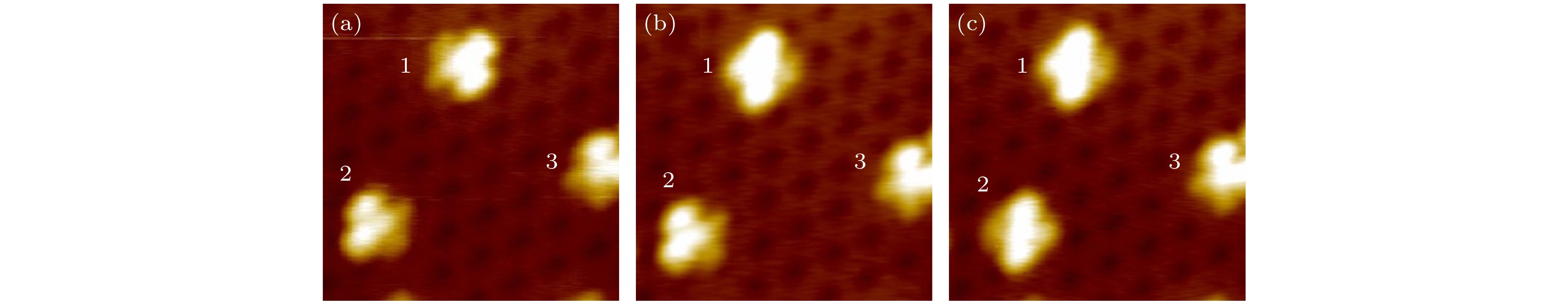
图 A1 在–0.4 V的扫描状态下, 利用1.5 V, 5 ms的脉冲对(4 × 4)的硅烯超结构上吸附的CoPc分子进行操控, 实现CoPc分子的原位转动(a)未做任何脉冲操控前的分子形貌图;(b)对标记为1的分子完成脉冲操控后的形貌图;(c)对标记为2的分子完成脉冲操控后的形貌图
Figure A1. The scanning bias is –0.4 V, use a 1.5 V, 5 ms pulse to manipulate the CoPc molecules adsorbed on the (4 × 4) silicene superstructure to realize the in-situ rotation of the CoPc molecules: (a) The molecular topography before any pulse manipulation; (b) the topography of the molecule marked 1 after pulse manipulation; (c) the topography of the molecule marked 2 after pulse manipulation.
-
[1] Ternes M, Heinrich A J, Schneider W D 2008 J. Phys.: Condens. Matter 21 053001
[2] He Y, Gorman S K, Keith D, Kranz L, Keizer J G, Simmons M Y 2019 Nature 571 371
 Google Scholar
Google Scholar
[3] Fricke L, Hile S J, Kranz L, Chung Y, He Y, Pakkiam P, House M G, Keizer J G, Simmons MY 2021 Nat. Commun. 12 3323
 Google Scholar
Google Scholar
[4] Wang A, Li J, Zhang T 2018 Nat. Rev. Chem. 2 65
 Google Scholar
Google Scholar
[5] Samantaray M K, D'Elia V, Pump E, Falivene L, Harb M, Chikh S O, Cavallo L, Basset J M 2020 Chem. Rev. 120 734
 Google Scholar
Google Scholar
[6] Kharadi M A, Malik G F A, Khanday F A, Shah K A, Mittal S, KaushikB K 2020 ECS J. Solid State Sci. Technol. 9 115031
 Google Scholar
Google Scholar
[7] Gao N, Lu G Y, Jiang Q 2017 J. Mater. Chem. C 5 627
 Google Scholar
Google Scholar
[8] Guo Z X, Furuya S, Iwata J, Oshiyama A 2013 Phys. Rev. B 87 235435
 Google Scholar
Google Scholar
[9] Lin C L, Kim B K, Ahn Y H, Kim J J, Choi M S, Bae M H, Kang K, Lim J S, López R, KimN 2013 Phys. Rev. Lett. 110 076803
 Google Scholar
Google Scholar
[10] Stephan R, Hanf M C, Sonnet P 2015 J. Phys.:Condens. Matter 27 015002
 Google Scholar
Google Scholar
[11] Vogt P, Padova P D, Quaresima C, Avila J, Frantzeskakis E, Asensio M C, Resta A, Ealet B, LayG L 2012 Phys. Rev. Lett. 108 155501
 Google Scholar
Google Scholar
[12] Feng B J, Ding Z J, Meng S, Yao Y G, He X Y, Cheng P, Chen L, Wu K H 2012 Nano Lett. 12 3507
 Google Scholar
Google Scholar
[13] Enriquez H, Vizzini S, Kara A, Lalmi B, Oughaddou H 2012 J. Phys.:Condens. Matter 24 314211
 Google Scholar
Google Scholar
[14] Resta A, Leoni T, Barth C, Ranguis A, Becker C, Bruhn T, Vogt P, Lay GL 2013 Sci. Rep. 3 2399
 Google Scholar
Google Scholar
[15] Kawahara K, Shirasawa T, Arafune R, Lin C L, Takahashi T, Kawai M, Takagi N 2014 Surf. Sci. 623 25
 Google Scholar
Google Scholar
[16] Ryuichi A, Liang L C, Kazuaki K, Noriyuki T, Emi M, Kim Y, Noriaki T, Maki K 2013 Surf. Sci. 608 297
 Google Scholar
Google Scholar
[17] Liu ZL, Wang MX, Liu C H, Jia JF, Vogt P, Quaresima C, Ottaviani C, Olivieri B, DePadova P, Lay G L 2014 APL Mat. 2 092513
 Google Scholar
Google Scholar
[18] RahmanMS, Nakagawa T, MizunoS 2015 Jpn. J. Appl. Phys. 54 015502
 Google Scholar
Google Scholar
[19] Jamgotchian H, Ealet B, Colignon Y, Maradj H, Hoarau JY, Biberian JP, AufrayB 2015 J. Phys. Condens. Matter 27 395002
 Google Scholar
Google Scholar
[20] Curcella A, Bernard R, Borensztein Y, Resta A, Lazzeri M, Prévot G 2016 Phys. Rev. B 94 165438
 Google Scholar
Google Scholar
[21] Curcella A, Bernard R, Borensztein Y, Resta A, Lazzeri M, Prévot G 2019 Phys. Rev. B 99 205411
 Google Scholar
Google Scholar
[22] Pawlak R, Drechsel C, D’Astolfo P, Kisiel M, Meyer E, Cerda J I 2020 PNAS 117 228
 Google Scholar
Google Scholar
[23] Krull C, Robles R, Mugarza A, GambardellaP 2013 Nat. Mater. 12 337
 Google Scholar
Google Scholar
[24] Liu L W, Yang K, Jiang Y H, Song B Q, Xiao W D, Song S R, Du S X, Ouyang M, Hofer WA, Neto AHC, Gao HJ 2015 Phys. Rev. Lett. 114 126601
 Google Scholar
Google Scholar
[25] Wintterlin J, BocquetM L 2009 Surf. Sci. 603 1841
 Google Scholar
Google Scholar
[26] Li Z Y, Li B, Yang J L, Hou J G 2010 Acc. Chem. Res. 43 954
 Google Scholar
Google Scholar
[27] Mugarza A, Robles R, Krull C, Korytr R, Lorente N, GambardellaP 2012 Phys. Rev. B 85 155437
 Google Scholar
Google Scholar
Catalog
Metrics
- Abstract views: 6584
- PDF Downloads: 102
- Cited By: 0

























 DownLoad:
DownLoad: