-
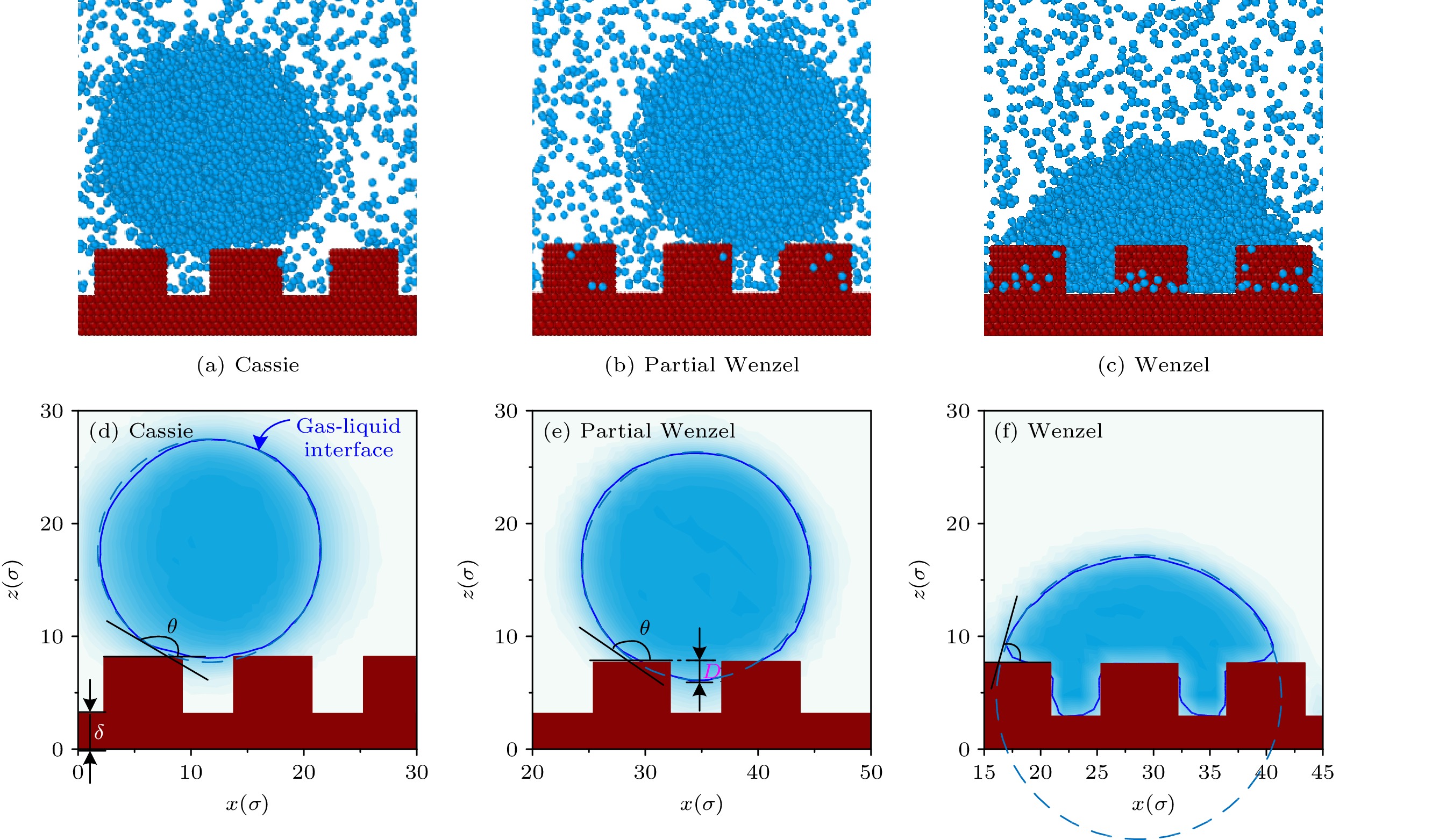
The wetting modes of droplet on nanostructure surface including Cassie, Partial Wenzel, and Wenzel are of great importance in enhancing the condensation heat transfer, surface self-cleaning and oil-water separation. Previous studies focused mainly on the behaviors of droplets on the surface of nano-pillar structures. In this work, the wetting behaviors of argon nanodroplet on platinum surface is investigated by the molecular dynamics simulations. The effects of nanostructure geometry parameters and characteristic contact angle θe on the wetting mode and the transition between different modes are investigated. The three-dimensional simulation box includes a bottom wall containing trapezoid wires (TWs) with different geometry parameters and other five surfaces. The TWs are populated on the wall based on the array arrangement. The periodic boundary conditions are imposed on the four side surfaces of the simulation box. The base angles of the side surface of TW with respect to horizontal plane are chosen as 60° (inverted TW), 90° (rectangular pin fin) and 120° (TW), respectively. For all the three base angles, the nanostructure surface can be completely wetted by liquid, behaving as the Wenzel mode when θe < 118°, under which the gaps of nanostructures are filled with liquid. However, when the characteristic contact angle θe is in a range of 118°–145°, the base angles of nanostructures have different effects on wetting modes. The surface with inverted TWs (60° base angle) is conducive to keeping droplet in Cassie mode, in which the liquid does not penetrate into any gap of nanostructures. The surface with rectangular pin fins behaves as either Partial Wenzel mode or Cassie mode. The transition between the two modes takes place at θe ~130°. The surface with TWs (120° base angle) keeps the droplet in Partial Wenzel mode, in which the gaps of nanostructures are partially wetted by liquid. For θe larger than 145°, the dewetting process takes place on the surface of the nanostructure, in which the droplet leaves the solid surface. We conclude that the wetting modes on nanostructured surface satisfy the minimum surface energy principle. Our work discloses a new finding that the surface with inverted TWs is easy to maintain Cassie mode, which is good for dropwise condensation applications.
-
Keywords:
- droplet /
- nano-structure /
- wetting mode /
- molecular dynamics
[1] 强伟丽 2018 硕士学位论文 (大连: 大连理工大学)
Qiang W L 2018 M. S. Thesis (Dalian: Dalian University of Technology) (in Chinese)
[2] Wen R F, Zhong L, Peng B L, Xu W, Ma X H 2015 Appl. Therm. Eng. 88 265
 Google Scholar
Google Scholar
[3] Birbarah P, Miljkovic N 2017 Int. J. Heat Mass Transfer 114 1025
 Google Scholar
Google Scholar
[4] Neinhuis C, Barthlott W 1997 Ann. Bot. 79 667
 Google Scholar
Google Scholar
[5] Lin F, Li S H, Li Y S, Li H J, Zhang L J, Zhai J, Song Y L, Liu B Q, Jiang L, Zhu D B 2002 Adv. Mater. 14 24
 Google Scholar
Google Scholar
[6] Blossey R 2003 Nat. Mater. 2 301
 Google Scholar
Google Scholar
[7] Xie J, Xu J L, Shang W, Zhang K 2018 Int. J. Heat Mass Transfer 127 1170
 Google Scholar
Google Scholar
[8] Wenzel R N 1936 J. Ind. Eng. Chem. 28 988
 Google Scholar
Google Scholar
[9] Cassie A B D, Baxter S 1944 Trans. Faraday Soc. 40 546
 Google Scholar
Google Scholar
[10] Zhuang Y X, Hansen O, Knieling T, Wang C, Rombach P, Lang W, Benecke W, Kehlenbeck M, Koblitz J 2007 J. Microelectromech. Syst. 16 1451
 Google Scholar
Google Scholar
[11] Nishino T, Meguro M, Nakamae K, Matsushita M, Ueda Y 1999 Langmuir 15 4321
 Google Scholar
Google Scholar
[12] Li Y S, Quéré D, Lv C J, Zheng Q S 2017 Mono Super Mater. 114 3387
 Google Scholar
Google Scholar
[13] Fu T, Wu N N, Lu C, Wang J B, Wang Q L 2019 Mol. Simulat. 45 35
 Google Scholar
Google Scholar
[14] Shahraz A, Borhan A, Fichthorn K A 2013 Langmuir 29 11632
 Google Scholar
Google Scholar
[15] Chen S, Wang J, Chen D 2014 J. Phys. Chem. C 118 18529
 Google Scholar
Google Scholar
[16] Wang J J, Li T, Li Y F, Duan Y R, Jiang Y Y, Arandiyan H, Li H 2018 Molecules 23 2407
 Google Scholar
Google Scholar
[17] Mao Y, Chen C L, Zhang Y 2013 Appl. Phys. A 111 747
 Google Scholar
Google Scholar
[18] Saha J K, Matin M A, Jang J, Jang J 2013 Bull. Korean Chem. Soc. 34 1047
 Google Scholar
Google Scholar
[19] Li Q, Wang B, Chen Y, Zhao Z 2016 Chem. Phys. Lett. 662 73
 Google Scholar
Google Scholar
[20] Wang J, Chen S, Chen D 2015 Phys. Chem. Chem. Phys. 17 30533
 Google Scholar
Google Scholar
[21] Marmur A 2004 Langmuir 20 3517
 Google Scholar
Google Scholar
[22] He B, Patankar N A, Lee J 2003 Langmuir 19 4999
 Google Scholar
Google Scholar
[23] Bico J, Uwe T, Quéré D 2002 Colloids Surf., A 206 41
 Google Scholar
Google Scholar
[24] Shi B, Dhir V K 2009 J. Chem. Phys. 130 204715
 Google Scholar
Google Scholar
[25] Yong X, Zhang L T 2009 Langmuir 25 5045
 Google Scholar
Google Scholar
[26] Savoy E S, Escobedo F A 2012 Langmuir 28 16080
 Google Scholar
Google Scholar
[27] Wang Z, Lin K, Zhao Y 2019 Colloid Interface Sci. 552 563
 Google Scholar
Google Scholar
[28] Zhu C Q, Gao Y R, Huang Y Y, Li H, Meng S, Francisco J S, Zeng X C 2017 Nanoscale 9 18240
 Google Scholar
Google Scholar
[29] 章佳健 2020 博士学位论文 (合肥: 中国科学技术大学)
Zhang J J 2020 Ph. D. Dissertation (Anhui: University of Science and Technology of China) (in Chinese)
[30] Niu Z X, Huang T, Chen Y 2018 Front. Phys.-Beijng 13 137804
 Google Scholar
Google Scholar
[31] Nagayama G, Kawagoe M, Tokunaga A, Tsuruta T 2010 Int. J. Therm. Sci. 49 59
 Google Scholar
Google Scholar
[32] Chen J X, Chen W Y, Xie Y J, Wang Z G, Qin J B 2017 Appl. Surf. Sci. 396 1058
 Google Scholar
Google Scholar
[33] 王文辉 2018 硕士学位论文 (哈尔滨: 哈尔滨工程大学)
Wang W H 2018 M. S. Thesis (Haerbin: Harbin Engineering University) (in Chinese)
[34] Li W, Amirfazli A 2005 J. Colloid Interface Sci. 292 195
 Google Scholar
Google Scholar
[35] Shahraz A, Borhan A, Fichthorn A K A 2012 Langmuir 28 14227
 Google Scholar
Google Scholar
-
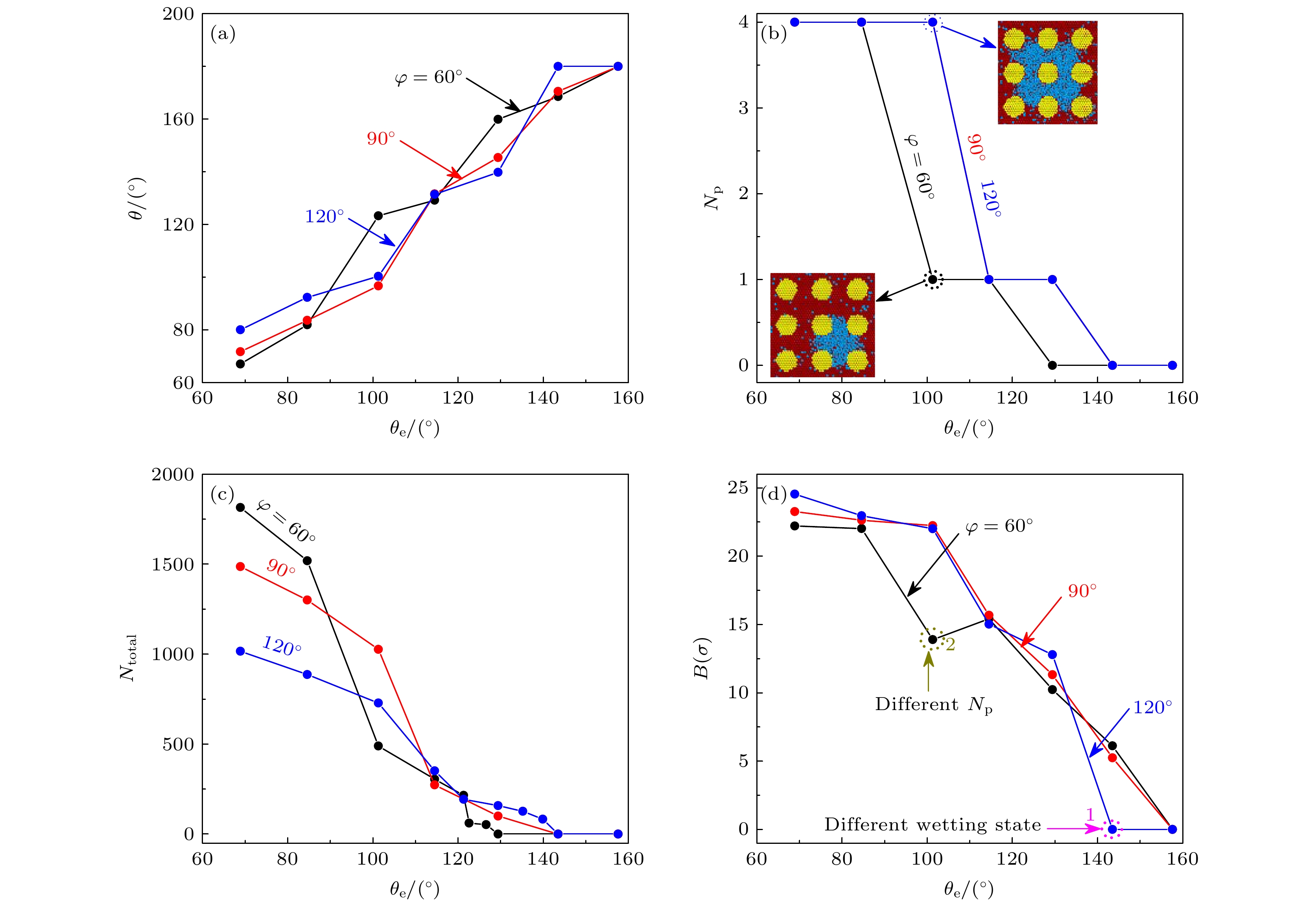
图 6 (a)当φ = 60°, 90°, 120°时, 表观接触角随θe的变化曲线; (b)液体润湿凹槽数目随θe的变化曲线, 其中, φ = 90°和120°时的曲线完全重合; (c)液体原子渗入到凹槽的数量随θe的变化曲线; (d)液滴基底圆半径随θe的变化曲线
Figure 6. (a) Curves of apparent contact angle with θe when φ = 60°, 90°, 120°; (b) change curves of the number of liquid wetting grooves with θe, where the curves at φ = 90° and 120° are completely coincident; (c) amount of liquid atoms entering the groove varies with θe; (d) change curves of the droplet base circle radius with θe.
表 1 三种粗糙表面对应的结构参数
Table 1. Structural parameters corresponding to three rough surfaces.
φ/(°) w /σ s /σ h /σ r f 60 6.9 4.6 4.025 1.758 0.283 90 6.9 4.6 4.025 1.660 0.283 120 6.9 4.6 4.025 1.509 0.283 -
[1] 强伟丽 2018 硕士学位论文 (大连: 大连理工大学)
Qiang W L 2018 M. S. Thesis (Dalian: Dalian University of Technology) (in Chinese)
[2] Wen R F, Zhong L, Peng B L, Xu W, Ma X H 2015 Appl. Therm. Eng. 88 265
 Google Scholar
Google Scholar
[3] Birbarah P, Miljkovic N 2017 Int. J. Heat Mass Transfer 114 1025
 Google Scholar
Google Scholar
[4] Neinhuis C, Barthlott W 1997 Ann. Bot. 79 667
 Google Scholar
Google Scholar
[5] Lin F, Li S H, Li Y S, Li H J, Zhang L J, Zhai J, Song Y L, Liu B Q, Jiang L, Zhu D B 2002 Adv. Mater. 14 24
 Google Scholar
Google Scholar
[6] Blossey R 2003 Nat. Mater. 2 301
 Google Scholar
Google Scholar
[7] Xie J, Xu J L, Shang W, Zhang K 2018 Int. J. Heat Mass Transfer 127 1170
 Google Scholar
Google Scholar
[8] Wenzel R N 1936 J. Ind. Eng. Chem. 28 988
 Google Scholar
Google Scholar
[9] Cassie A B D, Baxter S 1944 Trans. Faraday Soc. 40 546
 Google Scholar
Google Scholar
[10] Zhuang Y X, Hansen O, Knieling T, Wang C, Rombach P, Lang W, Benecke W, Kehlenbeck M, Koblitz J 2007 J. Microelectromech. Syst. 16 1451
 Google Scholar
Google Scholar
[11] Nishino T, Meguro M, Nakamae K, Matsushita M, Ueda Y 1999 Langmuir 15 4321
 Google Scholar
Google Scholar
[12] Li Y S, Quéré D, Lv C J, Zheng Q S 2017 Mono Super Mater. 114 3387
 Google Scholar
Google Scholar
[13] Fu T, Wu N N, Lu C, Wang J B, Wang Q L 2019 Mol. Simulat. 45 35
 Google Scholar
Google Scholar
[14] Shahraz A, Borhan A, Fichthorn K A 2013 Langmuir 29 11632
 Google Scholar
Google Scholar
[15] Chen S, Wang J, Chen D 2014 J. Phys. Chem. C 118 18529
 Google Scholar
Google Scholar
[16] Wang J J, Li T, Li Y F, Duan Y R, Jiang Y Y, Arandiyan H, Li H 2018 Molecules 23 2407
 Google Scholar
Google Scholar
[17] Mao Y, Chen C L, Zhang Y 2013 Appl. Phys. A 111 747
 Google Scholar
Google Scholar
[18] Saha J K, Matin M A, Jang J, Jang J 2013 Bull. Korean Chem. Soc. 34 1047
 Google Scholar
Google Scholar
[19] Li Q, Wang B, Chen Y, Zhao Z 2016 Chem. Phys. Lett. 662 73
 Google Scholar
Google Scholar
[20] Wang J, Chen S, Chen D 2015 Phys. Chem. Chem. Phys. 17 30533
 Google Scholar
Google Scholar
[21] Marmur A 2004 Langmuir 20 3517
 Google Scholar
Google Scholar
[22] He B, Patankar N A, Lee J 2003 Langmuir 19 4999
 Google Scholar
Google Scholar
[23] Bico J, Uwe T, Quéré D 2002 Colloids Surf., A 206 41
 Google Scholar
Google Scholar
[24] Shi B, Dhir V K 2009 J. Chem. Phys. 130 204715
 Google Scholar
Google Scholar
[25] Yong X, Zhang L T 2009 Langmuir 25 5045
 Google Scholar
Google Scholar
[26] Savoy E S, Escobedo F A 2012 Langmuir 28 16080
 Google Scholar
Google Scholar
[27] Wang Z, Lin K, Zhao Y 2019 Colloid Interface Sci. 552 563
 Google Scholar
Google Scholar
[28] Zhu C Q, Gao Y R, Huang Y Y, Li H, Meng S, Francisco J S, Zeng X C 2017 Nanoscale 9 18240
 Google Scholar
Google Scholar
[29] 章佳健 2020 博士学位论文 (合肥: 中国科学技术大学)
Zhang J J 2020 Ph. D. Dissertation (Anhui: University of Science and Technology of China) (in Chinese)
[30] Niu Z X, Huang T, Chen Y 2018 Front. Phys.-Beijng 13 137804
 Google Scholar
Google Scholar
[31] Nagayama G, Kawagoe M, Tokunaga A, Tsuruta T 2010 Int. J. Therm. Sci. 49 59
 Google Scholar
Google Scholar
[32] Chen J X, Chen W Y, Xie Y J, Wang Z G, Qin J B 2017 Appl. Surf. Sci. 396 1058
 Google Scholar
Google Scholar
[33] 王文辉 2018 硕士学位论文 (哈尔滨: 哈尔滨工程大学)
Wang W H 2018 M. S. Thesis (Haerbin: Harbin Engineering University) (in Chinese)
[34] Li W, Amirfazli A 2005 J. Colloid Interface Sci. 292 195
 Google Scholar
Google Scholar
[35] Shahraz A, Borhan A, Fichthorn A K A 2012 Langmuir 28 14227
 Google Scholar
Google Scholar
Catalog
Metrics
- Abstract views: 10807
- PDF Downloads: 327
- Cited By: 0















 DownLoad:
DownLoad: