-
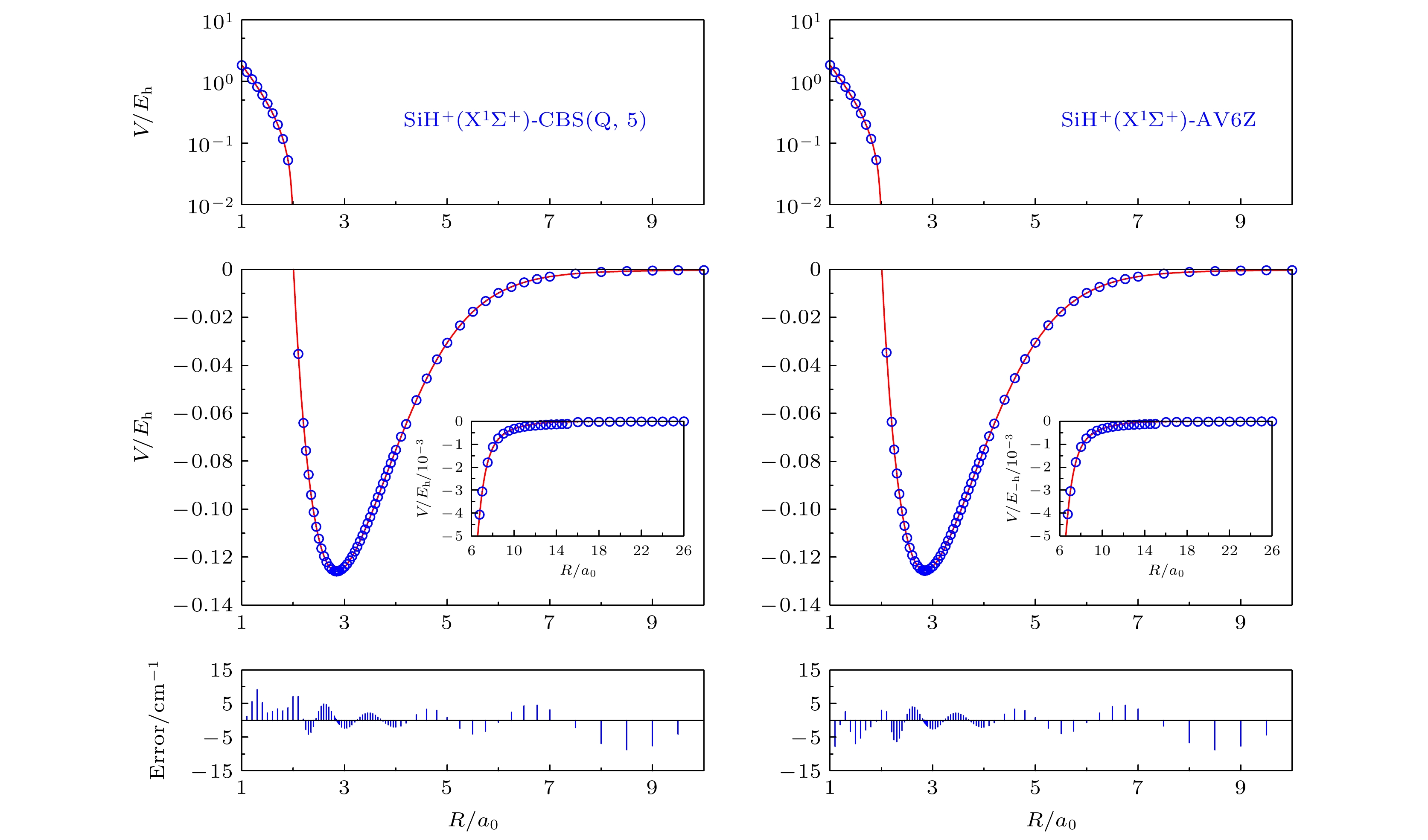
The analytical potential energy function (APEF) of SiH+(X1Σ+) is fitted by Aguado-Paniagua function with 112 ab initio energy points, which are calculated by Molpro 2012 Package with the multi-reference configuration interaction including the Davidson correction method using AVX Z and AVX dZ (X = Q, 5, 6) basis sets. Moreover, the calculated ab initio energy points are subsequently extrapolated to complete basis set (CBS) limit to avoid the basis set superposition error. All the fitting parameters of APEFs for AV6Z, CBS(Q, 5), AV6dZ, CBS(Qd, 5d), SA-AV6dZ and SOC-AV6dZ methods are gathered. The potential energy curves (PEC) and the corresponding ab initio points are also shown. As can be seen, the PECs show excellent agreement with the ab initio points and a smooth behavior both in short range and long range, which ensures the high quality of fitting process for the current APEFs. Based on these APEFs of different basis sets and methods including AVQZ, AV5Z, AV6Z, CBS(Q, 5), AVQdZ, AV5dZ, AV6dZ and CBS(Qd, 5d), the spectral constants De, Re, ωe, Be, αe and ωeχe are obtained. In addition, the effects of spin-orbit coupling interaction (SOC) on the system are also investigated. By comparing the spectral constants of SA-AV6dZ with the ones of SOC-AV6dZ, it is found that the effect of SOC on SiH+(X1Σ+) is small and can be ignored. We also compare the spectral constants in this work with the experimental values and other theoretical results. The results of this work accord well with the corresponding experimental and other theoretical results. It is worth noting that the deviation of dissociation energy between the theoretical calculations and experimental values is relatively large. Based on this conclusion, we suggest that the spectral constants including the dissociation energy for SiH+(X1Σ+) should be remeasured. Based on the APEF of SOC-AV6dZ which should be more accurate than others in theory, the top 23 vibrational states (j = 0) of SiH+(X1Σ+) are calculated first by solving the radial Schrödinger equation. All the vibrational energy levels, classical turning points, rotation constants and six centrifugal distortion constants are also provided. The results of this work can provide significant references for the experimental and other theoretical work. -
Keywords:
- SiH+(X1Σ+) ion /
- potential energy curve /
- spectral constant /
- spin-orbit coupling interaction
[1] Grevesse N, Sauval A J 1970 Astron. Astrophys. 9 232
[2] Douglas A E, Lutz B L 1970 Can. J. Phys. 48 247
 Google Scholar
Google Scholar
[3] Grevesse N, Sauval A J 1971a J. Quant. Spectrosc. Radiat. Transf. 11 65
 Google Scholar
Google Scholar
[4] Almeida A A, Sing P D 1978 Astrophys. Space Sci. 56 415
 Google Scholar
Google Scholar
[5] Gao W, Wang B B, Hu X J, Chai S, Han Y C, Greenwood J B 2017 Phys. Rev. A 96 013426
 Google Scholar
Google Scholar
[6] Wang B B, Han Y C, Gao W, Cong S L 2017 Phys. Chem. Chem. Phys. 19 22926
 Google Scholar
Google Scholar
[7] Moore P L, Browne J C, Matsen F A 1965 J. Chem. Phys. 43 903
 Google Scholar
Google Scholar
[8] Cosby P C, Helm H, Moseley J T 1980 Astrophys. J. 235 52
 Google Scholar
Google Scholar
[9] Barinovs G, Hemert M C V 2006 Astrophys. J. 636 923
 Google Scholar
Google Scholar
[10] Ram R S, Engleman R, Bernath P F 1998 J. Mol. Spectrosc. 190 341
 Google Scholar
Google Scholar
[11] Singh P D, Vanlandingham F G 1978 Astron. Astrophys. 66 87
 Google Scholar
Google Scholar
[12] Carlson T A, Copley J, Duric N, Elander N, Erman P, Larsson M, Lyyra M 1980 Astron. Astrophys. 83 238
[13] Hishikawa A, Karawajczyk A 1993 J. Mol. Spectrosc. 158 479
 Google Scholar
Google Scholar
[14] Davies P B, Martineau P M 1988 J. Chem. Phys. 88 485
 Google Scholar
Google Scholar
[15] Mosnier J P, Kennedy E T, Kampen P V, Cubaynes D, Guilbaud S, Sisourat N, Puglisi A, Carniato S, Bizau J M 2016 Phys. Rev. A 93 061401
 Google Scholar
Google Scholar
[16] Hirst D M 1986 Chem. Phys. Lett. 128 504
 Google Scholar
Google Scholar
[17] Langhoff S R, Davidson E R 1974 Int. J. Quantum Che. 8 61
 Google Scholar
Google Scholar
[18] Matos J M O, Kello V, Roos B O, Sadlej A J 1988 J. Chem. Phys. 89 423
 Google Scholar
Google Scholar
[19] Sannigrahi A B, Buenker R J, Hirsch G, Gu J P 1995 Chem. Phys. Lett. 237 204
 Google Scholar
Google Scholar
[20] Werner H J, Knowles P J, Lindh R, Manby F R, Schutz M, et al. Molpro, A Package of ab initio Programs (Version 2015.1) http://www.molpro.net [2021-03-08]
[21] Zhang Y G, Dou G, Cui J, Yu Y 2018 J. Mol. Struct. 1165 318
 Google Scholar
Google Scholar
[22] Neese F 2011 Wiley Interdisci. Rev. Comput. Mol. Sci. 2 73
[23] Biglari Z, Shayesteh A, Ershadifar S 2018 J. Quant. Spectrosc. Radiat. Transf. 221 80
 Google Scholar
Google Scholar
[24] Werner H J, Knowles P J, Lindh R, Manby F R, Schutz M, et al. Molpro, A Package of ab initio Programs (Version 2012.1) http://www.molpro.net [2021-03-08]
[25] Aguado A, Paniagua M 1992 J. Chem. Phys. 96 1265
 Google Scholar
Google Scholar
[26] Aguado A, Tablero C, Paniagua M 1998 Comput. Phys. Commun. 108 259
 Google Scholar
Google Scholar
[27] Varandas A J C 2007 J. Chem. Phys. 126 244105
 Google Scholar
Google Scholar
[28] Varandas A J C 2000 J. Chem. Phys. 113 8880
 Google Scholar
Google Scholar
[29] Jansen H B, Ross P 1969 Chem. Phys. Lett. 3 140
 Google Scholar
Google Scholar
[30] Liu B, McLean A D 1973 J. Chem. Phys. 59 4557
 Google Scholar
Google Scholar
[31] Karton A, Martin J M L 2006 Theor. Chem. ACC. 115 330
 Google Scholar
Google Scholar
[32] Yang C L, Huang Y J, Zhang X, Han K L 2003 J. Mol. Struc. Theochem. 625 289
 Google Scholar
Google Scholar
[33] Yang C L, Zhang X, Han K L 2004 J. Mol. Struc. Theochem. 676 209
 Google Scholar
Google Scholar
[34] Huber K P, Herzberg G 1979 Molecular Spectra and Molecular Structure (Vol. IV) (New York: Springer) p600
[35] Roy R J L 2017 J. Quant. Spectrosc. Radiat. Transf. 186 167
 Google Scholar
Google Scholar
-
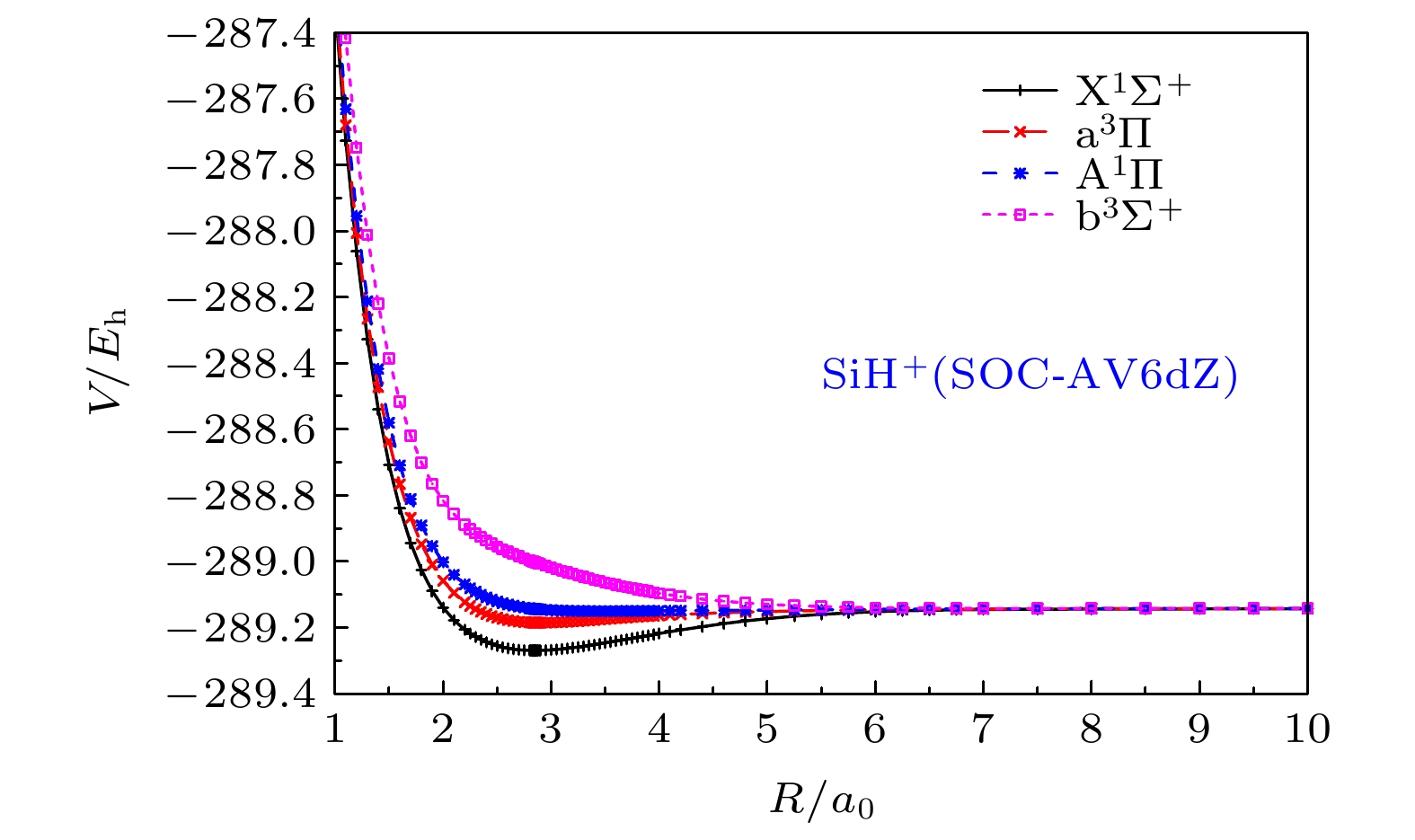
图 3 SiH+离子的
${{\rm{X}}^1}{\Sigma ^ + }$ ,${{\rm{A}}^1}\Pi$ ,${\rm{b}}{}^3{\Sigma ^ + }$ 和${{\rm{a}}^3}\Pi$ 态在SOC-AV6dZ基组下的从头算能量点Figure 3. The ab initio points of
${{\rm{X}}^1}{\Sigma ^ + }$ ,${{\rm{A}}^1}\Pi$ ,${\rm{b}}{}^3{\Sigma ^ + }$ and${{\rm{a}}^3}\Pi$ states for SiH+ cation at SOC-AV6dZ results.表 1 SiH+(X1Σ+) APEFs的拟合参数
Table 1. Parameters of APEFs for SiH+(X1Σ+).
AV6Z CBS(Q, 5) AV6dZ CBS(Qd, 5d) SA-AV6dZ SOC-AV6dZ a0 0.50876826×101 0.50552062×101 0.50858283×101 0.50691706×101 0.50773275×101 0.50772768×101 a1 –0.13804619×100 –0.14563907×100 –0.13801387×100 –0.14215220×100 –0.13720269×100 –0.15322183×100 a2 –0.13563746×101 –0.14531223×101 –0.13570613×101 –0.14215828×101 –0.16375066×101 0.30937784×100 a3 –0.51802913×102 –0.49687570×102 –0.51738027×102 –0.50317640×102 –0.42586263×102 –0.92938545×102 a4 0.62749955×103 0.59645776×103 0.62624067×103 0.60422890×103 0.45856480×103 0.11152320×104 a5 –0.48068238×104 –0.45486708×104 –0.47942732×104 –0.45972031×104 –0.30344319×104 –0.81653091×104 a6 0.26148147×105 0.24858810×105 0.26073093×105 0.24994077×105 0.14706913×105 0.40426343×105 a7 –0.98935973×105 –0.94946694×105 –0.98651660×105 –0.94886015×105 –0.51962969×105 –0.13681997×106 a8 0.24960293×106 0.24200403×106 0.24891691×106 0.24047472×106 0.12676883×106 0.31028426×106 a9 –0.39722347×106 –0.38879401×106 –0.39620641×106 –0.38442238×106 –0.19927330×106 –0.44979467×106 a10 0.35937312×106 0.35468541×106 0.35853489×106 0.34921672×106 0.18029764×106 0.37617755×106 a11 –0.14069370×106 –0.13986829×106 –0.14040272×106 –0.13721966×106 –0.71149416×106 –0.13802294×106 β1 0.6890 0.6800 0.6890 0.6840 0.6870 0.6870 β2 0.7470 0.7470 0.7470 0.7470 0.7470 0.7470 ∆Ermsd/
(kcal·mol–1)1.60176420×10–2 1.61755859×10–2 1.60042370×10–2 1.627559848×10–2 9.45767662×10–3 1.11170443×10–2 表 2 SiH+(X1Σ+)的平衡键长Re, 解离能De, 振动频率ωe, 光谱常数ωeχe, αe和βe
Table 2. Spectroscopic constants compared with the experimental values and other theoretical results for SiH+(X1Σ+).
基组 De(Eh) Re(a0) ωe/cm–1 βe/cm–1 αe/cm–1 ωeχe/cm–1 AVQZ 0.124640 2.851925 2153.245 7.607440 0.219327 42.373 AV5Z 0.125388 2.848589 2155.792 7.625272 0.218960 42.220 AV6Z 0.125584 2.848001 2156.686 7.628410 0.218833 42.189 CBS(Q, 5) 0.125856 2.847053 2157.189 7.633502 0.218622 42.117 AVQdZ 0.125067 2.848877 2156.187 7.623729 0.219427 42.343 AV5dZ 0.125461 2.848067 2156.737 7.628067 0.219011 42.232 AV6dZ 0.125622 2.847728 2157.046 7.629881 0.218849 42.190 CBS(Qd, 5d) 0.125801 2.847782 2156.602 7.629593 0.218534 42.112 SA-AV6dZ 0.127264 2.848531 2163.448 7.625581 0.216725 41.893 SOC-AV6dZ 0.126533 2.848382 2164.033 7.626378 0.217885 42.158 Expe[12,34] 0.123203 2.842338 2157.17 7.6603 0.2096 34.24 Theory[16] 0.118665 2.834590 2155.4 7.6786 0.2082 38.8 Theory[18] 0.123982 2.844039 2172.0 — — — Theory[21] 0.125317 2.834590 2177.9 7.6984 — 36.7 Theory[23] 0.124980 2.842149 2154.3 7.6609 0.2032 35.0 表 3 SiH+(X1Σ+)离子在SOC-AV6dZ方法下, j = 0时的前23个振动能级G(v)、经典拐点和惯性转动常数Bv
Table 3. Vibrational levels G(v), classical turn point androtational constant Bv for SiH+(X1Σ+) when j = 0 at SOC-AV6dZ result.
v G(v)/ cm–1 Rmin(a0) Rmax(a0) Bv/ cm–1 0 1074.467 2.62981 3.11141 7.530487 1 3171.224 2.49347 3.33862 7.336827 2 5200.543 2.40984 3.51594 7.142529 3 7162.231 2.34756 3.67498 6.947281 4 9055.742 2.29761 3.82512 6.750433 5 10880.124 2.25595 3.97091 6.551101 6 12634.005 2.22031 3.91505 6.348250 7 14315.600 2.18934 4.26009 6.140735 8 15922.726 2.16213 4.40742 5.927312 9 17452.802 2.13803 4.55893 5.706603 10 18902.835 2.11661 4.71650 5.477032 11 20269.364 2.09752 4.88227 5.236702 12 21548.363 2.08051 5.05888 4.983201 13 22735.066 2.06540 5.24986 4.713296 14 23823.694 2.05206 5.46016 4.422419 15 24807.010 2.04041 5.69731 4.103776 16 25675.599 2.03041 5.97379 3.746622 17 26416.625 2.02208 6.31278 3.332604 18 27011.579 2.01553 6.76545 2.826988 19 27432.546 2.01096 7.48310 2.158867 20 27650.956 2.00861 9.05975 1.323582 21 27734.462 2.00771 11.47553 0.838944 22 27769.256 2.00734 16.60576 0.360244 表 4 SiH+(X1Σ+)离子在SOC-AV6dZ方法下, j = 0时的前23个振动能级的6个离心畸变常数Dv, Hv, Lv, Mv, Nv和Ov
Table 4. Six centrifugal distortion constants Dv, Hv, Lv, Mv, Nv和Ov for the top 23 vibrational states of SiH+(X1Σ+) when j = 0 at SOC-AV6dZ result.
v Dv (× 10–4) Hv (× 10–8) Lv Mv Nv Ov 0 –3.7712102 1.4824556 –9.1045 × 10–13 4.1069 × 10–17 –2.4011 × 10–21 6.9631 × 10–26 1 –3.7362090 1.4243863 –8.8032 × 10–13 3.5297 × 10–17 –4.1863 × 10–21 1.8363 × 10–25 2 –3.7039865 1.3618539 –8.9866 × 10–13 2.9262 × 10–17 –5.1444 × 10–21 1.9171 × 10–25 3 –3.6778687 1.2884893 –9.4914 × 10–13 2.3397 × 10–17 –5.8593 × 10–21 9.6796 × 10–26 4 –3.6608093 1.2011917 –1.0216 × 10–12 1.6648 × 10–17 –6.8904 × 10–21 –1.1678 × 10–25 5 –3.6553038 1.0982022 –1.1140 × 10–12 6.8522 × 10–18 –8.7371 × 10–21 –4.8697 × 10–25 6 –3.6635199 0.9774540 –1.2320 × 10–12 –9.1302 × 10–18 –1.2003 × 10–20 –1.1229 × 10–24 7 –3.6875661 0.8351772 –1.3901 × 10–12 –3.5900 × 10–17 –1.7702 × 10–20 –2.2781 × 10–24 8 –3.7298613 0.6645057 –1.6143 × 10–12 –8.0925 × 10–17 –2.7799 × 10–20 –4.5064 × 10–24 9 –3.7936180 0.4536483 –1.9481 × 10–12 –1.5748 × 10–16 –4.6321 × 10–20 –9.0505 × 10–24 10 –3.8835055 1.8293995 –2.4663 × 10–12 –2.9088 × 10–16 –8.1928 × 10–20 –1.8869 × 10–23 11 –4.0066389 –0.1804552 –3.3026 × 10–12 –5.3251 × 10–16 –1.5445 × 10–19 –4.1577 × 10–23 12 –4.1741837 –0.6927552 –4.7113 × 10–12 –9.9426 × 10–16 –3.1311 × 10–19 –9.8710 × 10–23 13 –4.4041727 –1.4546951 –7.2135 × 10–12 –1.9417 × 10–15 –6.9300 × 10–19 –2.5871 × 10–22 14 –4.7268845 –2.6591579 –1.1978 × 10–11 –4.0783 × 10–15 –1.7160 × 10–18 –7.7417 × 10–22 15 –5.1961278 –4.7106022 –2.1959 × 10–11 –9.5527 × 10–15 –4.9431 × 10–18 –2.7809 × 10–21 16 –5.9157772 –8.5688303 –4.5904 × 10–11 –2.6336 × 10–14 –1.7665 × 10–17 –1.2991 × 10–20 17 –7.1117968 –16.938203 –1.1615 × 10–10 –9.3420 × 10–14 –8.7454 × 10–17 –9.0301 × 10–20 18 –9.3643712 –39.467832 –3.9578 × 10–10 –4.9264 × 10–13 –7.1396 × 10–16 –1.1444 × 10–18 19 –14.323856 –114.68916 –1.7949 × 10–9 –3.4005 × 10–12 –7.1696 × 10–15 –1.6154 × 10–17 20 –18.399913 –47.324191 1.5501 × 10–9 –7.5768 × 10–13 –3.7548 × 10–14 –1.9274 × 10–17 21 –14.637253 –291.40012 –2.0665 × 10–8 –1.4452 × 10–10 –1.3084 × 10–12 –1.3806 × 10–14 22 –57.213156 –20756.133 –1.6655 × 10–5 –1.7512 × 10–6 –2.1241 × 10–7 –2.8187 × 10–8 -
[1] Grevesse N, Sauval A J 1970 Astron. Astrophys. 9 232
[2] Douglas A E, Lutz B L 1970 Can. J. Phys. 48 247
 Google Scholar
Google Scholar
[3] Grevesse N, Sauval A J 1971a J. Quant. Spectrosc. Radiat. Transf. 11 65
 Google Scholar
Google Scholar
[4] Almeida A A, Sing P D 1978 Astrophys. Space Sci. 56 415
 Google Scholar
Google Scholar
[5] Gao W, Wang B B, Hu X J, Chai S, Han Y C, Greenwood J B 2017 Phys. Rev. A 96 013426
 Google Scholar
Google Scholar
[6] Wang B B, Han Y C, Gao W, Cong S L 2017 Phys. Chem. Chem. Phys. 19 22926
 Google Scholar
Google Scholar
[7] Moore P L, Browne J C, Matsen F A 1965 J. Chem. Phys. 43 903
 Google Scholar
Google Scholar
[8] Cosby P C, Helm H, Moseley J T 1980 Astrophys. J. 235 52
 Google Scholar
Google Scholar
[9] Barinovs G, Hemert M C V 2006 Astrophys. J. 636 923
 Google Scholar
Google Scholar
[10] Ram R S, Engleman R, Bernath P F 1998 J. Mol. Spectrosc. 190 341
 Google Scholar
Google Scholar
[11] Singh P D, Vanlandingham F G 1978 Astron. Astrophys. 66 87
 Google Scholar
Google Scholar
[12] Carlson T A, Copley J, Duric N, Elander N, Erman P, Larsson M, Lyyra M 1980 Astron. Astrophys. 83 238
[13] Hishikawa A, Karawajczyk A 1993 J. Mol. Spectrosc. 158 479
 Google Scholar
Google Scholar
[14] Davies P B, Martineau P M 1988 J. Chem. Phys. 88 485
 Google Scholar
Google Scholar
[15] Mosnier J P, Kennedy E T, Kampen P V, Cubaynes D, Guilbaud S, Sisourat N, Puglisi A, Carniato S, Bizau J M 2016 Phys. Rev. A 93 061401
 Google Scholar
Google Scholar
[16] Hirst D M 1986 Chem. Phys. Lett. 128 504
 Google Scholar
Google Scholar
[17] Langhoff S R, Davidson E R 1974 Int. J. Quantum Che. 8 61
 Google Scholar
Google Scholar
[18] Matos J M O, Kello V, Roos B O, Sadlej A J 1988 J. Chem. Phys. 89 423
 Google Scholar
Google Scholar
[19] Sannigrahi A B, Buenker R J, Hirsch G, Gu J P 1995 Chem. Phys. Lett. 237 204
 Google Scholar
Google Scholar
[20] Werner H J, Knowles P J, Lindh R, Manby F R, Schutz M, et al. Molpro, A Package of ab initio Programs (Version 2015.1) http://www.molpro.net [2021-03-08]
[21] Zhang Y G, Dou G, Cui J, Yu Y 2018 J. Mol. Struct. 1165 318
 Google Scholar
Google Scholar
[22] Neese F 2011 Wiley Interdisci. Rev. Comput. Mol. Sci. 2 73
[23] Biglari Z, Shayesteh A, Ershadifar S 2018 J. Quant. Spectrosc. Radiat. Transf. 221 80
 Google Scholar
Google Scholar
[24] Werner H J, Knowles P J, Lindh R, Manby F R, Schutz M, et al. Molpro, A Package of ab initio Programs (Version 2012.1) http://www.molpro.net [2021-03-08]
[25] Aguado A, Paniagua M 1992 J. Chem. Phys. 96 1265
 Google Scholar
Google Scholar
[26] Aguado A, Tablero C, Paniagua M 1998 Comput. Phys. Commun. 108 259
 Google Scholar
Google Scholar
[27] Varandas A J C 2007 J. Chem. Phys. 126 244105
 Google Scholar
Google Scholar
[28] Varandas A J C 2000 J. Chem. Phys. 113 8880
 Google Scholar
Google Scholar
[29] Jansen H B, Ross P 1969 Chem. Phys. Lett. 3 140
 Google Scholar
Google Scholar
[30] Liu B, McLean A D 1973 J. Chem. Phys. 59 4557
 Google Scholar
Google Scholar
[31] Karton A, Martin J M L 2006 Theor. Chem. ACC. 115 330
 Google Scholar
Google Scholar
[32] Yang C L, Huang Y J, Zhang X, Han K L 2003 J. Mol. Struc. Theochem. 625 289
 Google Scholar
Google Scholar
[33] Yang C L, Zhang X, Han K L 2004 J. Mol. Struc. Theochem. 676 209
 Google Scholar
Google Scholar
[34] Huber K P, Herzberg G 1979 Molecular Spectra and Molecular Structure (Vol. IV) (New York: Springer) p600
[35] Roy R J L 2017 J. Quant. Spectrosc. Radiat. Transf. 186 167
 Google Scholar
Google Scholar
Catalog
Metrics
- Abstract views: 7417
- PDF Downloads: 105
- Cited By: 0














 DownLoad:
DownLoad: