-
Dynamics of exciton-exciton annihilation (EEA) in molecular aggregates is closely related to its luminescence characteristics and energy transfer. It is meaningful to uncover energy and charge transfer process in molecular systems. Therefore, studying the dynamics of exciton is important for simulating photosynthesis in nature and analyzing the transport process of photocarriers. In this paper the weak coupling approximation is adopted to obtain the rate equation in the framework of density matrix theory. The relation among the intermolecular distance, exciton state density, excited state dipole moment and exciton-exciton annihilation dynamics is studied by the rate equations. It is found that the decrease of intermolecular distance leads the generation rate of higher-order excited states to increase, resulting in the obvious S-shaped decay characteristics. Moreover, the dipole moment of the higher-order excited state is the key factor of the exciton fusion process, and the greater the exciton density, the more easily the exciton fusion process occurs. Therefore, the reduction of intermolecular distance and the increase of the dipole moment of the higher-order excited state make the nearest neighbor molecules have a strong coupling, resulting in a high generation rate of the higher-order excited state. It is found that the evolution processes of the first excited state in different exciton densities are consistent with the experimental results of the excitation of OPPV7 monomer (PPV oligomers of 7) at a low excitation energy, and the excitation of OPPV7 aggregates at different excitation energy levels. It can be observed that the exciton decay rate is faster under the excitation of the strong external field. Using the quantum wave packet under optical excitation as the initial state, the excited state dynamics is simulated at different exciton energy levels. It is found that the exciton state can maintain good locality within a few hundreds of femtoseconds, which shows that the exciton state is a coherent superposition state, and its local characteristics are related to the excitation energy level. These conclusions are applicable to the aggregations whose single molecule has an energy level of
${E_{mf}} \approx 2{E_{me}}$ , and also provide a reasonable reference for the exciton-exciton annihilation process under optical excitation.-
Keywords:
- exciton-exciton annihilation /
- quantum wave packet /
- molecular aggregates /
- energy transfer
[1] Cook S, Liyuan H, Furube A, Katoh R 2010 J. Phys. Chem. C 114 10962
 Google Scholar
Google Scholar
[2] Peckus D, Devizis A, Hertel D, Meerholz K, Gulbinas V 2012 Chem. Phys. 404 42
 Google Scholar
Google Scholar
[3] Ginas S, Kirkpatrick J, Howard I A, Johnson K, Wilson M W B, Friend R H, Silva C 2013 J. Phys. Chem. B 117 4649
 Google Scholar
Google Scholar
[4] Dai D C, Monkman A P 2013 Phys. Rev. B 87 045308
 Google Scholar
Google Scholar
[5] Völker S F, Schmiedel A, Holzapfel M, Renziehausen K, Engel V, Lambert C 2014 J. Phys. Chem. C 111 17467
[6] Fennel F, Lochbrunner S 2015 Phys. Rev. B 92 140301
 Google Scholar
Google Scholar
[7] Engel E, Leo K, Hoffmann M 2006 Chem. Phys. 325 170
 Google Scholar
Google Scholar
[8] Linardy E, Yadav D, Vella D, Verzhbitskiy I A, Watanabe K, Taniguchi T, Pauly F, Trushin M 2020 Nano Lett. 20 1647
 Google Scholar
Google Scholar
[9] Kühn O, Renger T, May V, Voigt J, Pullerits T, Sundström V 1997 Trends in Photochem. Photobiol. 4 213
[10] Hader K, May V, Lambert, Engel V 2016 Phys. Chem. Chem. Phys. 18 13368
 Google Scholar
Google Scholar
[11] Peteanu L A, Chowdhury S, Wildeman J, Sfeir M Y 2017 J. Phys. Chem. B 121 1707
 Google Scholar
Google Scholar
[12] Feist J, Garcia-Vidal F J 2015 Phys. Rev. Lett. 114 196402
 Google Scholar
Google Scholar
[13] Schachenmayer J, Genes C, Tignone E, Pupillo G 2015 Phys. Rev. Lett. 114 196403
 Google Scholar
Google Scholar
[14] Xu L, Ji Y, Shi X, Wang L, Gao K 2020 Org. Electron. 85 105886
 Google Scholar
Google Scholar
[15] Spano F C 1992 Phys. Rev. B 46 13017
 Google Scholar
Google Scholar
[16] Juzeliunas G, Knoester J 2000 J. Chem. Phys. 112 2325
 Google Scholar
Google Scholar
[17] Mukamel S, Berman O 2003 J. Chem. Phys. 119 12194
 Google Scholar
Google Scholar
[18] Mukamel S, Abramavicius D 2004 Chem. Rev. 104 2073
 Google Scholar
Google Scholar
[19] Wang L, Plehn T, May V 2020 Phys. Rev. B 102 075401
 Google Scholar
Google Scholar
[20] Wang L, May V 2016 Phys. Rev. B 94 195413
 Google Scholar
Google Scholar
[21] Tempelaar R, Jansen T L C, Knoester J 2017 J. Phys. Chem. Lett. 8 6113
 Google Scholar
Google Scholar
[22] May V 2014 J. Chem. Phys. 140 054103
 Google Scholar
Google Scholar
[23] 茅江奇, 范旭阳, 路彦珍, 王鹿霞 2021 70 047302
Mao J Q, Fan X Y, Lu Y Z, Wang L X 2021 Acta Phys. Sin. 70 047302
-
图 2 不同分子间距离
${\varDelta _{m, m \pm 1}}$ 下的J型分子聚集体的平均第一激发态和高阶激发态占据数动力学过程(${m_{m\left( n \right)}}= $ $ 0.8~\rm D$ ).插图: 前100 fs的J型分子聚集体的平均激发态占据数和时间的线性关系图Figure 2. Population of the average first excited state and higher excited state of the J-type molecular aggregates with the distance between molecules
${\varDelta _{m, m \pm 1}}$ (${m_{m\left( n \right)}}= $ $ 0.8~\rm D$ ). Inset: Linear graph of the population of the average first excited state and higher excited state by the J-type molecular aggregates in the first 100 fs versus time.图 3 不同高阶激发态偶极矩
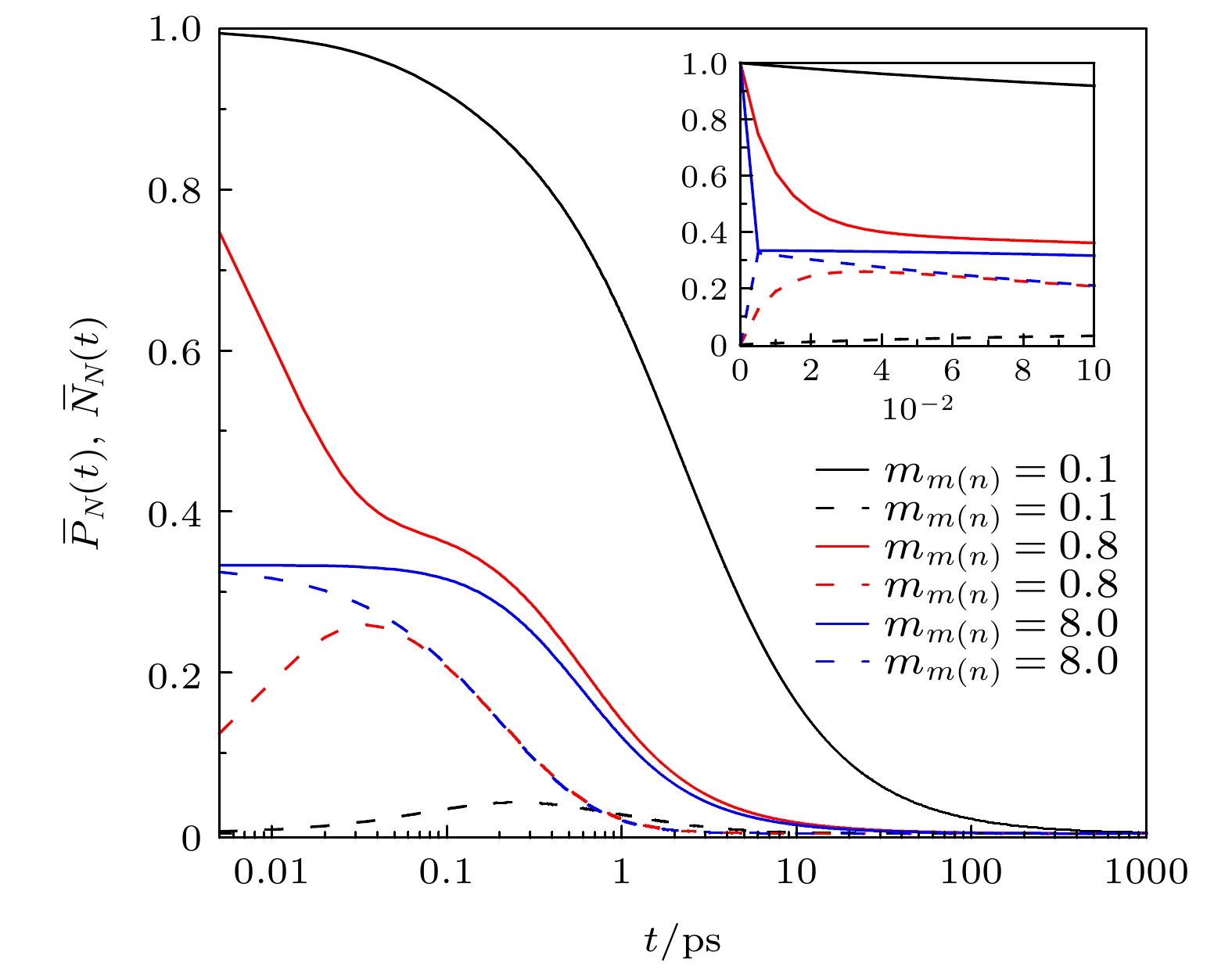
${m_{m\left( n \right)}}$ 下的J型分子聚集体的平均第一激发态和高阶激发态占据数动力学过程; 插图: 前100 fs的J型分子聚集体平均激发态占据数和时间的线性关系图Figure 3. Population of the average first excited state and higher excited state of the J-type molecular aggregateswith different dipole moment of higher excited state
${m_{m\left( n \right)}}$ .Inset: Linear graph of the population of the average first excited state and higher excited state of the J-type molecular aggregates in the first 100 fs versus time.图 4 不同激子数下J型分子聚集体的总第一激发态和总高阶激发态占据数动力学过程(
${m_{m\left( n \right)}}=0.8~\rm D$ ); 插图: 前100 fs的J型分子聚集体总第一激发态占据数和总高阶激发态占据数和时间的线性关系图Figure 4. Thepopulation of the total first excited state and higher excited state of the J-type molecular aggregates with different numbers of excitons(
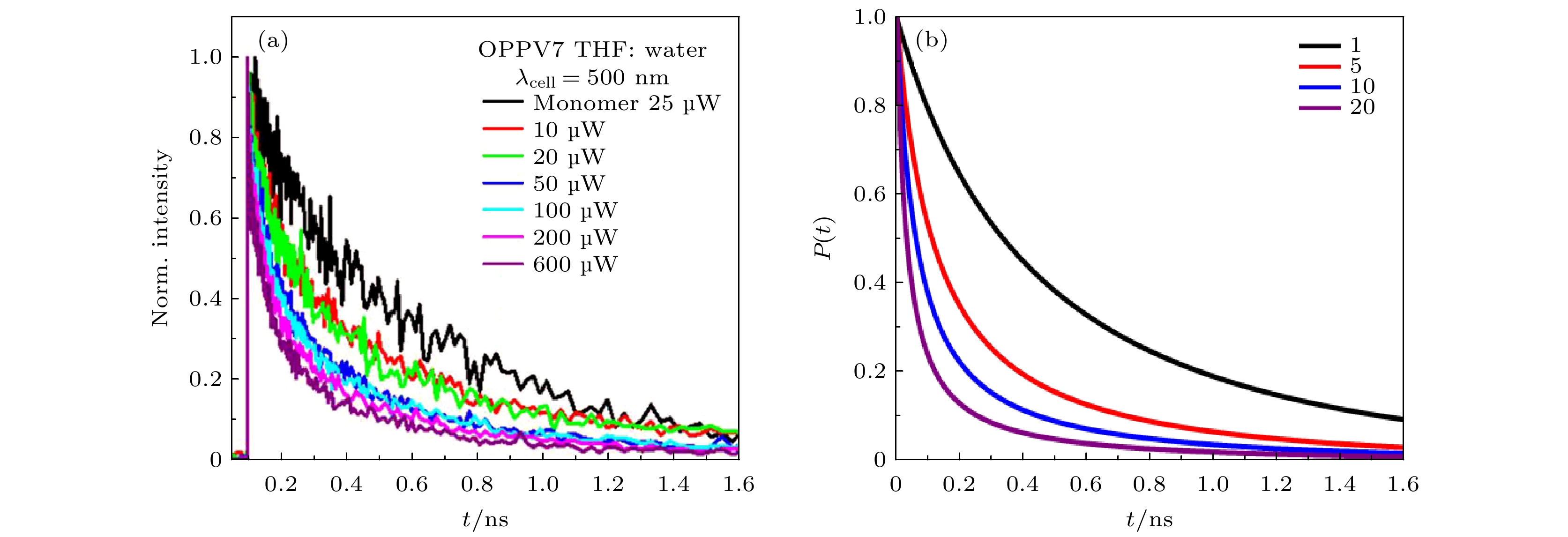
${m_{m\left( n \right)}}=0.8~\rm D$ ). Inset: Linear graph of the population of the total first excited state and higher excited state of the J-type molecular aggregates in the first 100 fs versus time.图 5 (a)OPPV7单体和OPPV7(THF: water)聚集体的发射衰减速率[11]; (b)不同激子数下J型分子聚集体激发时的第一激发态湮灭过程(
${m_{m\left( n \right)}}=0.1~\rm D$ ,${r^{}}{\text{ = 200 p}}{{\text{s}}^{{{ - 1}}}}$ )Figure 5. (a) Emission decays of OPPV7 monomer and OPPV7 (THF: water) Aggregates [11]; (b) annihilation process of the first excited state when the J-type molecular aggregates are excited in different excitons(
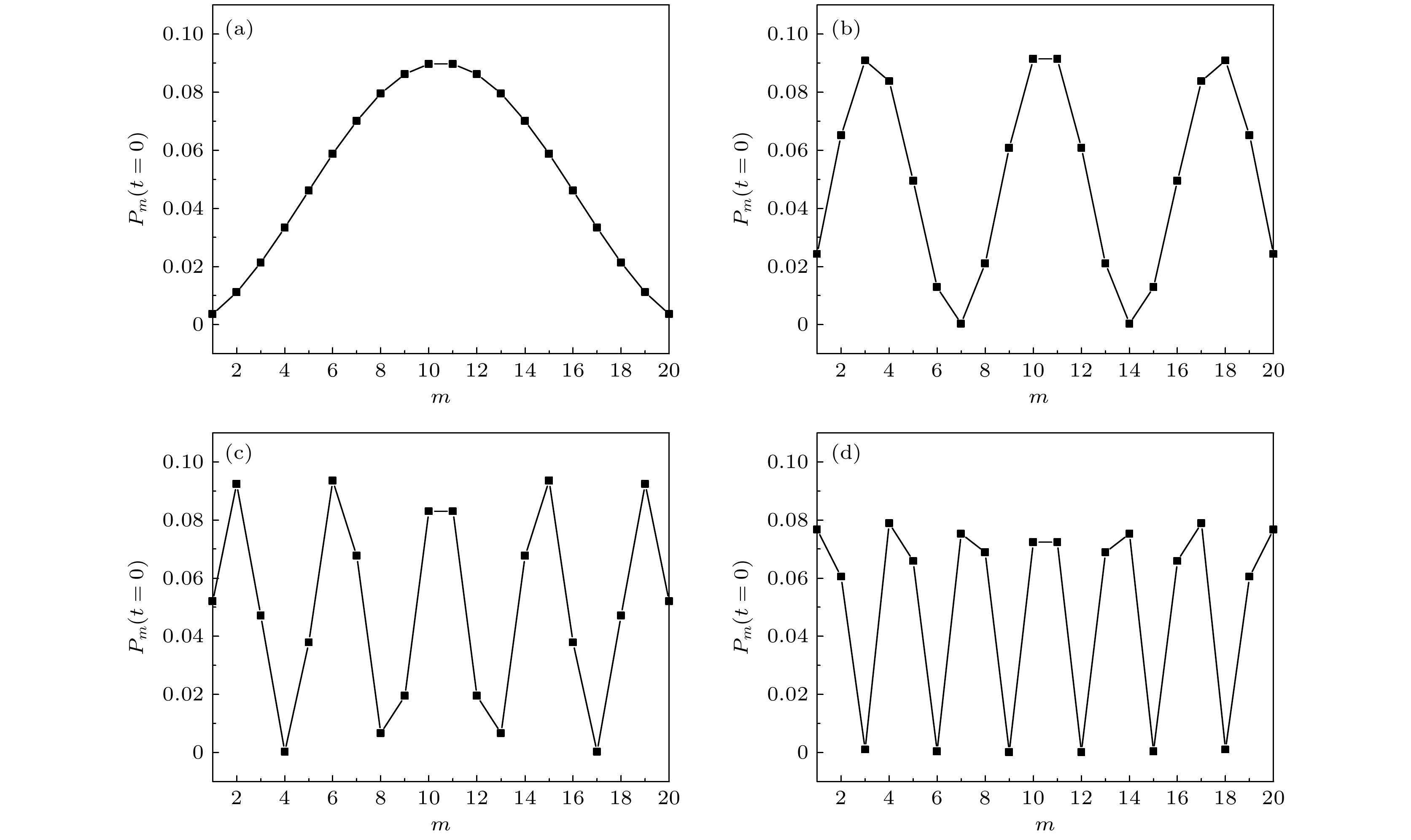
${m_{m\left( n \right)}}=0.1~\rm D$ ,${r^{}}{{ = 200~ {\rm{p}}}}{{\text{s}}^{{{ - 1}}}}$ ).图 6 J型分子聚集体哈密顿量对角化的前四个明能级对应的波包分布概率
${\left| {{C_m}} \right|^2}$ 对应率方程的4种初始激发位形 (a)第一能级; (b)第三能级; (c)第五能级; (d)第七能级Figure 6. Four initial excitation configurations of the wave packet distribution corresponding (
${\left| {{C_m}} \right|^2}$ ) to the first four bright energy levels corresponding to the diagonalization of the Hamiltonian of the J-type molecular aggregates: (a) The first energy level; (b) the third energy level; (c) the fifth energy level; (d) the seventh energy level.图 7 J型分子聚集体(10个激子)激发时的第一激发态占据数演变(
${m_{m\left( n \right)}}=0.8~\rm D$ ,${\varDelta _{m, m \pm 1}}$ = 1.2 nm) (a)第一能级; (b)第三能级; (c)第五能级; (d)第七能级Figure 7. Population evolution of the first excited state when J-type molecular aggregates are excited (
${m_{m\left( n \right)}}=0.8~\rm D$ ,${\varDelta _{m, m \pm 1}}$ = 1.2 nm): (a) The first energy level; (b) the third energy level; (c) the fifth energy level; (d) the seventh energy level.图 8 J型分子聚集体(10个激子)激发时的高阶激发态占据数演变(
${m_{m\left( n \right)}}=0.8~\rm D$ ,${\varDelta _{m, m \pm 1}}$ = 1.2 nm) (a)第一能级; (b)第三能级; (c)第五能级; (d)第七能级Figure 8. Population evolution of the higher excited state when J-type molecular aggregates are excited(
${m_{m\left( n \right)}}=0.8~\rm D$ ,${\varDelta _{m, m \pm 1}}$ = 1.2 nm): (a) The first energy level; (b) the third energy level; (c) the fifth energy level; (d) the seventh energy level. -
[1] Cook S, Liyuan H, Furube A, Katoh R 2010 J. Phys. Chem. C 114 10962
 Google Scholar
Google Scholar
[2] Peckus D, Devizis A, Hertel D, Meerholz K, Gulbinas V 2012 Chem. Phys. 404 42
 Google Scholar
Google Scholar
[3] Ginas S, Kirkpatrick J, Howard I A, Johnson K, Wilson M W B, Friend R H, Silva C 2013 J. Phys. Chem. B 117 4649
 Google Scholar
Google Scholar
[4] Dai D C, Monkman A P 2013 Phys. Rev. B 87 045308
 Google Scholar
Google Scholar
[5] Völker S F, Schmiedel A, Holzapfel M, Renziehausen K, Engel V, Lambert C 2014 J. Phys. Chem. C 111 17467
[6] Fennel F, Lochbrunner S 2015 Phys. Rev. B 92 140301
 Google Scholar
Google Scholar
[7] Engel E, Leo K, Hoffmann M 2006 Chem. Phys. 325 170
 Google Scholar
Google Scholar
[8] Linardy E, Yadav D, Vella D, Verzhbitskiy I A, Watanabe K, Taniguchi T, Pauly F, Trushin M 2020 Nano Lett. 20 1647
 Google Scholar
Google Scholar
[9] Kühn O, Renger T, May V, Voigt J, Pullerits T, Sundström V 1997 Trends in Photochem. Photobiol. 4 213
[10] Hader K, May V, Lambert, Engel V 2016 Phys. Chem. Chem. Phys. 18 13368
 Google Scholar
Google Scholar
[11] Peteanu L A, Chowdhury S, Wildeman J, Sfeir M Y 2017 J. Phys. Chem. B 121 1707
 Google Scholar
Google Scholar
[12] Feist J, Garcia-Vidal F J 2015 Phys. Rev. Lett. 114 196402
 Google Scholar
Google Scholar
[13] Schachenmayer J, Genes C, Tignone E, Pupillo G 2015 Phys. Rev. Lett. 114 196403
 Google Scholar
Google Scholar
[14] Xu L, Ji Y, Shi X, Wang L, Gao K 2020 Org. Electron. 85 105886
 Google Scholar
Google Scholar
[15] Spano F C 1992 Phys. Rev. B 46 13017
 Google Scholar
Google Scholar
[16] Juzeliunas G, Knoester J 2000 J. Chem. Phys. 112 2325
 Google Scholar
Google Scholar
[17] Mukamel S, Berman O 2003 J. Chem. Phys. 119 12194
 Google Scholar
Google Scholar
[18] Mukamel S, Abramavicius D 2004 Chem. Rev. 104 2073
 Google Scholar
Google Scholar
[19] Wang L, Plehn T, May V 2020 Phys. Rev. B 102 075401
 Google Scholar
Google Scholar
[20] Wang L, May V 2016 Phys. Rev. B 94 195413
 Google Scholar
Google Scholar
[21] Tempelaar R, Jansen T L C, Knoester J 2017 J. Phys. Chem. Lett. 8 6113
 Google Scholar
Google Scholar
[22] May V 2014 J. Chem. Phys. 140 054103
 Google Scholar
Google Scholar
[23] 茅江奇, 范旭阳, 路彦珍, 王鹿霞 2021 70 047302
Mao J Q, Fan X Y, Lu Y Z, Wang L X 2021 Acta Phys. Sin. 70 047302
Catalog
Metrics
- Abstract views: 8154
- PDF Downloads: 141
- Cited By: 0
















 DownLoad:
DownLoad: