-
The development potential of germanene-based integrated electronics originates from its high carrier mobility and compatibility with the existing silicon-based and germanium-based semiconductor industry. However, the small band gap energy band (Dirac point) of germanene greatly impedes its application. Thus, it is necessary to open a sizeable band gap without reducing the carrier mobility for the application in logic circuits. In this study, the effects of organic molecule (benzene or hexafluorobenzene) adsorption and substrate on the atomic structures and electronic properties of germanene under an external electric field are investigated by using density functional theory calculations with van der Waals correction. For benzene/germanene and hexafluorobenzene/germanene systems, four different adsorption sites are considered, with the center of the organic molecules lying directly atop the upper or lower Ge atoms of germanene, in the Ge-Ge bridge center, and on the central hollow ring. Meanwhile, different molecular orientations at each adsorption site are also considered. Thus, there are eight high-symmetry adsorption configurations of the systems, respectively. According to the adsorption energy, we can determine the most stable atomic structures of the above systems. The results show that the organic molecule adsorption can induce the larger buckling height in germanene. Both the adsorption energy and interlayer distance indicate that there is no chemical bond between the organic molecules and germanene. Mulliken population analysis shows that a charge redistribution in the two sublattices in germanene exists since benzene is an electron donor molecule and hexafluorobenzene is an electron acceptor molecule. As a result, the benzene/germanene system exhibits a relatively large band gap (0.036 eV), while hexafluorobenzene/germanene system displays a small band gap (0.005 eV). Under external electric field, germanene with organic molecule adsorption can exhibit a wide range of linear tunable band gaps, which is merely determined by the strength of electric field regardless of its direction. The charge transfer among organic molecules and two sublattices in germanene gradually rises with the increasing the strength of electric field, resulting in the electron density around the sublattices in germanene unequally distributed. Thus, according to the tight-binding model, a larger band gap at the K-point is opened. When germanane (fully hydrogenated germanene HGeH) substrate is applied, the band gaps further widen, where the band gap of benzene/ germanene/germanane system can increase to 0.152 eV, and that of hexafluorobenzene/germanene/germanane system can reach 0.105 eV. The sizable band gap in germanene is created due to the symmetry of two sublattices in germanene destroyed by the dual effects of organic molecule adsorption and substrate. Note that both of organic molecules and substrate are found to non-covalently functionalize the germanene. As the strength of the negative electric field increases, the band gaps can be further modulated effectively. Surprisingly, the band gaps of the above systems can be closed, and reopened under a critical electric field. These features are attributed to the build-in electric field due to the interlayer charge transfer of the systems, which breaks the equivalence between the two sublattices of germanene. More importantly, the high carrier mobility in germanene is still retained to a large extent. These results provide effective and reversible routes to engineering the band gap of germanene for the applications of germanene to field-effect transistor and other nanoelectronic devices.
-
Keywords:
- germanene /
- organic molecule adsorption /
- substrate /
- electronic structure
[1] 黄立, 杜世萱, 高鸿钧 2018 物理 47 173
 Google Scholar
Google Scholar
Huang L, Du S X, Gao H J 2018 Physics 47 173
 Google Scholar
Google Scholar
[2] Li L F, Lu S Z, Pan J B, Qin Z H, Wang Y Q, Wang Y L, Cao G Y, Du S X, Gao H J 2014 Adv. Mater. 26 4820
 Google Scholar
Google Scholar
[3] Dávila M E, Xian L, Cahangirov S, Rubio A, Lay Le G 2014 New J. Phys. 16 095002
 Google Scholar
Google Scholar
[4] Derivaz M, Dentel D, Stephan R, Hanf M C, Mehdaoui A, Sonnet P, Pirri C 2015 Nano Lett. 15 2510
 Google Scholar
Google Scholar
[5] Qin Z H, Pan J B, Lu S Z, Shao Y, Wang Y L, Du S X, Gao H J, Cao G Y 2017 Adv. Mater. 29 1606046
 Google Scholar
Google Scholar
[6] 武红, 李峰 2016 65 096801
 Google Scholar
Google Scholar
Wu H, Li F 2016 Acta Phys. Sin. 65 096801
 Google Scholar
Google Scholar
[7] Liu C C, Feng W X, Yao Y G 2011 Phys. Rev. Lett. 107 076802
 Google Scholar
Google Scholar
[8] Wang T H, Zhu Y F, Jiang Q 2013 J. Phys. Chem. C 117 12873
 Google Scholar
Google Scholar
[9] Zhu Y F, Dai Q Q, Zhao M, Jiang Q 2013 Sci. Rep. 3 1524
 Google Scholar
Google Scholar
[10] Garcia J C, de Lima D B, Assali L V C, Justo J F 2011 J. Phys. Chem. C 115 13242
 Google Scholar
Google Scholar
[11] Gao N, Zheng W T, Jiang Q 2012 Phys. Chem. Chem. Phys. 14 257
 Google Scholar
Google Scholar
[12] Wang R, Xu M S, Pi X D 2015 Chin. Phys. B 24 086807
 Google Scholar
Google Scholar
[13] Gao J F, Zhang J F, Liu H S, Zhang Q F, Zhao J J 2013 Nanoscale 5 9785
 Google Scholar
Google Scholar
[14] Li S, Wu Y F, Tu Y, Wang Y, Jiang T, Liu W, Zhao Y 2015 Sci. Rep. 5 7881
 Google Scholar
Google Scholar
[15] Liu H S, Feng H F, Du Y, Chen J, Wu K H, Zhao J J 2016 2D Mater. 3 025034
 Google Scholar
Google Scholar
[16] Hu T, Gerber I C 2013 J. Phys. Chem. C 117 2411
 Google Scholar
Google Scholar
[17] Wang Y P, Ji W X, Zhang C W, Li S S, Li F, Li P, Ren M J, Chen X L, Yuan M, Wang P J 2016 Mater. Chem. Phys. 173 379
 Google Scholar
Google Scholar
[18] Chen Q, Hu H, Chen X J, Wang J L 2011 Appl. Phys. Lett. 98 053102
 Google Scholar
Google Scholar
[19] Zhang Y B, Tang T T, Girit C, Hao Z, Martin M C, Zettl A, Crommie M F, Shen Y R, Wang F 2009 Nature 459 820
 Google Scholar
Google Scholar
[20] Ni Z, Liu Q H, Tang K C, Zheng J X, Zhou J, Qin R, Gao Z X, Yu P, Lu J 2012 Nano Lett. 12 113
 Google Scholar
Google Scholar
[21] Liu G, Luo W W, Wang X, Lei X L, Xu B, Ouyang C Y, Liu S B 2018 J. Mater. Chem. C 6 5937
 Google Scholar
Google Scholar
[22] Zhang D, Lou W K, Miao M S, Zhang S C, Chang K 2013 Phys. Rev. Lett. 111 156402
 Google Scholar
Google Scholar
[23] 张弦, 郭志新, 曹觉先, 肖思国, 丁建文 2015 64 186101
 Google Scholar
Google Scholar
Zhang X, Guo Z X, Cao J X, Xiao S G, Ding J W 2015 Acta Phys. Sin. 64 186101
 Google Scholar
Google Scholar
[24] Li X D, Wu S Q, Zhou S, Zhu Z Z 2014 Nanoscale Res. Lett. 9 110
 Google Scholar
Google Scholar
[25] Ye H, Hu F F, Tang H Y, Yang L W, Chen X P, Wang L G, Zhang G Q 2018 Phys. Chem. Chem. Phys. 20 16067
 Google Scholar
Google Scholar
[26] Fan Y C, Liu X B, Wang J R, Ai H Q, Zhao M W 2018 Phys. Chem. Chem. Phys. 20 11369
 Google Scholar
Google Scholar
[27] Gao N, Lu G Y, Wen Z, Jiang Q 2017 J. Mater. Chem. C 5 627
 Google Scholar
Google Scholar
[28] Zhou S, Zhao J 2016 J. Phys. Chem. C 120 21691
 Google Scholar
Google Scholar
[29] Delley B 1990 J. Chem. Phys. 92 508
 Google Scholar
Google Scholar
[30] Delley B 2000 J. Chem. Phys. 113 7756
 Google Scholar
Google Scholar
[31] Perdew J P, Burke K, Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[32] Koelling D D, Hartreermon B N 1977 J. Phys. C 10 3107
 Google Scholar
Google Scholar
[33] Monkhorst H J, Pack J D 1976 Phys. Rev. B 13 5188
 Google Scholar
Google Scholar
[34] Grimme S 2006 J. Comput. Chem. 27 1787
 Google Scholar
Google Scholar
[35] Li S, Wu Y F, Liu W, Zhao Y H 2014 Chem. Phys. Lett. 609 161
 Google Scholar
Google Scholar
[36] Xie X Q, Ao Z M, Su D W, Zhang J Q, Wang G X 2015 Adv. Funct. Mater. 25 1393
 Google Scholar
Google Scholar
[37] 陈庆玲, 戴振宏, 刘兆庆, 安玉凤, 刘悦林 2016 65 136101
 Google Scholar
Google Scholar
Chen Q L, Dai Z H, Liu Z Q, An Y F, Liu Y L 2016 Acta Phys. Sin. 65 136101
 Google Scholar
Google Scholar
[38] Shen T, Ren J C, Liu X, Li S, Liu W 2019 J. Am. Chem. Soc. 141 3110
 Google Scholar
Google Scholar
[39] He C, Han F S, Zhang J H, Zhang W X 2020 J. Mater. Chem. C 8 6923
 Google Scholar
Google Scholar
[40] Rubio-Pereda P, Takeuchi N 2015 J. Phys. Chem. C 119 27995
 Google Scholar
Google Scholar
[41] Su G R, Yang S, Jiang Y D, Li J T, Ren J C, Liu W 2019 Prog. Surf. Sci. 4 100561
 Google Scholar
Google Scholar
[42] Gao W, Chen Y, Li B, Liu S P, Liu X, Jiang Q 2020 Nat. Commun. 11 1196
 Google Scholar
Google Scholar
[43] Ye J P, Liu G, Han Y, Luo W W, Sun B Z, Lei X L, Xu B, Ouyang C Y, Zhang H L 2019 Phys. Chem. Chem. Phys. 21 20287
 Google Scholar
Google Scholar
[44] Ye X S, Shao Z G, Zhao H B, Yang L, Wang C L 2014 RSC Adv. 4 21216
 Google Scholar
Google Scholar
[45] Quhe R, Fei R, Liu Q H, Zheng J X, Li H, Xu C Y, Ni Z Y, Wang Y Y, Yu D P, Gao Z X, Lu J 2012 Sci. Rep. 2 853
 Google Scholar
Google Scholar
[46] Yan J A, Gao S P, Stein R, Coard G 2015 Phys. Rev. B 91 245403
 Google Scholar
Google Scholar
[47] 陈艳珊, 刘兴斌 2013 低温与特气 31 33
 Google Scholar
Google Scholar
Chen Y S, Liu X B 2013 Low Temp. Spec. Gases 31 33
 Google Scholar
Google Scholar
[48] Amamou W, Odenthal P M, Bushong E J, O’hara D J, Luo Y K, Baren J V, Pinchuck I, Wu Y, Ahmed A S, Katoch J, Bockrath M W 2015 2D Mater. 2 035012
 Google Scholar
Google Scholar
[49] Jiang S S, Bianco E, Goldberger J E 2014 J. Mater. Chem. C 2 3185
 Google Scholar
Google Scholar
[50] Bianco E, Butler S, Jiang S S, Restrepo O D, Windl W F, Goldberger J E 2013 ACS Nano 7 4414
 Google Scholar
Google Scholar
-
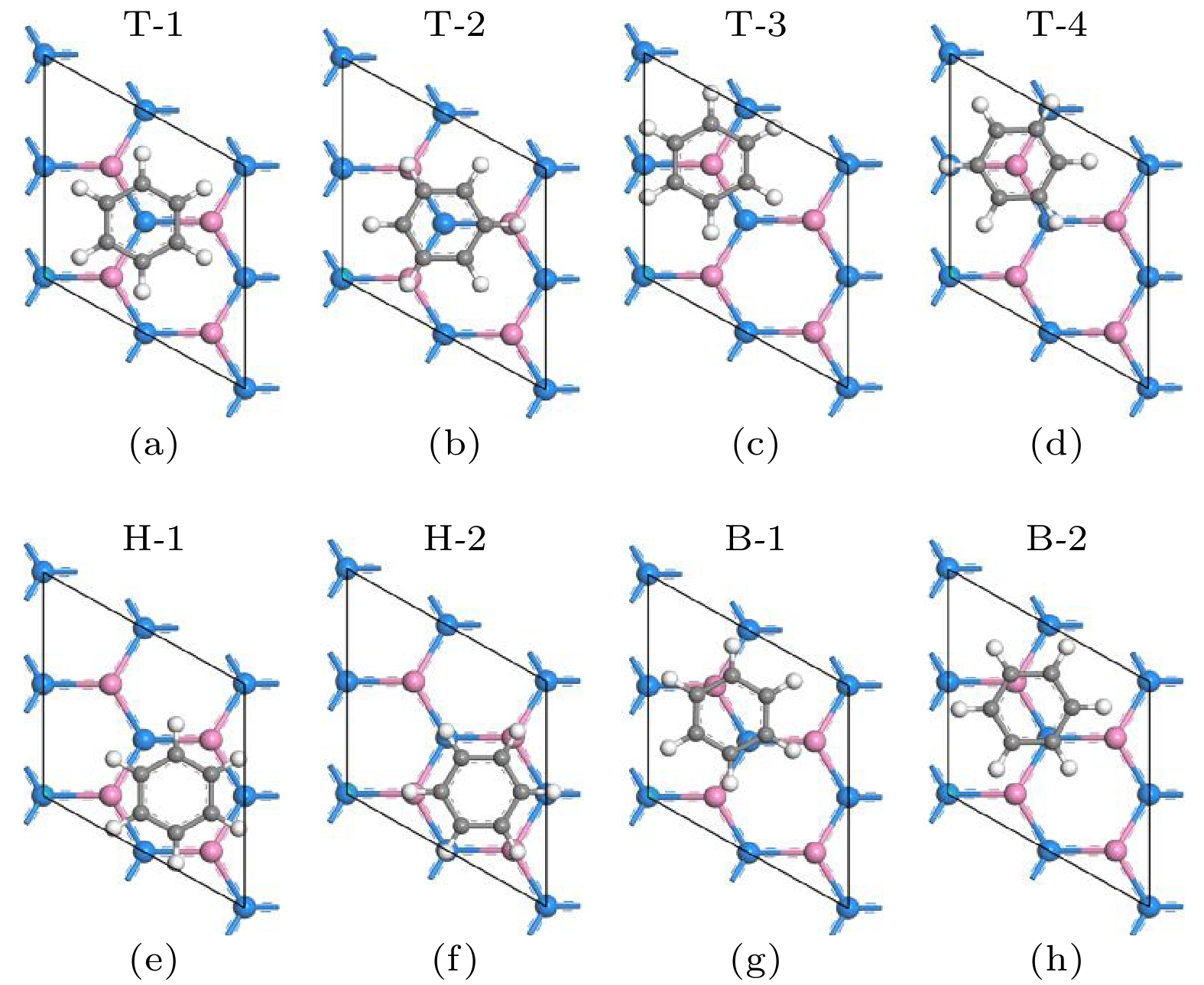
图 1 有机分子吸附在2 × 2锗烯超晶胞表面的八种吸附构型示意图(粉色球和蓝色球分别表示锗烯中上层锗原子和下层锗原子, 灰色球表示C原子, 而白色球表示H原子或F原子)
Figure 1. Schematic view of the eight adsorption configurations of the organic molecules adsorbed on the 2 × 2 supercell of germanene, where the pink balls represent the upper Ge atoms of the germanene, the blue balls represent the lower Ge atoms of the germanene, the gray balls represent the C atom, and the white balls represent the H or F atoms.
图 2 (a) T-1构型的苯/锗烯体系的俯视图和主视图. (b) T-4构型的六氟苯/锗烯体系的俯视图和主视图. 粉色球和蓝色球分别表示锗烯上层和下层Ge原子, 灰色球表示C原子, 白色球和黄色球分别表示H原子和F原子; Eex表示垂直于锗烯的外电场强度, 从锗原子指向有机分子方向的电场为正电场, 反之为负电场. (c) T-1构型的苯/锗烯体系的能带结构图和部分态密度(PDOS)图. (d) T-4构型的六氟苯/锗烯体系的能带结构图和部分态密度图
Figure 2. (a) Top and side views of the benzene/germanene systems with T-1 configuration. (b) The top and side views of the hexafluorobenzene/germanene systems with T-4 configuration. The pink and blue balls represent the upper and lower Ge atoms of the germanene, respectively. The gray balls represent the C atom. The white and yellow balls represent the H and F atoms, respectively. External electric field Eex perpendicular to the germanene is applied along the upward direction (defined as positive “+”, i.e., the electric field direction is from the germanene to the organic molecules at positive electric field) or the downward direction (defined as negative “–”). (c) Band structure and partial density of states (PDOS) of benzene/germanene with T-1 configuration. (d) Band structure and PDOS of hexafluobenzene/germanene with T-4 configuration.
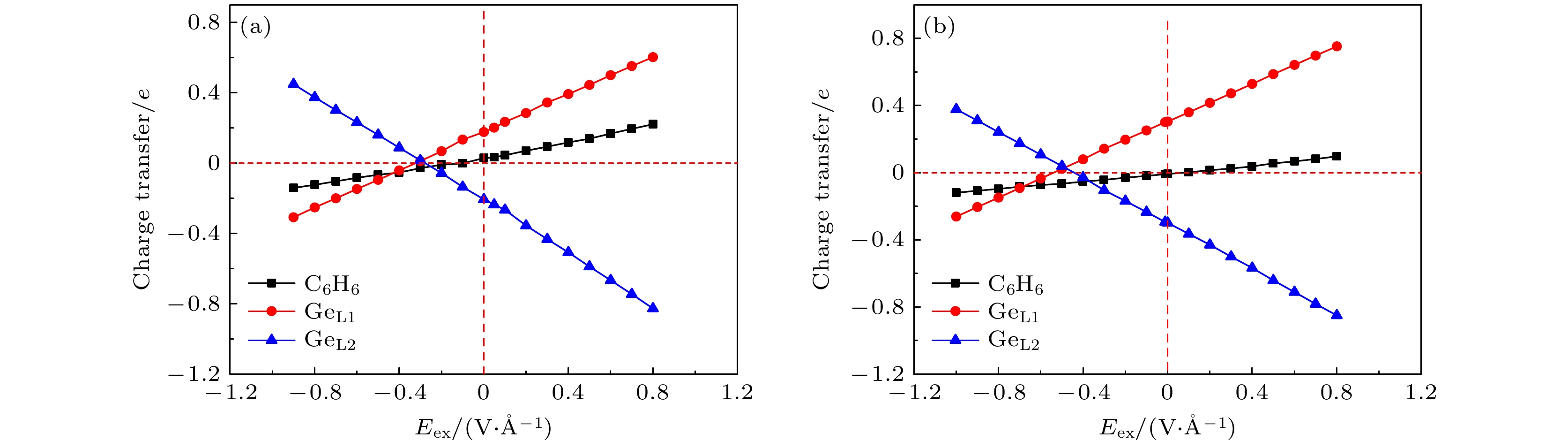
图 3 (a) T-1构型的苯/锗烯体系和(b) T-4构型的六氟苯/锗烯体系的带隙Eg随外电场强度Eex的变化图, 插图a—d分别显示了两种体系在负电场和正电场作用下具有最大带隙时的能带结构, e和f分别显示了两种体系在临界外电场下具有零带隙时的能带结构图
Figure 3. Band gaps Eg of (a) benzene/germanene system with T-1 configuration and (b) hexafluorobenzene/germanene system with T-4 configuration as a function of the strength of the external electric field. The inserts a–d show the band structures of both systems with the maximum band gap under negative and positive electric field, while e and f show the band structure of both systems with the zero-gap under the critical external electric field.
图 6 (a)苯/锗烯/HGeH体系的俯视图和主视图. (b)六氟苯/锗烯/HGeH体系的俯视图和主视图, 其中粉色球和蓝色球表示锗烯上层和下层Ge原子, 灰色球表示C原子, 白色球和黄色球表示有机分子中H原子和F原子, 橙色球和黑色球表示HGeH上层H原子和下层H原子, 绿色球和红色球表示HGeH上层和下层Ge原子; Eex表示垂直于锗烯的外电场, 从HGeH指向有机分子方向的电场为正电场, 反之为负电场. (c)苯/锗烯/HGeH体系的能带结构图和部分态密度(PDOS)图. (d)六氟苯/锗烯/HGeH体系的能带结构图和部分态密度图
Figure 6. (a) Top and side views of the benzene/germanene/HGeH system. (b) Top and side views of the hexafluorobenzene/germanene/HGeH systems. The pink and blue balls represent the upper and lower Ge atoms of the germanene, respectively. The gray balls represent the C atom. The white and yellow balls represent the H or F atoms of organic molecules. The orange and black balls represent the upper and lower H atoms of HGeH. The green and red balls represent the upper and lower Ge atoms of HGeH, respectively. External electric field Eex perpendicular to the germanene is applied along the upward direction (defined as positive “+”, i.e., the electric field direction is from the HGeH to the organic molecules at positive electric field) or the downward direction (defined as negative “–”). (c) Band structure and PDOS of benzene/germanene/HGeH. (d) Band structure and PDOS of hexafluobenzene/germanene/HGeH.
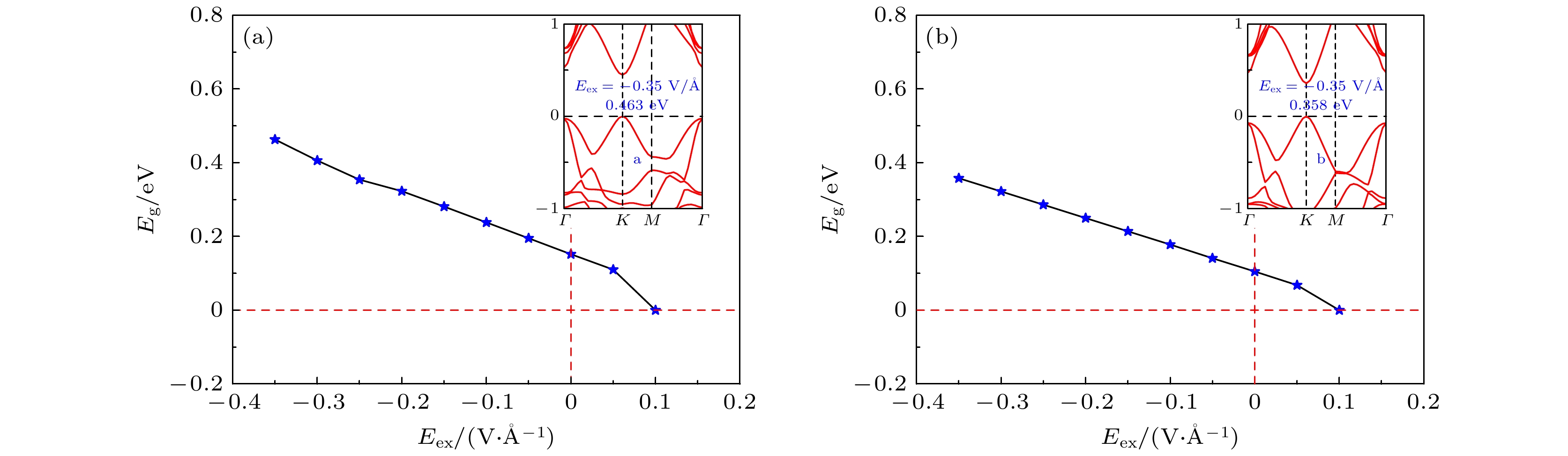
图 7 (a)苯/锗烯/HGeH体系和(b)六氟苯/锗烯/HGeH体系的带隙Eg随外电场强度Eex的变化关系图, 其中插图显示了两个体系具有最大带隙时的能带结构
Figure 7. Band gaps Eg of (a) benzene/germanene/HGeH system and (b) hexafluorobenzene/germanene/HGeH system as a function of the strength of the external electric field Eex. The inserts show the band structures of both systems with the maximum band gaps.
表 1 苯/锗烯体系和六氟苯/锗烯体系的八种高对称吸附构型的吸附能Ead、吸附距离H、翘曲高度d和带隙Eg
Table 1. Adsorption energy Ead, adsorption distance H, buckling height d and band gap Eg of eight highly symmetric adsorption configurations of benzene/germanene and hexafluobenzene/germanene systems.
八种构型 T-1 T-2 T-3 T-4 H-1 H-2 B-1 B-2 苯/锗烯 Ead/eV 0.676 0.662 0.605 0.617 0.525 0.522 0.640 0.638 H/Å 3.060 3.063 3.146 3.090 3.352 3.445 2.977 3.060 d/Å 0.804 0.795 0.741 0.739 0.720 0.720 0.793 0.783 Eg/eV 0.036 0.035 0.041 0.039 0.009 0.010 0.044 0.044 六氟苯/锗烯 Ead/eV 0.593 0.588 0.589 0.656 0.521 0.569 0.647 0.631 H/Å 3.005 3.160 3.020 2.970 3.114 3.141 2.982 3.054 d/Å 0.780 0.786 0.732 0.763 0.765 0.781 0.762 0.776 Eg/eV 0.014 0.022 0.039 0.005 0.006 0.018 0.016 0.035 -
[1] 黄立, 杜世萱, 高鸿钧 2018 物理 47 173
 Google Scholar
Google Scholar
Huang L, Du S X, Gao H J 2018 Physics 47 173
 Google Scholar
Google Scholar
[2] Li L F, Lu S Z, Pan J B, Qin Z H, Wang Y Q, Wang Y L, Cao G Y, Du S X, Gao H J 2014 Adv. Mater. 26 4820
 Google Scholar
Google Scholar
[3] Dávila M E, Xian L, Cahangirov S, Rubio A, Lay Le G 2014 New J. Phys. 16 095002
 Google Scholar
Google Scholar
[4] Derivaz M, Dentel D, Stephan R, Hanf M C, Mehdaoui A, Sonnet P, Pirri C 2015 Nano Lett. 15 2510
 Google Scholar
Google Scholar
[5] Qin Z H, Pan J B, Lu S Z, Shao Y, Wang Y L, Du S X, Gao H J, Cao G Y 2017 Adv. Mater. 29 1606046
 Google Scholar
Google Scholar
[6] 武红, 李峰 2016 65 096801
 Google Scholar
Google Scholar
Wu H, Li F 2016 Acta Phys. Sin. 65 096801
 Google Scholar
Google Scholar
[7] Liu C C, Feng W X, Yao Y G 2011 Phys. Rev. Lett. 107 076802
 Google Scholar
Google Scholar
[8] Wang T H, Zhu Y F, Jiang Q 2013 J. Phys. Chem. C 117 12873
 Google Scholar
Google Scholar
[9] Zhu Y F, Dai Q Q, Zhao M, Jiang Q 2013 Sci. Rep. 3 1524
 Google Scholar
Google Scholar
[10] Garcia J C, de Lima D B, Assali L V C, Justo J F 2011 J. Phys. Chem. C 115 13242
 Google Scholar
Google Scholar
[11] Gao N, Zheng W T, Jiang Q 2012 Phys. Chem. Chem. Phys. 14 257
 Google Scholar
Google Scholar
[12] Wang R, Xu M S, Pi X D 2015 Chin. Phys. B 24 086807
 Google Scholar
Google Scholar
[13] Gao J F, Zhang J F, Liu H S, Zhang Q F, Zhao J J 2013 Nanoscale 5 9785
 Google Scholar
Google Scholar
[14] Li S, Wu Y F, Tu Y, Wang Y, Jiang T, Liu W, Zhao Y 2015 Sci. Rep. 5 7881
 Google Scholar
Google Scholar
[15] Liu H S, Feng H F, Du Y, Chen J, Wu K H, Zhao J J 2016 2D Mater. 3 025034
 Google Scholar
Google Scholar
[16] Hu T, Gerber I C 2013 J. Phys. Chem. C 117 2411
 Google Scholar
Google Scholar
[17] Wang Y P, Ji W X, Zhang C W, Li S S, Li F, Li P, Ren M J, Chen X L, Yuan M, Wang P J 2016 Mater. Chem. Phys. 173 379
 Google Scholar
Google Scholar
[18] Chen Q, Hu H, Chen X J, Wang J L 2011 Appl. Phys. Lett. 98 053102
 Google Scholar
Google Scholar
[19] Zhang Y B, Tang T T, Girit C, Hao Z, Martin M C, Zettl A, Crommie M F, Shen Y R, Wang F 2009 Nature 459 820
 Google Scholar
Google Scholar
[20] Ni Z, Liu Q H, Tang K C, Zheng J X, Zhou J, Qin R, Gao Z X, Yu P, Lu J 2012 Nano Lett. 12 113
 Google Scholar
Google Scholar
[21] Liu G, Luo W W, Wang X, Lei X L, Xu B, Ouyang C Y, Liu S B 2018 J. Mater. Chem. C 6 5937
 Google Scholar
Google Scholar
[22] Zhang D, Lou W K, Miao M S, Zhang S C, Chang K 2013 Phys. Rev. Lett. 111 156402
 Google Scholar
Google Scholar
[23] 张弦, 郭志新, 曹觉先, 肖思国, 丁建文 2015 64 186101
 Google Scholar
Google Scholar
Zhang X, Guo Z X, Cao J X, Xiao S G, Ding J W 2015 Acta Phys. Sin. 64 186101
 Google Scholar
Google Scholar
[24] Li X D, Wu S Q, Zhou S, Zhu Z Z 2014 Nanoscale Res. Lett. 9 110
 Google Scholar
Google Scholar
[25] Ye H, Hu F F, Tang H Y, Yang L W, Chen X P, Wang L G, Zhang G Q 2018 Phys. Chem. Chem. Phys. 20 16067
 Google Scholar
Google Scholar
[26] Fan Y C, Liu X B, Wang J R, Ai H Q, Zhao M W 2018 Phys. Chem. Chem. Phys. 20 11369
 Google Scholar
Google Scholar
[27] Gao N, Lu G Y, Wen Z, Jiang Q 2017 J. Mater. Chem. C 5 627
 Google Scholar
Google Scholar
[28] Zhou S, Zhao J 2016 J. Phys. Chem. C 120 21691
 Google Scholar
Google Scholar
[29] Delley B 1990 J. Chem. Phys. 92 508
 Google Scholar
Google Scholar
[30] Delley B 2000 J. Chem. Phys. 113 7756
 Google Scholar
Google Scholar
[31] Perdew J P, Burke K, Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[32] Koelling D D, Hartreermon B N 1977 J. Phys. C 10 3107
 Google Scholar
Google Scholar
[33] Monkhorst H J, Pack J D 1976 Phys. Rev. B 13 5188
 Google Scholar
Google Scholar
[34] Grimme S 2006 J. Comput. Chem. 27 1787
 Google Scholar
Google Scholar
[35] Li S, Wu Y F, Liu W, Zhao Y H 2014 Chem. Phys. Lett. 609 161
 Google Scholar
Google Scholar
[36] Xie X Q, Ao Z M, Su D W, Zhang J Q, Wang G X 2015 Adv. Funct. Mater. 25 1393
 Google Scholar
Google Scholar
[37] 陈庆玲, 戴振宏, 刘兆庆, 安玉凤, 刘悦林 2016 65 136101
 Google Scholar
Google Scholar
Chen Q L, Dai Z H, Liu Z Q, An Y F, Liu Y L 2016 Acta Phys. Sin. 65 136101
 Google Scholar
Google Scholar
[38] Shen T, Ren J C, Liu X, Li S, Liu W 2019 J. Am. Chem. Soc. 141 3110
 Google Scholar
Google Scholar
[39] He C, Han F S, Zhang J H, Zhang W X 2020 J. Mater. Chem. C 8 6923
 Google Scholar
Google Scholar
[40] Rubio-Pereda P, Takeuchi N 2015 J. Phys. Chem. C 119 27995
 Google Scholar
Google Scholar
[41] Su G R, Yang S, Jiang Y D, Li J T, Ren J C, Liu W 2019 Prog. Surf. Sci. 4 100561
 Google Scholar
Google Scholar
[42] Gao W, Chen Y, Li B, Liu S P, Liu X, Jiang Q 2020 Nat. Commun. 11 1196
 Google Scholar
Google Scholar
[43] Ye J P, Liu G, Han Y, Luo W W, Sun B Z, Lei X L, Xu B, Ouyang C Y, Zhang H L 2019 Phys. Chem. Chem. Phys. 21 20287
 Google Scholar
Google Scholar
[44] Ye X S, Shao Z G, Zhao H B, Yang L, Wang C L 2014 RSC Adv. 4 21216
 Google Scholar
Google Scholar
[45] Quhe R, Fei R, Liu Q H, Zheng J X, Li H, Xu C Y, Ni Z Y, Wang Y Y, Yu D P, Gao Z X, Lu J 2012 Sci. Rep. 2 853
 Google Scholar
Google Scholar
[46] Yan J A, Gao S P, Stein R, Coard G 2015 Phys. Rev. B 91 245403
 Google Scholar
Google Scholar
[47] 陈艳珊, 刘兴斌 2013 低温与特气 31 33
 Google Scholar
Google Scholar
Chen Y S, Liu X B 2013 Low Temp. Spec. Gases 31 33
 Google Scholar
Google Scholar
[48] Amamou W, Odenthal P M, Bushong E J, O’hara D J, Luo Y K, Baren J V, Pinchuck I, Wu Y, Ahmed A S, Katoch J, Bockrath M W 2015 2D Mater. 2 035012
 Google Scholar
Google Scholar
[49] Jiang S S, Bianco E, Goldberger J E 2014 J. Mater. Chem. C 2 3185
 Google Scholar
Google Scholar
[50] Bianco E, Butler S, Jiang S S, Restrepo O D, Windl W F, Goldberger J E 2013 ACS Nano 7 4414
 Google Scholar
Google Scholar
Catalog
Metrics
- Abstract views: 7601
- PDF Downloads: 92
- Cited By: 0














 DownLoad:
DownLoad: