-
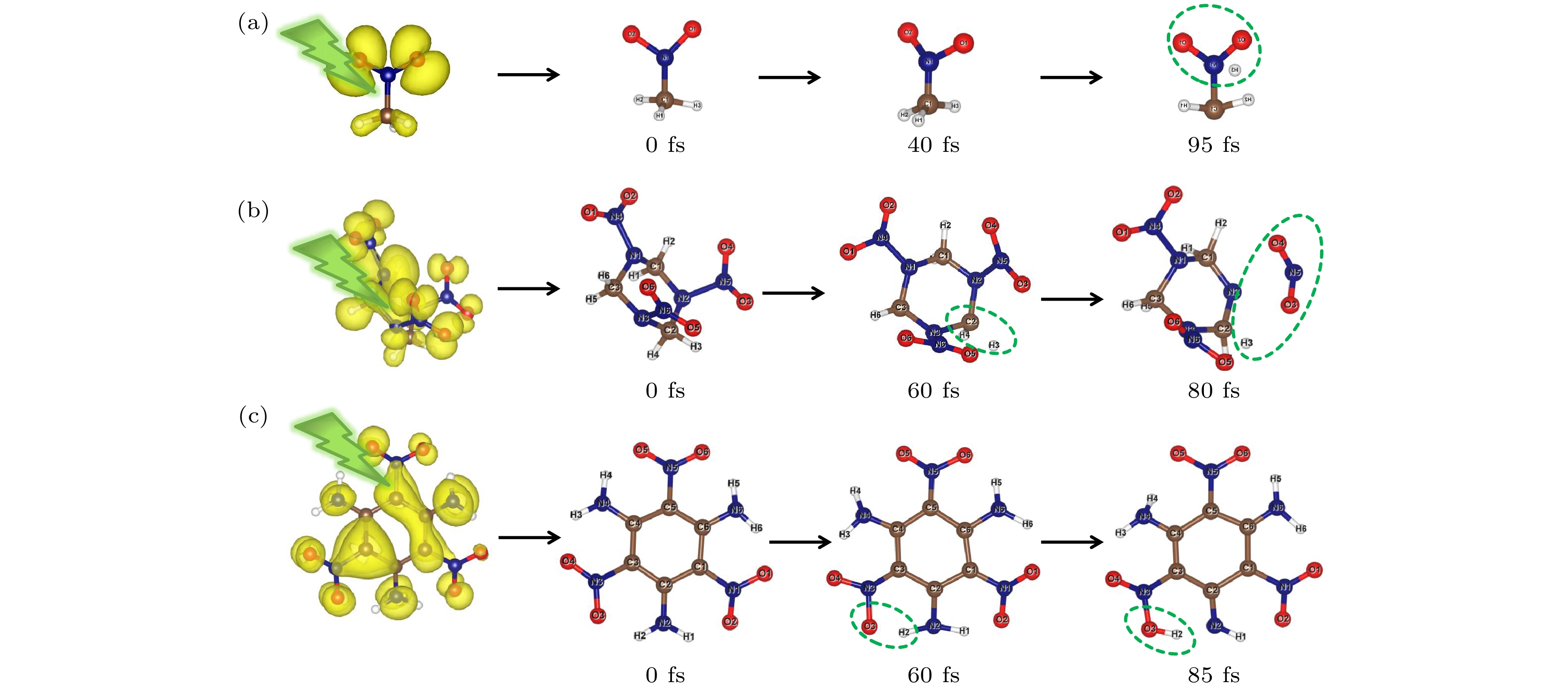
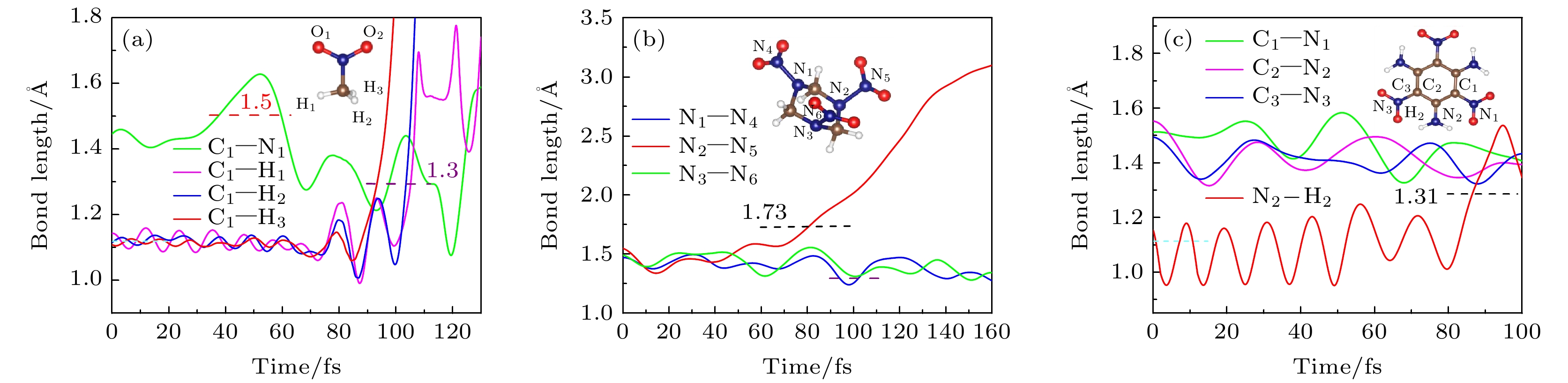
Nitro explosive is a main type of energetic material which can release a large amount of energy when detonated under extreme conditions. Further study of the excited state dynamics of photo-induced nitro explosive can provide an effective method to understand the complex process of ultrafast detonation physics. In this paper, the initial step of photodissociation at the first excited electron state of some typical nitro explosives including nitromethane (NM), cyclotrimethylenetrinitramine (RDX) and triaminotrinitrobenzene (TATB) is studied using the time-dependent density functional theory and the molecular dynamic method. The transient structures of energetic molecules and time evolutions of excited energy levels are observed. It is found that the structural relaxation of energetic molecules occurs immediately after the electronic excitation, and the entire photoexcitation process comes into being within a range of 200 fs. At the same time, the positions of molecular energy levels change to various degrees with the oscillations of different frequencies, such as the overlap between HOMO and LUMO, which is related to the obvious change of molecular configuration, indicating that the energy of excited carriers transfers to atoms in the form of heat through electron-phonon coupling, and the energy is redistributed through vibration relaxation in the initial stage of photodissociation which causes the chemical bonds of C—H, N—N and N—N to rupture, and the hydrogen atoms dissociated from methyl, methylene or amino groups, and the nearest nitro group to form some new intermediate states. In this process, the energy levels near the excited electron and hole energy also change significantly with time, suggesting that the coupling between electron and electron also plays a role in the dissociation process. Comparing with NM and RDX, the evolution of the excited energy level of TATB has obvious lower-frequency (phonon frequency) oscillations, showing that the coupling between electronic state and phonon of TATB is weak and thus makes it more difficult to dissociate. Our study can deepen the understanding of the structural relaxation of excited states and the time evolution of excitation energy levels in energetic materials, and provide a new understanding of the photoinduced reaction and the initial steps of laser ignition in energetic materials.
-
Keywords:
- nitro explosives /
- time-dependent density functional /
- excited carriers /
- photo dissocation
[1] Field J E 1992 Acc. Chem. Res. 25 489
 Google Scholar
Google Scholar
[2] Zhang S Q, Wang Y Q, Zheng X M 2006 Acta Phys-Chim Sin. 22 1489
 Google Scholar
Google Scholar
[3] Bhattacharya A, Guo Y, Bernstein E R 2010 Acc. Chem. Res. 43 1476
 Google Scholar
Google Scholar
[4] Fang X, McLuckie W G 2015 J. Hazard. Mater. 285 375
 Google Scholar
Google Scholar
[5] Gruzdkov Y A, Gupta Y M 1998 J. Phys. Chem. A 102 8325
 Google Scholar
Google Scholar
[6] Aduev B P, Nurmukhametov D R, Belokurov G M, Nelyubina N V, Kalenskii A V, Aluker N L 2017 Russ. J. Phys. Chem. B 11 460
 Google Scholar
Google Scholar
[7] Jordan M J T, Kable S H 2012 Science 335 1054
 Google Scholar
Google Scholar
[8] Spighi G, Gaveau MA, Mestdagh JM, Poisson L, Soep B 2014 Physi. Chem. Chem. Phys. 16 9610
 Google Scholar
Google Scholar
[9] Parada G A, Markle T F, Glover S D, Hammarstrom L, Ott S, Zietz B 2015 Chem. Eur. J 21 6362
 Google Scholar
Google Scholar
[10] Zhang W, Sang J, Cheng J, Ge S, Yuan S, Lo G V, Dou Y 2018 Molecules 23 1593
 Google Scholar
Google Scholar
[11] Rehwoldt M C, Wang H, Kline D J, Wu T, Eckman N, Wang P, Agrawal N R, Zachariah M R 2020 Combust. Flame 211 260
 Google Scholar
Google Scholar
[12] Cabalo J, Sausa R 2005 Appl. Optics 44 1084
 Google Scholar
Google Scholar
[13] Mattos E C, Diniz M F, Nakamura N M, Dutra R d C L 2009 J. Aerosp. Technol. Manag. 1 167
 Google Scholar
Google Scholar
[14] Rom N, Zybin S V, van Duin A C T, Goddard W A, III, Zeiri Y, Katz G, Kosloff R 2011 J. Phys. Chem. A 115 10181
 Google Scholar
Google Scholar
[15] Blais N C, Engelke R, Sheffield S A 1997 J. Phys. Chem. A 101 8285
 Google Scholar
Google Scholar
[16] Citroni M, Bini R, Pagliai M, Cardini G, Schettino V 2010 J. Phys. Chem. B 114 9420
 Google Scholar
Google Scholar
[17] Kuklja M M, Aduev B P, Aluker E D, Krasheninin V I, Krechetov A G, Mitrofanov A Y 2001 J. Appl. Phys. 89 4156
 Google Scholar
Google Scholar
[18] Guo Y Q, Greenfield M, Bhattacharya A, Bernstein E R 2007 J. Chem. Phys. 127 154301
 Google Scholar
Google Scholar
[19] Owens F J, Sharma J 1980 J. Appl. Phys. 51 1494
 Google Scholar
Google Scholar
[20] Gares K L, Bykov S V, Brinzer T, Asher S A 2015 Appl. Spectrosc. 69 545
 Google Scholar
Google Scholar
[21] Tang T B, Chaudhri M M, Rees C S, Mullock S J 1987 J. Mater. Sci. 22 1037
 Google Scholar
Google Scholar
[22] Williams D L, Timmons J C, Woodyard J D, Rainwater K A, Lightfoot J M, Richardson B R, Burgess C E, Heh J L 2003 J. Phys. Chem. A 107 9491
 Google Scholar
Google Scholar
[23] Firsich D W 1984 J. Hazard. Mater. 9 133
 Google Scholar
Google Scholar
[24] Britt A D, Moniz W B, Chingas G C, Moore D W, Heller C A, Ko C L 1981 Propell. Explos. 6 94
 Google Scholar
Google Scholar
[25] Glascoe E A, Zaug J M, Armstrong M R, Crowhurst J C, Grant C D, Fried L E 2009 J. Phys. Chem. A 113 5881
 Google Scholar
Google Scholar
[26] Runge E, Gross E K U 1984 Phys. Rev. Lett. 52 997
 Google Scholar
Google Scholar
[27] Theilhaber J 1992 Phys. Rev. B 46 12990
 Google Scholar
Google Scholar
[28] Castro A, Appel H, Oliveira M, Rozzi C A, Andrade X, Lorenzen F, Marques M A L, Gross E K U, Rubio A 2006 Phys. Status Solidi. B 243 2465
 Google Scholar
Google Scholar
[29] Friend R H, Gymer R W, Holmes A B, Burroughes J H, Marks R N, Taliani C, Bradley D D C, Dos Santos D A, Bredas J L, Logdlund M, Salaneck W R 1999 Nature 397 121
 Google Scholar
Google Scholar
[30] Polyak I, Hutton L, Crespo-Otero R, Barbatt M, Knowles P J 2019 J. Chem. theory Comput. 15 3929
 Google Scholar
Google Scholar
[31] Kolesov G, Granas O, Hoyt R, Vinichenko D, Kaxiras E 2016 J. Chem. theory Comput. 12 466
 Google Scholar
Google Scholar
[32] Nelson T, Fernandez-Alberti S, Chernyak V, Roitberg A E, Tretiak S 2011 J. Phys. Chem. B 115 5402
 Google Scholar
Google Scholar
[33] Ghosh J, Gajapathy H, Konar A, Narasimhaiah G M, Bhattacharya A 2017 J. Chem. Phys. 147 204302
 Google Scholar
Google Scholar
[34] Myers T W, Bjorgaard J A, Brown K E, Chavez D E, Hanson S K, Scharff R J, Tretiak S, Veauthier J M 2016 J. Am. Chem. Soc. 138 4685
 Google Scholar
Google Scholar
[35] Soler J M, Artacho E, Gale J D, Garcia A, Junquera J, Ordejon P, Sanchez-Portal D 2002 J. Phys-Condens. Mat. 14 2745
 Google Scholar
Google Scholar
[36] Sugino O, Miyamoto Y 1999 Phys. Rev. B 59 2579
 Google Scholar
Google Scholar
[37] Perdew J P, Burke K, Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[38] Grimme S 2006 J. Comput. Chem. 27 1787
 Google Scholar
Google Scholar
[39] Troullier N, Martins J L 1991 Phys. Rev. B 43 8861
 Google Scholar
Google Scholar
[40] Gorse D, Cavagnat D, Pesquer M, Lapouge C 1993 J. Phys. Chem. 97 4262
 Google Scholar
Google Scholar
[41] Choi C S, Mapes J E, Prince E 1972 Acta Crystallogr. B 28 1357
 Google Scholar
Google Scholar
[42] Cady H H, Larson A C 1965 Acta Crystallogr. 18 485
 Google Scholar
Google Scholar
[43] Zhong M, Liu Q-J, Qin H, Jiao Z, Zhao F, Shang H-L, Liu F-S, Liu Z-T 2017 Eur. Phys. J. B 90 115
 Google Scholar
Google Scholar
[44] Fan J, Su Y, Zheng Z, Zhang Q, Zhao J 2019 J. Raman Spectrosc. 50 889
 Google Scholar
Google Scholar
[45] Su Y, Fan J, Zheng Z, Zhao J, Song H 2018 Chin. Phys. B 27 056401
 Google Scholar
Google Scholar
[46] Flicker W M, Mosher O A, Kuppermann A 1979 Chem. Phys. Lett. 60 518
 Google Scholar
Google Scholar
[47] Whitley V H 2006 AIP Conf. Proc. 845 1357
 Google Scholar
Google Scholar
[48] Kakar S, Nelson A J, Treusch R, Heske C, van Buuren T, Jimenez I, Pagoria P, Terminello L J 2000 Phys. Rev. B 62 15666
 Google Scholar
Google Scholar
[49] Zhang W, Shen R, Ye Y, Wu L, Hu Y, Zhu P 2014 Spectrosc. Lett. 47 611
 Google Scholar
Google Scholar
[50] Nelson T, Bjorgaard J, Greenfield M, Bolme C, Brown K, McGrane S, Scharff R J, Tretiak S 2016 J. Phys. Chem. A 120 519
 Google Scholar
Google Scholar
-
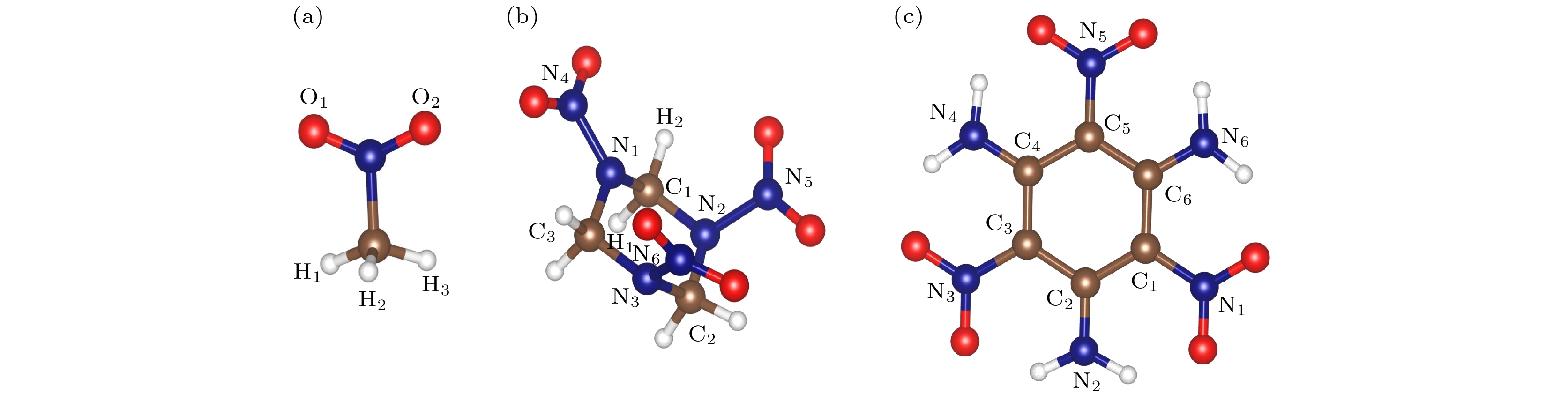
图 1 基态含能分子结构示意图 (a) 硝基甲烷(NM); (b) 环三亚甲基三硝胺(RDX); (c) 三氨基三硝基苯(TATB); 其中蓝色为N原子, 红色为O原子, 棕色为C原子, 白色为H原子
Figure 1. Structure diagrams of (a) nitromethane (NM); (b) cyclotrimethylenetrinitramine (RDX); (c) triaminotrinitrobenzene (TATB) at ground state. Blue ball is N atom, red ball is O atom, brown ball is C atom, and white ball is H atom.
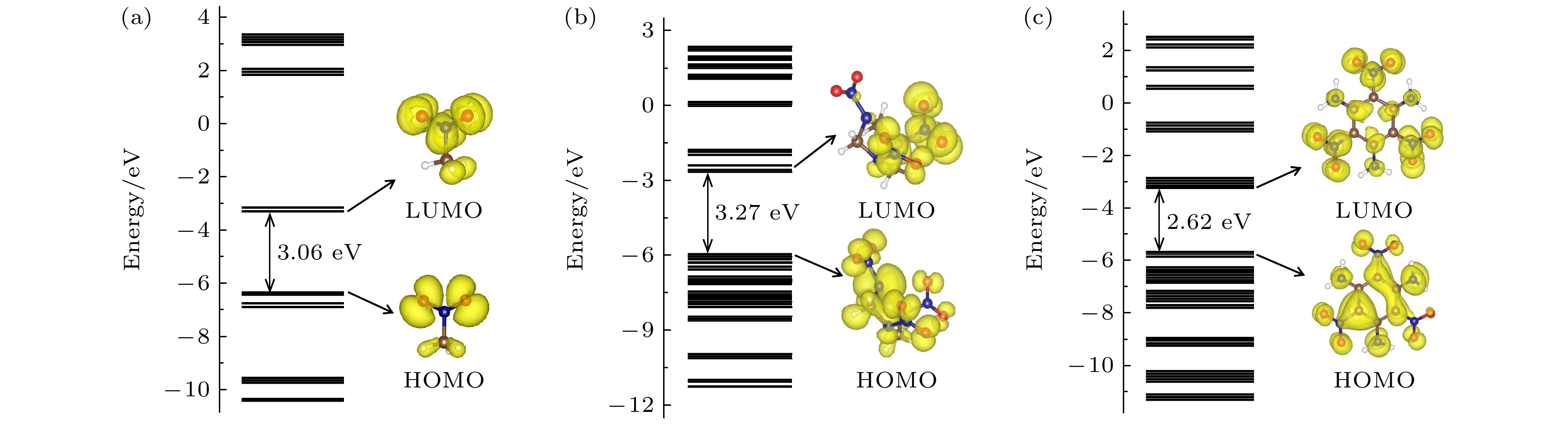
图 2 三种含能分子的基态轨道能级排布及最高占据态轨道(HOMO)与最低非占据态轨道(LUMO)的电荷密度分布图 (a) 硝基甲烷(NM); (b) 环三亚甲基三硝胺(RDX); (c) 三氨基三硝基苯(TATB)
Figure 2. The ground state molecular orbital (MO) energy levels and charge density of the highest occupied state orbitals and the lowest unoccupied state orbitals of (a) nitromethane (NM), (b) cyclotrimethylenetrinitramine (RDX), (c) triaminotrinitrobenzene (TATB).
图 5 分子轨道能级随时间的演化图 (a) 硝基甲烷(NM)分子HOMO, LUMO能级演化; (b), (c) 环三亚甲基三硝胺(RDX)分子HOMO, LUMO及其附近能级演化; (d) 三氨基三硝基苯(TATB)分子HOMO, LUMO能级演化. 绿色实线为最高占据态轨道, 对应激发空穴所在能级, 红色实线为最低非占据态轨道, 对应激发电子所在能级, 灰色实线为附近的其他能级
Figure 5. Time evolution of excited energy level: (a) HOMO and LUMO of Nitromethane (NM); (b), (c) HOMO, LUMO and nearby orbitals of cyclotrimethylenetrinitramine (RDX); (d) HOMO and LUMO of Triaminotrinitrobenzene (TATB). Green solid line denotes the highest occupied molecular orbit corresponding to the excited hole, red solid line denotes the lowest unoccupied molecular orbit corresponding to the excited electron, gray solid lines denote other molecular orbit.
表 1 三种含能分子基态的键长信息
Table 1. Bond lengths of energetic molecules at ground state
NM C—N C—H1 C—H2 C—H3 N—O1 N—O2 Length/Å This work 1.547 1.096 1.099 1.099 1.148 1.157 Exp.[40] 1.481 1.093 1.092 1.092 1.223 1.224 RDX C—N N1—N2 N3—N4 N5—N6 C1—H1 C1—H2 Length/Å This work 1.443 1.443 1.471 1.472 1.182 1.183 Exp.[41] 1.464 1.351 1.352 1.398 1.085 1.087 TATB C—C C—Nnitro C—Namino N—O N—H O···H Length/Å This work 1.425 1.436 1.318 1.216 1.021 1.646 Exp.[42] 1.441 1.442 1.316 1.243 0.925 1.780 表 2 300 K与0 K下RDX和TATB的键角对比
Table 2. Bond angles of RDX and TATB under 300 K and compared with 0 K
RDX Angle/(°) TATB Angle/(°) 0 K 300 K Change 0 K 300 K Change α1 113.7 112.1 –1.6 θ 118.6 119.2 +0.6 α2 114.8 109.8 –5.0 γ1 179.9 173.3 –6.6 δ 140.6 135.1 –5.5 γ2 179.1 174.4 –4.7 -
[1] Field J E 1992 Acc. Chem. Res. 25 489
 Google Scholar
Google Scholar
[2] Zhang S Q, Wang Y Q, Zheng X M 2006 Acta Phys-Chim Sin. 22 1489
 Google Scholar
Google Scholar
[3] Bhattacharya A, Guo Y, Bernstein E R 2010 Acc. Chem. Res. 43 1476
 Google Scholar
Google Scholar
[4] Fang X, McLuckie W G 2015 J. Hazard. Mater. 285 375
 Google Scholar
Google Scholar
[5] Gruzdkov Y A, Gupta Y M 1998 J. Phys. Chem. A 102 8325
 Google Scholar
Google Scholar
[6] Aduev B P, Nurmukhametov D R, Belokurov G M, Nelyubina N V, Kalenskii A V, Aluker N L 2017 Russ. J. Phys. Chem. B 11 460
 Google Scholar
Google Scholar
[7] Jordan M J T, Kable S H 2012 Science 335 1054
 Google Scholar
Google Scholar
[8] Spighi G, Gaveau MA, Mestdagh JM, Poisson L, Soep B 2014 Physi. Chem. Chem. Phys. 16 9610
 Google Scholar
Google Scholar
[9] Parada G A, Markle T F, Glover S D, Hammarstrom L, Ott S, Zietz B 2015 Chem. Eur. J 21 6362
 Google Scholar
Google Scholar
[10] Zhang W, Sang J, Cheng J, Ge S, Yuan S, Lo G V, Dou Y 2018 Molecules 23 1593
 Google Scholar
Google Scholar
[11] Rehwoldt M C, Wang H, Kline D J, Wu T, Eckman N, Wang P, Agrawal N R, Zachariah M R 2020 Combust. Flame 211 260
 Google Scholar
Google Scholar
[12] Cabalo J, Sausa R 2005 Appl. Optics 44 1084
 Google Scholar
Google Scholar
[13] Mattos E C, Diniz M F, Nakamura N M, Dutra R d C L 2009 J. Aerosp. Technol. Manag. 1 167
 Google Scholar
Google Scholar
[14] Rom N, Zybin S V, van Duin A C T, Goddard W A, III, Zeiri Y, Katz G, Kosloff R 2011 J. Phys. Chem. A 115 10181
 Google Scholar
Google Scholar
[15] Blais N C, Engelke R, Sheffield S A 1997 J. Phys. Chem. A 101 8285
 Google Scholar
Google Scholar
[16] Citroni M, Bini R, Pagliai M, Cardini G, Schettino V 2010 J. Phys. Chem. B 114 9420
 Google Scholar
Google Scholar
[17] Kuklja M M, Aduev B P, Aluker E D, Krasheninin V I, Krechetov A G, Mitrofanov A Y 2001 J. Appl. Phys. 89 4156
 Google Scholar
Google Scholar
[18] Guo Y Q, Greenfield M, Bhattacharya A, Bernstein E R 2007 J. Chem. Phys. 127 154301
 Google Scholar
Google Scholar
[19] Owens F J, Sharma J 1980 J. Appl. Phys. 51 1494
 Google Scholar
Google Scholar
[20] Gares K L, Bykov S V, Brinzer T, Asher S A 2015 Appl. Spectrosc. 69 545
 Google Scholar
Google Scholar
[21] Tang T B, Chaudhri M M, Rees C S, Mullock S J 1987 J. Mater. Sci. 22 1037
 Google Scholar
Google Scholar
[22] Williams D L, Timmons J C, Woodyard J D, Rainwater K A, Lightfoot J M, Richardson B R, Burgess C E, Heh J L 2003 J. Phys. Chem. A 107 9491
 Google Scholar
Google Scholar
[23] Firsich D W 1984 J. Hazard. Mater. 9 133
 Google Scholar
Google Scholar
[24] Britt A D, Moniz W B, Chingas G C, Moore D W, Heller C A, Ko C L 1981 Propell. Explos. 6 94
 Google Scholar
Google Scholar
[25] Glascoe E A, Zaug J M, Armstrong M R, Crowhurst J C, Grant C D, Fried L E 2009 J. Phys. Chem. A 113 5881
 Google Scholar
Google Scholar
[26] Runge E, Gross E K U 1984 Phys. Rev. Lett. 52 997
 Google Scholar
Google Scholar
[27] Theilhaber J 1992 Phys. Rev. B 46 12990
 Google Scholar
Google Scholar
[28] Castro A, Appel H, Oliveira M, Rozzi C A, Andrade X, Lorenzen F, Marques M A L, Gross E K U, Rubio A 2006 Phys. Status Solidi. B 243 2465
 Google Scholar
Google Scholar
[29] Friend R H, Gymer R W, Holmes A B, Burroughes J H, Marks R N, Taliani C, Bradley D D C, Dos Santos D A, Bredas J L, Logdlund M, Salaneck W R 1999 Nature 397 121
 Google Scholar
Google Scholar
[30] Polyak I, Hutton L, Crespo-Otero R, Barbatt M, Knowles P J 2019 J. Chem. theory Comput. 15 3929
 Google Scholar
Google Scholar
[31] Kolesov G, Granas O, Hoyt R, Vinichenko D, Kaxiras E 2016 J. Chem. theory Comput. 12 466
 Google Scholar
Google Scholar
[32] Nelson T, Fernandez-Alberti S, Chernyak V, Roitberg A E, Tretiak S 2011 J. Phys. Chem. B 115 5402
 Google Scholar
Google Scholar
[33] Ghosh J, Gajapathy H, Konar A, Narasimhaiah G M, Bhattacharya A 2017 J. Chem. Phys. 147 204302
 Google Scholar
Google Scholar
[34] Myers T W, Bjorgaard J A, Brown K E, Chavez D E, Hanson S K, Scharff R J, Tretiak S, Veauthier J M 2016 J. Am. Chem. Soc. 138 4685
 Google Scholar
Google Scholar
[35] Soler J M, Artacho E, Gale J D, Garcia A, Junquera J, Ordejon P, Sanchez-Portal D 2002 J. Phys-Condens. Mat. 14 2745
 Google Scholar
Google Scholar
[36] Sugino O, Miyamoto Y 1999 Phys. Rev. B 59 2579
 Google Scholar
Google Scholar
[37] Perdew J P, Burke K, Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[38] Grimme S 2006 J. Comput. Chem. 27 1787
 Google Scholar
Google Scholar
[39] Troullier N, Martins J L 1991 Phys. Rev. B 43 8861
 Google Scholar
Google Scholar
[40] Gorse D, Cavagnat D, Pesquer M, Lapouge C 1993 J. Phys. Chem. 97 4262
 Google Scholar
Google Scholar
[41] Choi C S, Mapes J E, Prince E 1972 Acta Crystallogr. B 28 1357
 Google Scholar
Google Scholar
[42] Cady H H, Larson A C 1965 Acta Crystallogr. 18 485
 Google Scholar
Google Scholar
[43] Zhong M, Liu Q-J, Qin H, Jiao Z, Zhao F, Shang H-L, Liu F-S, Liu Z-T 2017 Eur. Phys. J. B 90 115
 Google Scholar
Google Scholar
[44] Fan J, Su Y, Zheng Z, Zhang Q, Zhao J 2019 J. Raman Spectrosc. 50 889
 Google Scholar
Google Scholar
[45] Su Y, Fan J, Zheng Z, Zhao J, Song H 2018 Chin. Phys. B 27 056401
 Google Scholar
Google Scholar
[46] Flicker W M, Mosher O A, Kuppermann A 1979 Chem. Phys. Lett. 60 518
 Google Scholar
Google Scholar
[47] Whitley V H 2006 AIP Conf. Proc. 845 1357
 Google Scholar
Google Scholar
[48] Kakar S, Nelson A J, Treusch R, Heske C, van Buuren T, Jimenez I, Pagoria P, Terminello L J 2000 Phys. Rev. B 62 15666
 Google Scholar
Google Scholar
[49] Zhang W, Shen R, Ye Y, Wu L, Hu Y, Zhu P 2014 Spectrosc. Lett. 47 611
 Google Scholar
Google Scholar
[50] Nelson T, Bjorgaard J, Greenfield M, Bolme C, Brown K, McGrane S, Scharff R J, Tretiak S 2016 J. Phys. Chem. A 120 519
 Google Scholar
Google Scholar
Catalog
Metrics
- Abstract views: 8703
- PDF Downloads: 160
- Cited By: 0














 DownLoad:
DownLoad: