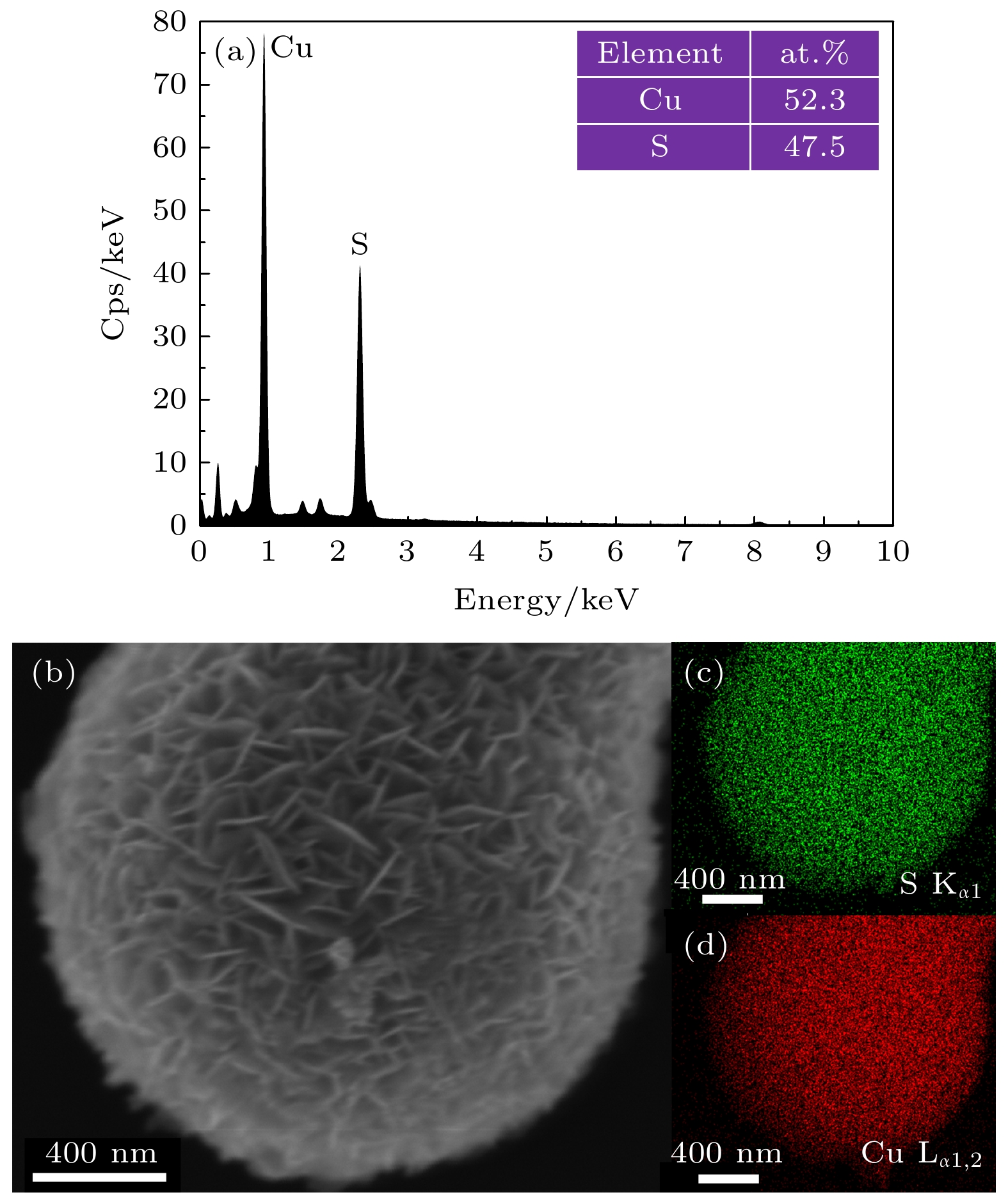
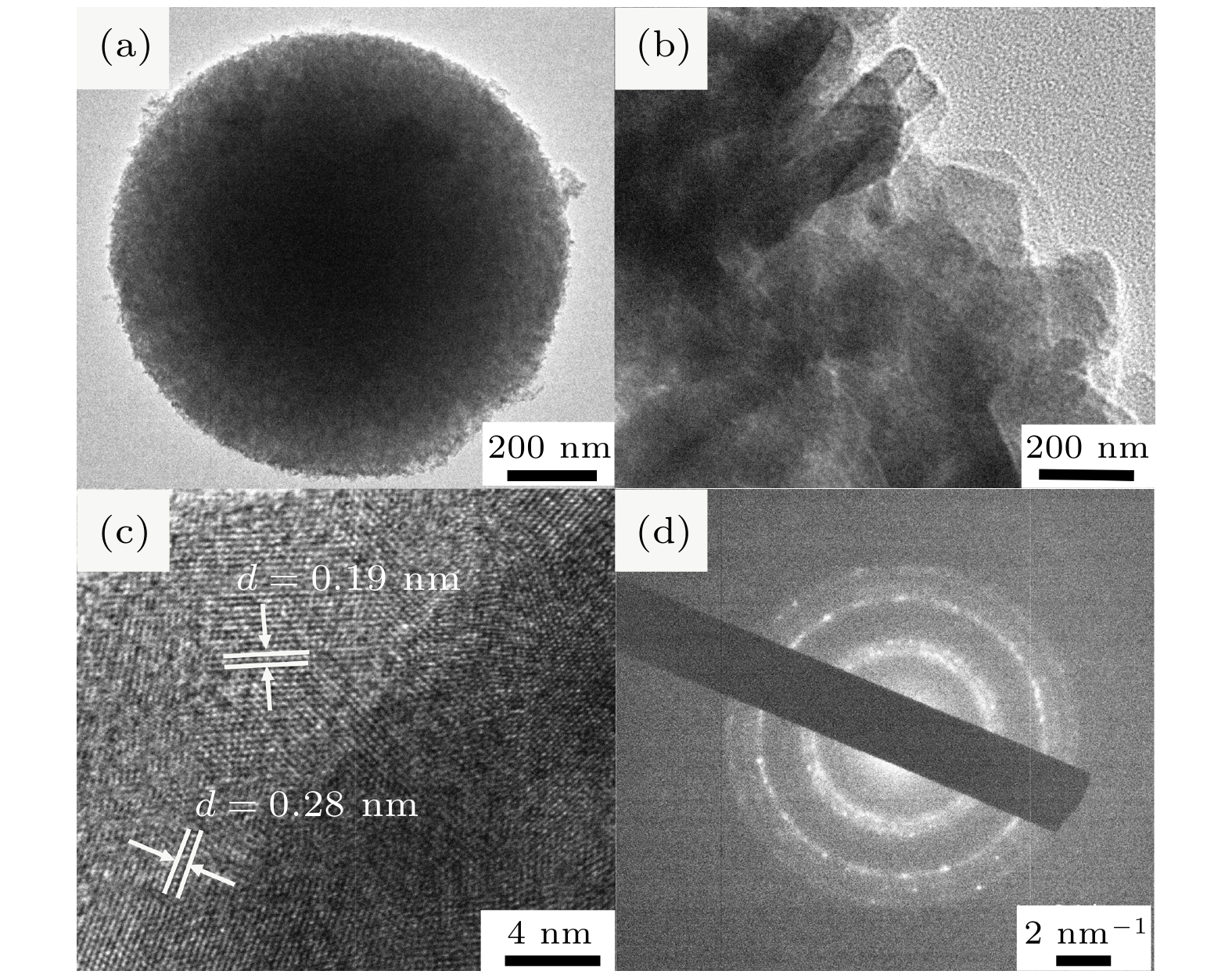
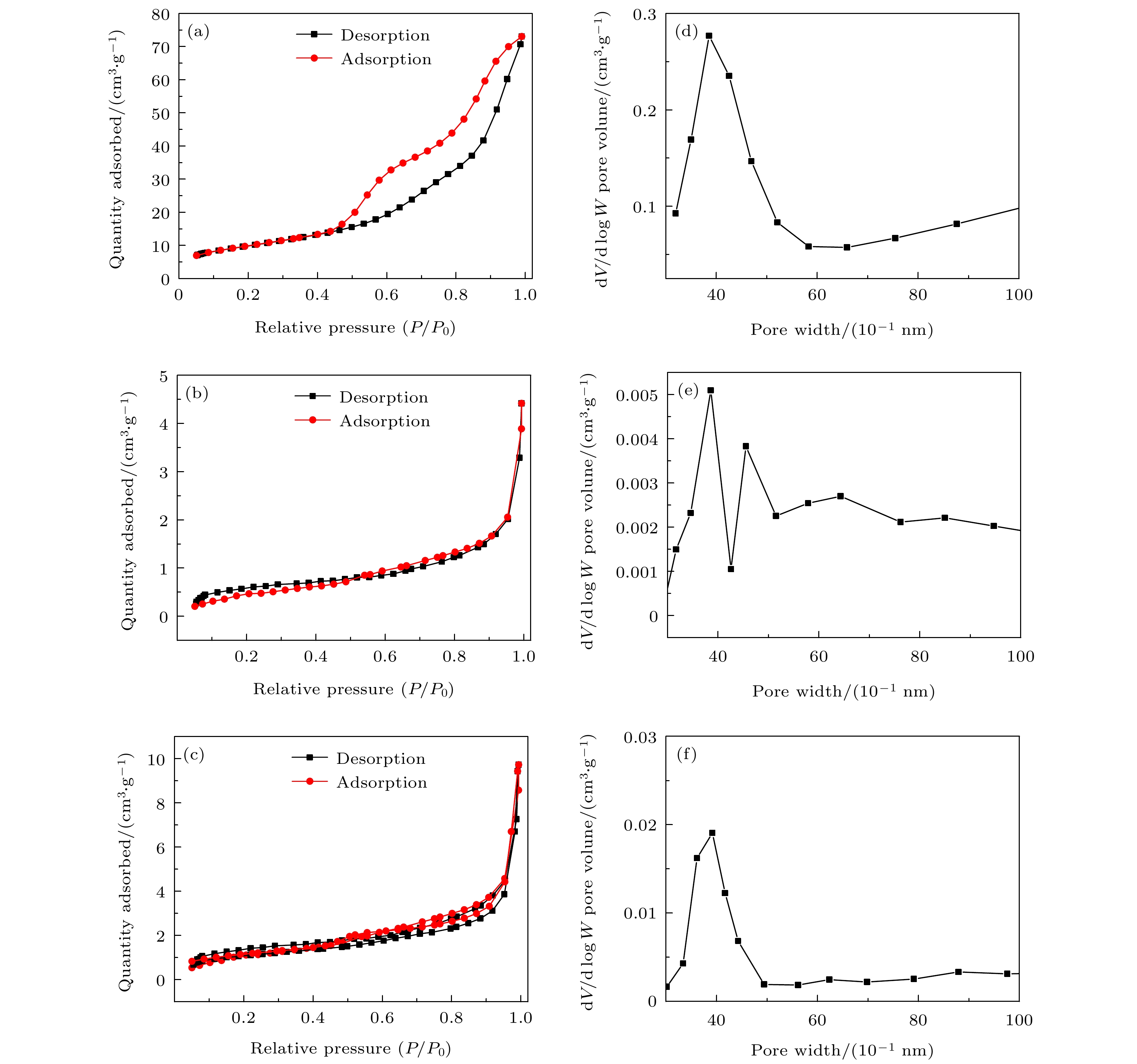
-
In recent decades, growing population and industrial development have led to releasing huge amounts of highly toxic chemical pollutants into the environment globally. Several approaches to handling the removal of contaminants from wastewater for environmental remediation, including biological, chemical, physical, and advanced oxidation processes have been employed. Among them, using nano-adsorbents as a tool for effectively removing organic contaminants represents a promising strategy in sewage purification field. More importantly, the nano-adsorbents with auto-deposition property can greatly improve the efficiency of sewage treatment. Therefore, the developing of environment friendly nano-adsorbents is thus an important issue to remove organic contaminants in water via simply adsorbing. Here in this work, porous flower-like copper sulfide (CuS) grade sub-nanomaterials are successfully fabricated by simply mixing two inorganic salts. Furthermore, the as-prepared nano-adsorbents with auto-deposition property can create a super adsorption capability for organic contaminants in wastewater. We further study the adsorption/auto-deposition characteristics of porous flower-like CuS grade sub-nanomaterials systematically by using various organic dyes (methyl blue, crystal violet, lemon yellow, sunset yellow and amaranth) as target molecules. For instance, in a typical procedure, 0.8-mg methyl blue can be removed 100% via adding 10-mg porous flower-like CuS grade sub-nanomaterials sample in 30 min. Therefore, the adsorption efficiency can be enhanced by 55% and 26% in comparison with the adsorption efficiency of CuS micro pompons and micron particles, respectively. Additionally, the porous flower-like CuS grade sub-nanomaterials can self-deposite on the bottom of the solution within 3 h after adsorption has finished, and the deposition efficiency can be improved by 95% and 3.17 times in comparison with the deposition efficiency of CuS micro pompons and micron particles, respectively. Comparing with micron particles, the unique self-depositing characteristics of porous flower-like grade sub-nanomaterials are attributed to larger specific surface area, greater porosity and stronger electrostatic adsorption capacity. Remarkably, this work provides an effective method of effectively removing various organic dyes from wastewater.
-
Keywords:
- self-deposition adsorption /
- porous sub-nanoflower /
- copper sulfide /
- self-assembly
[1] Srivastava S, Sinha R, Roy D 2004 Aquat. Toxicol. 66 319
 Google Scholar
Google Scholar
[2] Wu Y, Su M, Chen J, Xu Z, Tang J, Chang X, Chen D 2019 Dyes Pigm. 170 107591
 Google Scholar
Google Scholar
[3] Zhang H, Chen M, Wang D M, Xu L L, Liu X D 2016 Opt. Mater. Express 6 2573
 Google Scholar
Google Scholar
[4] Miao X, Tang Y, Wong C W 2015 Nature 518 483
[5] Ghorai S, Sarkar A, Raoufi M, Panda A B, Schönherr H, Pal S 2014 ACS Appl. Mater. Interfaces 6 4766
 Google Scholar
Google Scholar
[6] Wu Z, Joo H, Lee K 2005 Chem. Eng. J. 112 227
 Google Scholar
Google Scholar
[7] Xie Y J, Yan B, Xu H L, Chen J, Liu Q X, Deng Y H, Zeng H B 2014 ACS Appl. Mater. Interfaces 6 8845
 Google Scholar
Google Scholar
[8] Feng M, You W, Wu Z S, Chen Q D, Zhan H B 2013 ACS Appl. Mater. Interfaces 5 12654
 Google Scholar
Google Scholar
[9] Yousefi M, Villar-Rodil S, Paredes J I, Moshfegh A Z 2019 J. Alloy. Compd. 809 151783
 Google Scholar
Google Scholar
[10] Zhan Y, Wan X, He S, Yang Q, He Y 2018 Chem. Eng. J. 333 132
 Google Scholar
Google Scholar
[11] Reddy P A K, Reddy P V L, Kwon E, Kim K, Akter T, Kalagara S 2016 Environ. Int. 91 94
 Google Scholar
Google Scholar
[12] Verdin A, Sahraoui A L H, Durand R 2004 Int. Biodeterior. Biodegrad. 53 65
 Google Scholar
Google Scholar
[13] Zhang X, Zhang P Y, Wu Z, Zhang L, Zeng G M, Zhou C J 2013 Colloids Surf., A 435 85
 Google Scholar
Google Scholar
[14] Yagub M T, Sen T K, Afroze S, Ang H M 2014 Adv. Colloid. Interfaces Sci. 209 172
 Google Scholar
Google Scholar
[15] Feng M, You W, Wu Z S, Chen Q D, Zhan H B 2013 ACS Appl. Mater. Inter. 5 12654
[16] Massey A T, Gusain R, Kumari S, Khatri O P 2016 Ind. Eng. Chem. Res. 55 7124
 Google Scholar
Google Scholar
[17] Xie Y J, Yan B, Xu H L, et al. 2014 ACS Appl. Mater. Inter. 6 8845
[18] Liao W L, Ma Y Q, Chen A Y, Yang Y L 2015 Chem. Eng. J. 271 232
 Google Scholar
Google Scholar
[19] Song H J, You S, Jia X H, Yang J 2015 Ceram. Int. 8 23
[20] Bobbitt N S, Mendonca M L, Howarth A J, et al. 2017 Chem. Soc. Rev. 46 3357
 Google Scholar
Google Scholar
[21] Zhao W, Wang Z H, Zhou L, Liu N Q, Wang H X 2016 Front. Mater. Sci. Chin. 10 290
 Google Scholar
Google Scholar
[22] 赵娟, 胡慧芳, 曾亚萍, 程彩萍 2013 62 158104
 Google Scholar
Google Scholar
Zhao J, Hu H F, Zeng Y P, Cheng C P 2013 Acta Phys. Sin 62 158104
 Google Scholar
Google Scholar
[23] Mazaheri H, Ghaedi M, Asfaram A, Hajati S 2016 J. Mol. Liq. 219 667
 Google Scholar
Google Scholar
[24] Okpalugo T I T, Papakonstantinou P, Murphy H, McLaughlin J, Brown N M D 2005 Carbon 43 153
 Google Scholar
Google Scholar
[25] Dettlaff-Weglikowska U, Skakalova V, Graupner R, et al. 2005 J. Am. Chem. Soc. 127 5125
 Google Scholar
Google Scholar
[26] Tseng C H, Wang C C, Chen C Y 2006 Nanotechnology 17 5602
 Google Scholar
Google Scholar
[27] Nduna M K, Lewis A E, Nortier P 2014 Colloids Surf., A 441 643
 Google Scholar
Google Scholar
[28] Borthakur P, Boruah P K, Das M R 2021 J. Environ. Chem. Eng. 9 104635
 Google Scholar
Google Scholar
[29] Gqebe S, Rodriguez-Pascual M, Lewis A 2016 J. S. Afr. Inst. Min. Metall. 116 575
 Google Scholar
Google Scholar
[30] Zha Z B, Wang S M, Zhang S H, Qu E Z, Ke H T, Wang J R, Dai Z F 2013 Nanoscale 5 3216
 Google Scholar
Google Scholar
[31] Ayodhya D, Venkatesham M, Kumari A S, et al. 2016 J. Exp. Nanosci. 11 418
 Google Scholar
Google Scholar
[32] Wang T J, Zhang H, Xu L L, Wang X L, Chen M 2017 Opt. Mater. Express 7 3863
 Google Scholar
Google Scholar
[33] Fan Y, Liu P F, Huang Z Y, Jiang T W, Yao K L, Han R 2015 J. Power Sources 280 30
 Google Scholar
Google Scholar
[34] Velasco L F, Guillet-Nicolas R, Dobos G, Thommes M, Lodewyckx P 2016 Carbon 96 753
 Google Scholar
Google Scholar
[35] Gao S Y, Liu H Y, Geng K R, Wei X J 2015 Nano Energy 12 785
 Google Scholar
Google Scholar
[36] Eid K, Wang H J, He P, Wang K M, Ahamad T, Alshehri S M, Yamauchi Y, Wang L 2015 Nanoscale 7 16860
 Google Scholar
Google Scholar
[37] Wu K, Zhang Q, Sun D M, Zhu X S, Chen Y, Lu T H, Tang Y W 2015 Int. J. Hydrogen Energy 40 6530
 Google Scholar
Google Scholar
[38] Fu G T, Wu K, Lin J, Tang Y W, Chen Y, Zhou Y M, Lu T H 2013 J. Phys. Chem. C. 117 9826
 Google Scholar
Google Scholar
[39] Wang T J, Wang D M, Zhang H, Wang X L, Chen M 2017 Opt. Mater. Express 7 924
 Google Scholar
Google Scholar
[40] Gao P X, Ding Y, Mai W, Hughes W L, Lao C S, Wang Z L 2005 Science 309 1700
 Google Scholar
Google Scholar
-
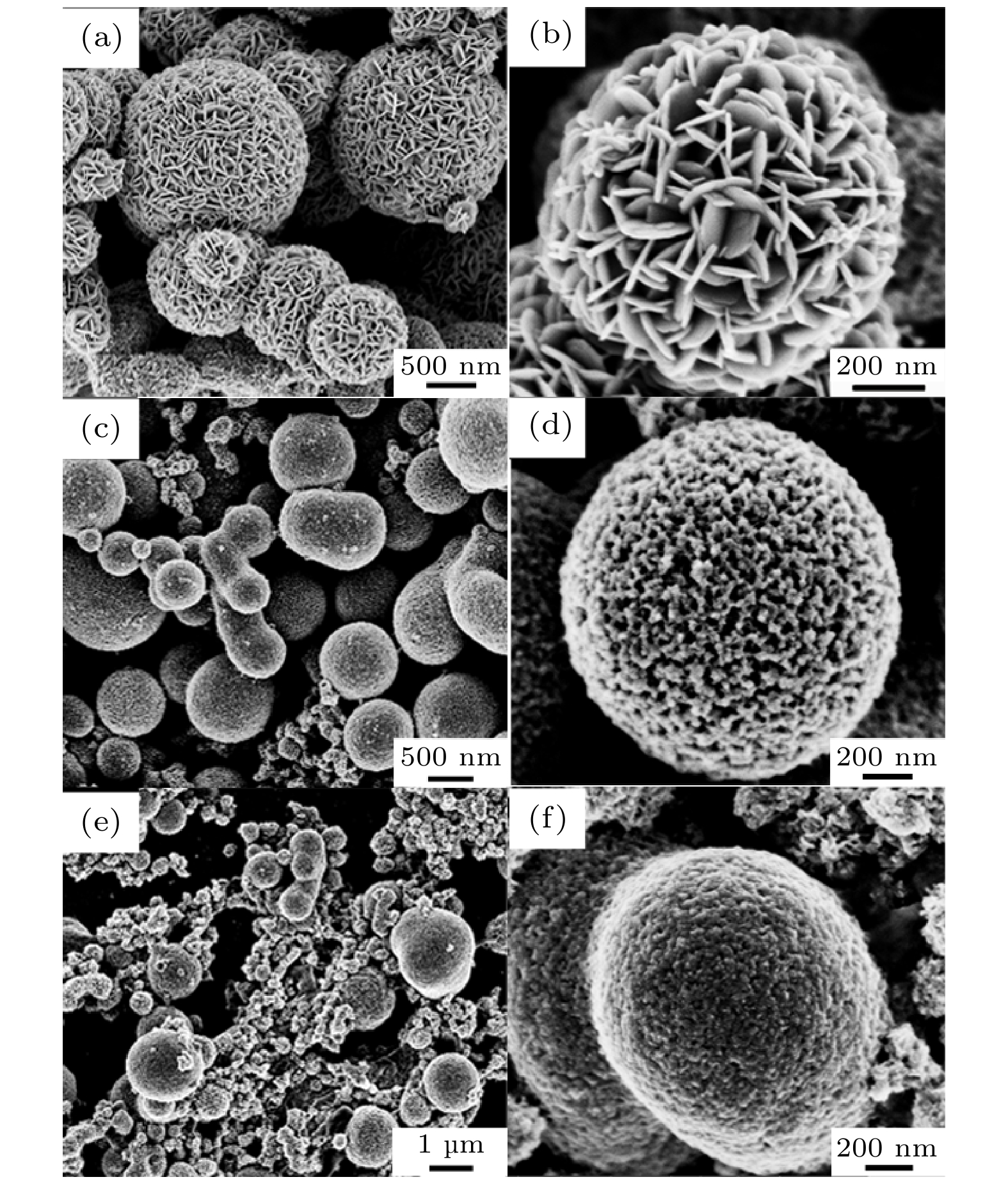
图 7 (a), (b)分别为纳米枝晶正在生长为纳米花瓣时的TEM图和SEM图; (c)硫源释放S2– 速率过快导致CuS直接团聚而未能得到纳米花瓣; (d)自组装过程因能量不足导致大量纳米片散落
Figure 7. (a), (b) TEM and SEM images when nanocrystals are growing into nanocrystals, respectively; (c) the S2– release rate of sulfur source is too fast, which leads to direct aggregation of CuS and fails to obtain nanopetals; (d) a large number of nanosheets were scattered due to lack of energy during the self-assembly process.
图 8 (a) CuS多孔级次纳米花, (b) CuS微米绒球, (c) CuS微米颗粒材料吸附MB溶液后上清液吸收谱随吸附时间的变化曲线; (d)表征三份材料对MB的吸附过程
Figure 8. The varied absorption spectra of supernatant after adding (a) porous grade sub-nanoflowers, (b) micron pompon and (c) micron particle material to adsorb MB with changing adsorption time; (d) the adsorption process of MB by adding three materials, respectively.
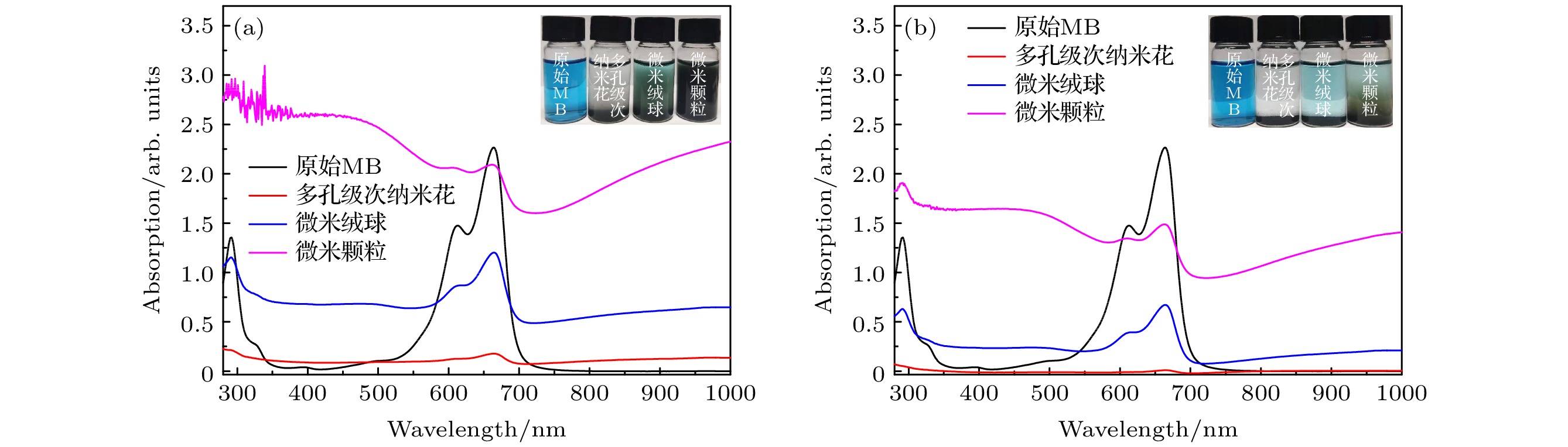
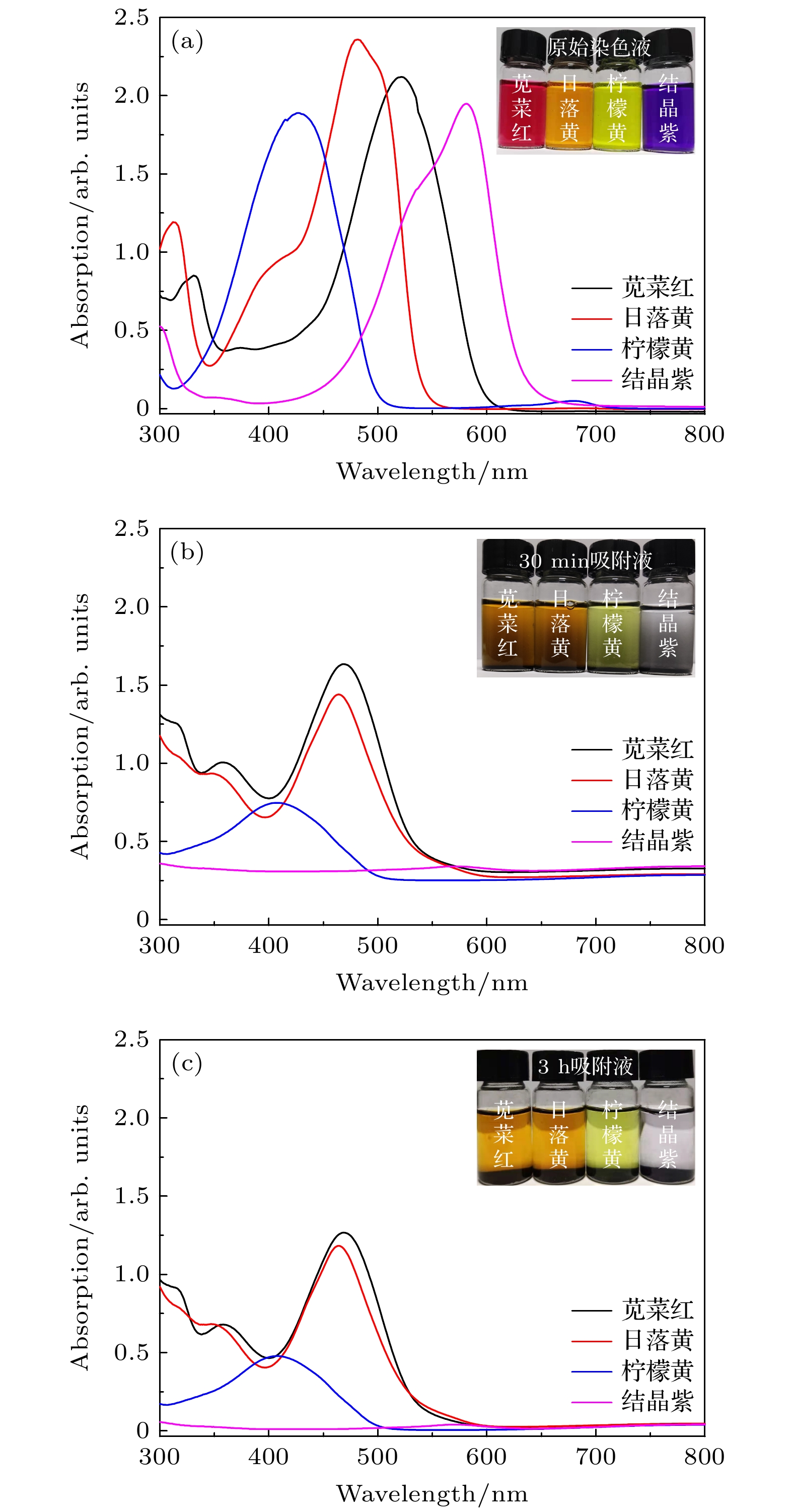
图 12 (a)为苋菜红、日落黄、柠檬黄和结晶紫的原始吸收谱; (b), (c)分别为加入多孔级次纳米花材料静置30 min和3 h后溶液吸收谱
Figure 12. (a) The original absorption spectra of amaranth, sunset yellow, lemon yellow and crystal violet; (b), (c) the absorption spectra of the solution after adding porous grade sub-nanoflowers materials for 30 min and 3 h, respectively.
表 1 三份样品的Zeta电势
Table 1. Zeta potential of three samples.
样品 Zeta电势/mV 级次纳米花 –13.3 微米绒球 –2.29 微米颗粒 –1.15 -
[1] Srivastava S, Sinha R, Roy D 2004 Aquat. Toxicol. 66 319
 Google Scholar
Google Scholar
[2] Wu Y, Su M, Chen J, Xu Z, Tang J, Chang X, Chen D 2019 Dyes Pigm. 170 107591
 Google Scholar
Google Scholar
[3] Zhang H, Chen M, Wang D M, Xu L L, Liu X D 2016 Opt. Mater. Express 6 2573
 Google Scholar
Google Scholar
[4] Miao X, Tang Y, Wong C W 2015 Nature 518 483
[5] Ghorai S, Sarkar A, Raoufi M, Panda A B, Schönherr H, Pal S 2014 ACS Appl. Mater. Interfaces 6 4766
 Google Scholar
Google Scholar
[6] Wu Z, Joo H, Lee K 2005 Chem. Eng. J. 112 227
 Google Scholar
Google Scholar
[7] Xie Y J, Yan B, Xu H L, Chen J, Liu Q X, Deng Y H, Zeng H B 2014 ACS Appl. Mater. Interfaces 6 8845
 Google Scholar
Google Scholar
[8] Feng M, You W, Wu Z S, Chen Q D, Zhan H B 2013 ACS Appl. Mater. Interfaces 5 12654
 Google Scholar
Google Scholar
[9] Yousefi M, Villar-Rodil S, Paredes J I, Moshfegh A Z 2019 J. Alloy. Compd. 809 151783
 Google Scholar
Google Scholar
[10] Zhan Y, Wan X, He S, Yang Q, He Y 2018 Chem. Eng. J. 333 132
 Google Scholar
Google Scholar
[11] Reddy P A K, Reddy P V L, Kwon E, Kim K, Akter T, Kalagara S 2016 Environ. Int. 91 94
 Google Scholar
Google Scholar
[12] Verdin A, Sahraoui A L H, Durand R 2004 Int. Biodeterior. Biodegrad. 53 65
 Google Scholar
Google Scholar
[13] Zhang X, Zhang P Y, Wu Z, Zhang L, Zeng G M, Zhou C J 2013 Colloids Surf., A 435 85
 Google Scholar
Google Scholar
[14] Yagub M T, Sen T K, Afroze S, Ang H M 2014 Adv. Colloid. Interfaces Sci. 209 172
 Google Scholar
Google Scholar
[15] Feng M, You W, Wu Z S, Chen Q D, Zhan H B 2013 ACS Appl. Mater. Inter. 5 12654
[16] Massey A T, Gusain R, Kumari S, Khatri O P 2016 Ind. Eng. Chem. Res. 55 7124
 Google Scholar
Google Scholar
[17] Xie Y J, Yan B, Xu H L, et al. 2014 ACS Appl. Mater. Inter. 6 8845
[18] Liao W L, Ma Y Q, Chen A Y, Yang Y L 2015 Chem. Eng. J. 271 232
 Google Scholar
Google Scholar
[19] Song H J, You S, Jia X H, Yang J 2015 Ceram. Int. 8 23
[20] Bobbitt N S, Mendonca M L, Howarth A J, et al. 2017 Chem. Soc. Rev. 46 3357
 Google Scholar
Google Scholar
[21] Zhao W, Wang Z H, Zhou L, Liu N Q, Wang H X 2016 Front. Mater. Sci. Chin. 10 290
 Google Scholar
Google Scholar
[22] 赵娟, 胡慧芳, 曾亚萍, 程彩萍 2013 62 158104
 Google Scholar
Google Scholar
Zhao J, Hu H F, Zeng Y P, Cheng C P 2013 Acta Phys. Sin 62 158104
 Google Scholar
Google Scholar
[23] Mazaheri H, Ghaedi M, Asfaram A, Hajati S 2016 J. Mol. Liq. 219 667
 Google Scholar
Google Scholar
[24] Okpalugo T I T, Papakonstantinou P, Murphy H, McLaughlin J, Brown N M D 2005 Carbon 43 153
 Google Scholar
Google Scholar
[25] Dettlaff-Weglikowska U, Skakalova V, Graupner R, et al. 2005 J. Am. Chem. Soc. 127 5125
 Google Scholar
Google Scholar
[26] Tseng C H, Wang C C, Chen C Y 2006 Nanotechnology 17 5602
 Google Scholar
Google Scholar
[27] Nduna M K, Lewis A E, Nortier P 2014 Colloids Surf., A 441 643
 Google Scholar
Google Scholar
[28] Borthakur P, Boruah P K, Das M R 2021 J. Environ. Chem. Eng. 9 104635
 Google Scholar
Google Scholar
[29] Gqebe S, Rodriguez-Pascual M, Lewis A 2016 J. S. Afr. Inst. Min. Metall. 116 575
 Google Scholar
Google Scholar
[30] Zha Z B, Wang S M, Zhang S H, Qu E Z, Ke H T, Wang J R, Dai Z F 2013 Nanoscale 5 3216
 Google Scholar
Google Scholar
[31] Ayodhya D, Venkatesham M, Kumari A S, et al. 2016 J. Exp. Nanosci. 11 418
 Google Scholar
Google Scholar
[32] Wang T J, Zhang H, Xu L L, Wang X L, Chen M 2017 Opt. Mater. Express 7 3863
 Google Scholar
Google Scholar
[33] Fan Y, Liu P F, Huang Z Y, Jiang T W, Yao K L, Han R 2015 J. Power Sources 280 30
 Google Scholar
Google Scholar
[34] Velasco L F, Guillet-Nicolas R, Dobos G, Thommes M, Lodewyckx P 2016 Carbon 96 753
 Google Scholar
Google Scholar
[35] Gao S Y, Liu H Y, Geng K R, Wei X J 2015 Nano Energy 12 785
 Google Scholar
Google Scholar
[36] Eid K, Wang H J, He P, Wang K M, Ahamad T, Alshehri S M, Yamauchi Y, Wang L 2015 Nanoscale 7 16860
 Google Scholar
Google Scholar
[37] Wu K, Zhang Q, Sun D M, Zhu X S, Chen Y, Lu T H, Tang Y W 2015 Int. J. Hydrogen Energy 40 6530
 Google Scholar
Google Scholar
[38] Fu G T, Wu K, Lin J, Tang Y W, Chen Y, Zhou Y M, Lu T H 2013 J. Phys. Chem. C. 117 9826
 Google Scholar
Google Scholar
[39] Wang T J, Wang D M, Zhang H, Wang X L, Chen M 2017 Opt. Mater. Express 7 924
 Google Scholar
Google Scholar
[40] Gao P X, Ding Y, Mai W, Hughes W L, Lao C S, Wang Z L 2005 Science 309 1700
 Google Scholar
Google Scholar
Catalog
Metrics
- Abstract views: 11215
- PDF Downloads: 156
- Cited By: 0














 DownLoad:
DownLoad: