-
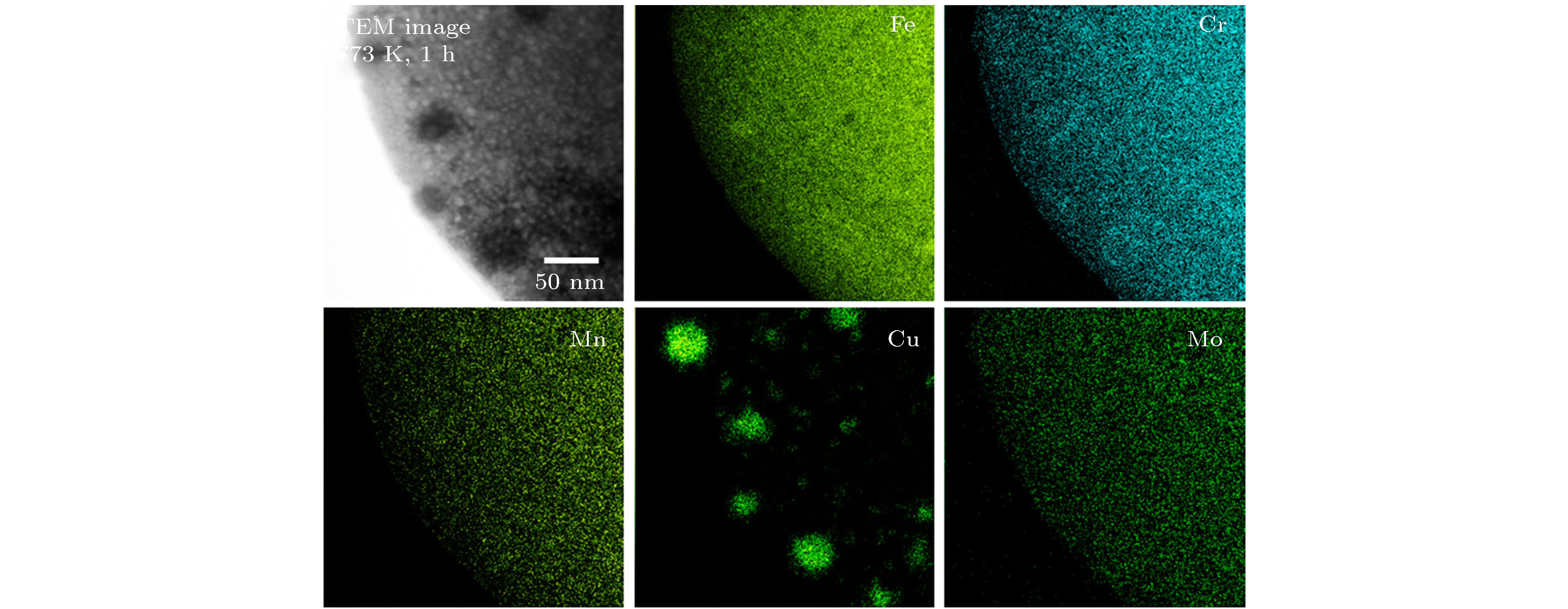
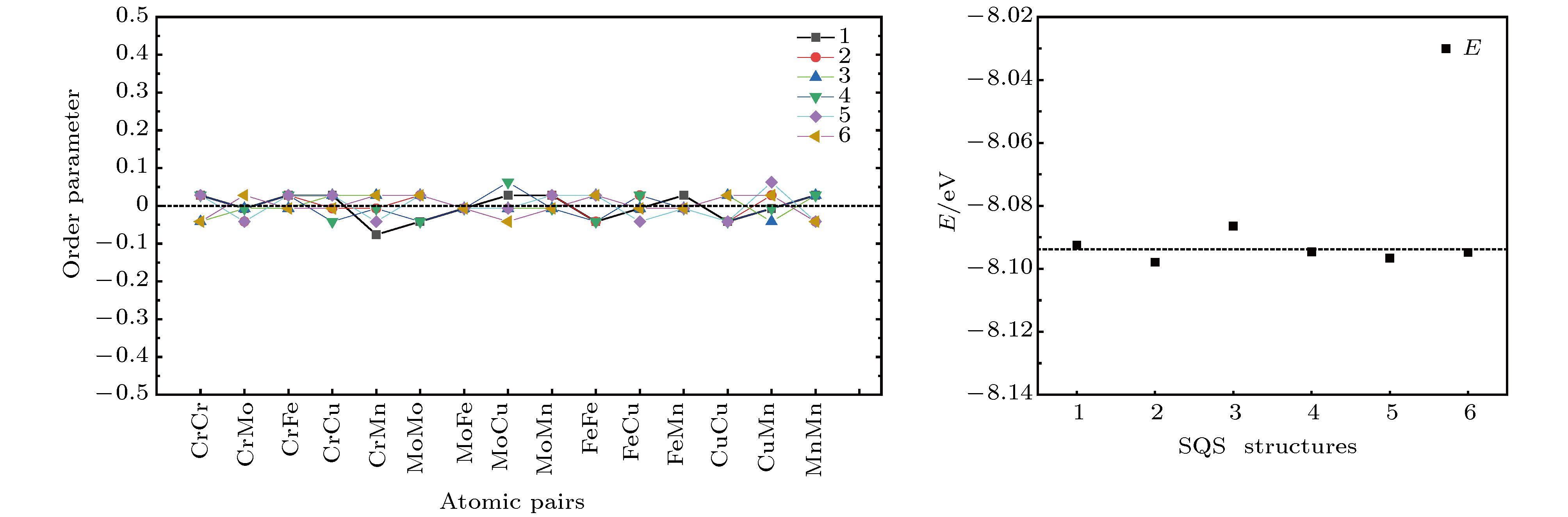
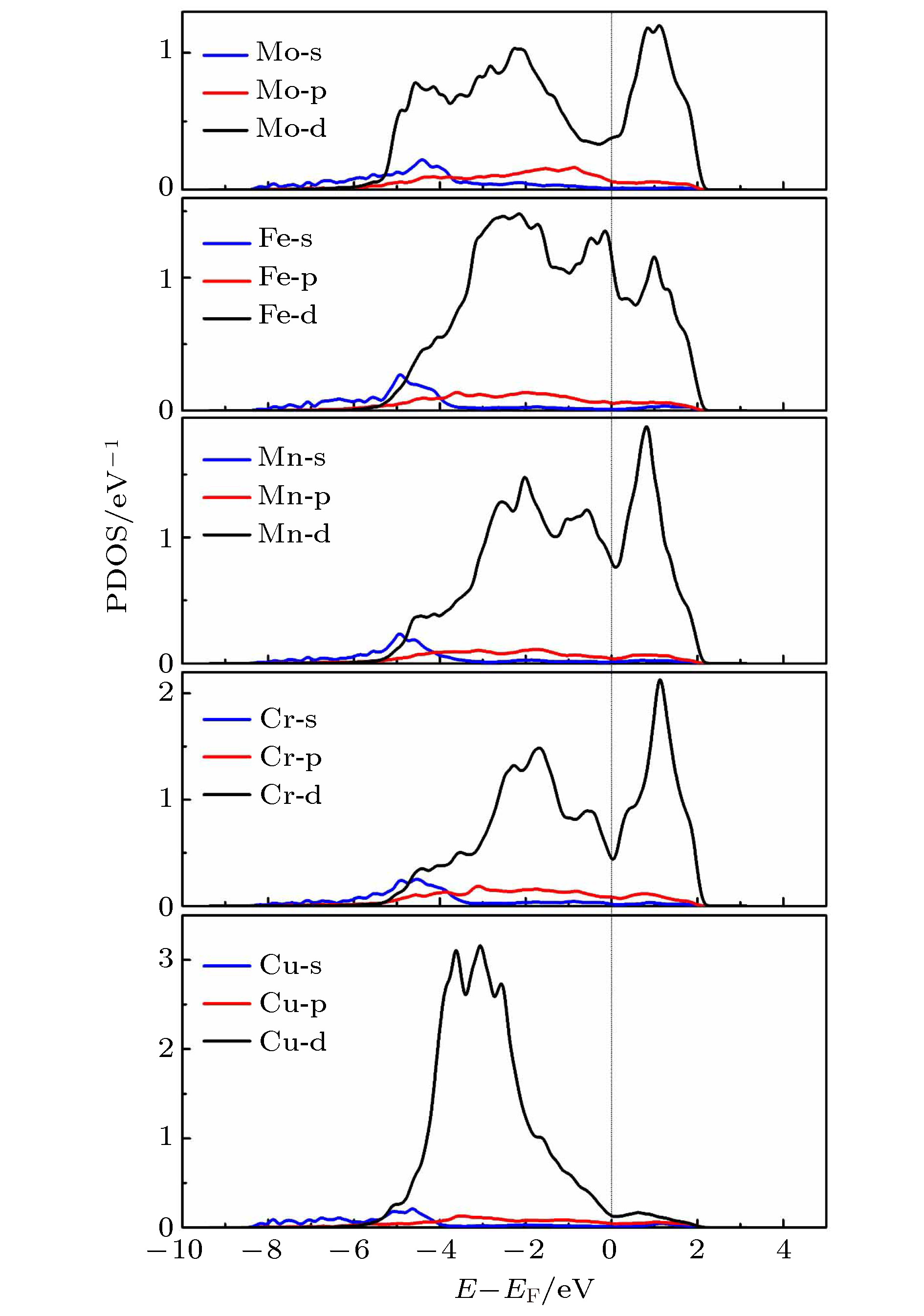
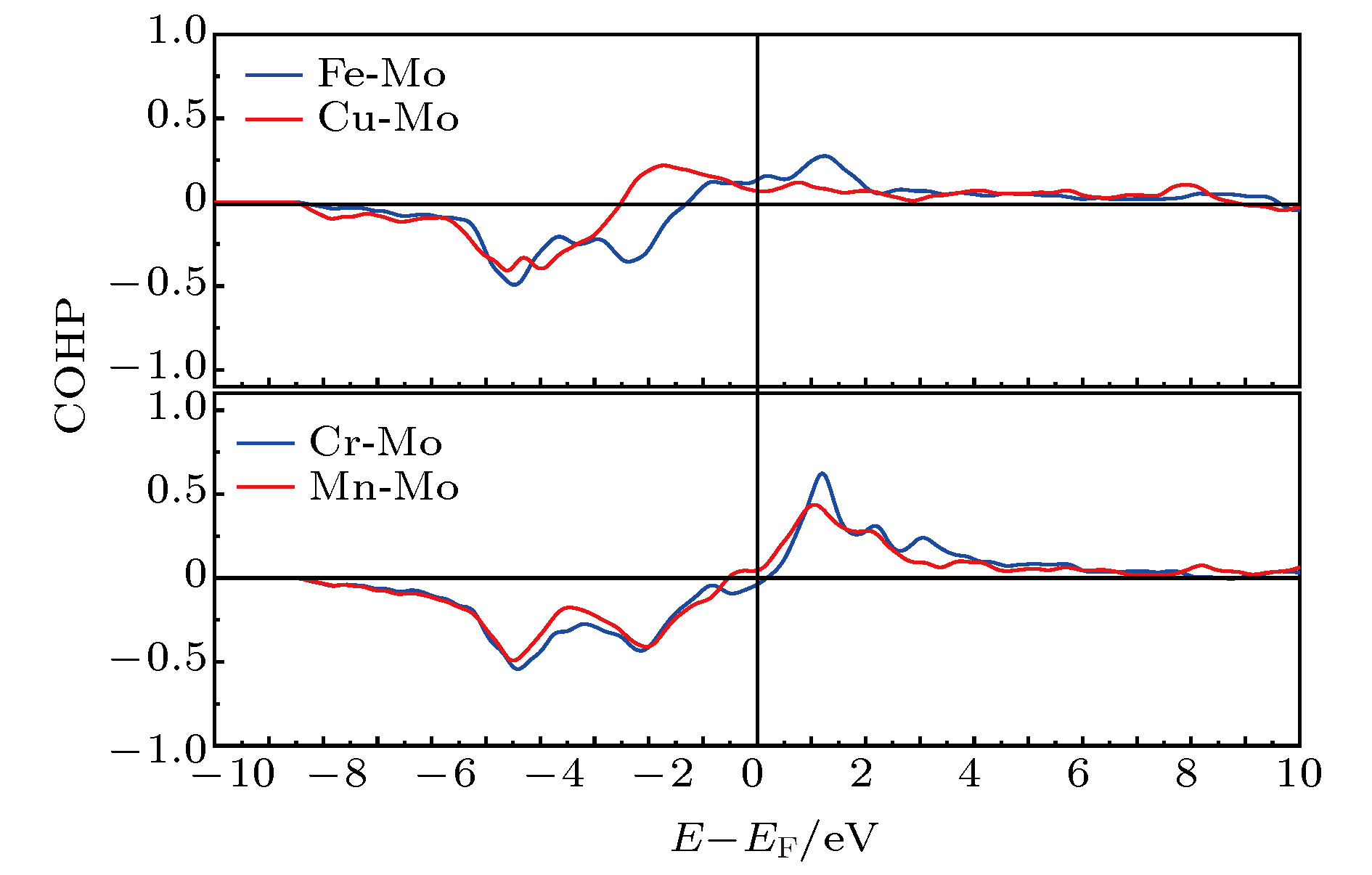
The prediction of stable state of high-entropy alloys (HEAs) is crucial to obtain fundamental insight to the excellent properties of HEAs. Taking a FeCuCrMnMo alloy as a case study, we combined Monte Carlo (MC) method with the density functional thoery (DFT) calculations (MC/DFT) to predict the equilibrium structure of high-entropy alloys in a finite unit cell. Instead of approaching the ideal random state obtained from special quasi-random approximation (SQS) method, physical factors such as atomic size, mixing enthalpy of atomic pairs, and interatomic interactions in the alloy are fully considered and implemented in our simulation by MC/DFT calculations. MC codes ensure the energy convergence of the system to the equilibrium state through the atom exchange process. The equilibrium structures exhibit Cu-rich short-range orders (SRO), which is consistent with the observation in experiments. Comparing with ideal random state structure, SRO structure is more stable in energy, and more closely packed in atomic arrangement. Moreover, the analyses of order parameters and radial distribution functions (RDFs) are performed to character the structure of high-entropy alloy. The order parameter of Cu-Cu atomic pair reaches to –0.53 in the SRO equilibrium structure, which indicates that Cu-rich regions appear in the alloy. The RDFs show that the atomic distance distribution of the SRO structure is between 2.25 Å to 2.7 Å, which is smaller than the range of 2.16 Å to 2.84 Å in the SQS structure, indicating that the lattice distortions is relatively small in the SRO structure after structural optimization. The appearing of SRO phenomena is attributed to the inherent characteristics of atoms, including (i) atomic size, (ii) interatomic mixing enthalpy and (iii) the interaction of atoms. Atomic sizes in the FeCuCrMnMo alloy are in the order of Fe (11.78) < Cu (11.81) < Cr (11.97) < Mn (14.38) < Mo (15.58), in unit of Å3/atom. The relatively large sizes of Mn and Mo atoms should disadvantage the pairing of Mo-Mo and Mn-Mn. The mixing enthalpy of Cu with other atoms are all positive values, indicating that Cu is not favor of pairing other elements and precipitate itself. The analyses of density of state (DOS) and Crystal Orbital Hamilton Population (COHP) also support the results. The reason is exactly attributed to the inactive valence electrons of Cu. Furthermore, the effect of SRO on the magnetic and mechanical properties are investigated. The existence of SRO decreases the mean value of magnetic moment, and results in an increase of elastic moduli (B, G and E) and a decrease in the ductility and anisotropy properties.
-
Keywords:
- first-principles calculations /
- Monte Carlo method /
- short range order /
- high entropy alloys
[1] Yeh J W, Chen S K, Lin S J, Gan J Y, Chin T S, Shun T T, Tsau C H, Chang S Y 2004 Adv. Eng. Mater. 6 299
 Google Scholar
Google Scholar
[2] Zhang W, Liaw P K, Zhang Y 2018 Sci. China. Mater. 61 2
 Google Scholar
Google Scholar
[3] Cantor B, Chang I T H, Knight P, Vincent A J B 2004 Mater. Sci. Eng. A 213 375
[4] Granberg F, Nordlund K, Ullah M W, Jin K, Lu C, Bei H, Wang L M, Djurabekova F, Weber W J, Zhang Y 2016 Phys. Rev. Lett. 116 135504
 Google Scholar
Google Scholar
[5] Lu C, Niu L, Chen N, Jin K, Yang T, Xiu P, Zhang Y, Gao F, Bei H, Shi S, He M R, Robertson I M, Weber W J, Wang L 2016 Nat. Commun. 7 13564
 Google Scholar
Google Scholar
[6] Kumar N A P K, Li C, Leonard K J, Bei H, Zinkle S J 2016 Acta Mater. 113 230
 Google Scholar
Google Scholar
[7] Zhong Z H, Xu Q, Mori K, Tokitani M 2019 Philos. Mag. 99 1515
 Google Scholar
Google Scholar
[8] Tian F 2017 Front. Mater. 4
 Google Scholar
Google Scholar
[9] Soven P 1967 Phys. Rev. 156 809
 Google Scholar
Google Scholar
[10] Abrikosov I A, Simak S I, Johansson B, Ruban A V, Skriver H L 1997 Phys. Rev. B 56 9319
 Google Scholar
Google Scholar
[11] Zunger A, Wei S, Ferreira L G, Bernard J E 1990 Phys. Rev. Lett. 65 353
 Google Scholar
Google Scholar
[12] van de Walle A, Tiwary P, de Jong M, Olmsted D L, Asta M, Dick A, Shin D, Wang Y, Chen L Q, Liu Z K 2013 Calphad 42 13
 Google Scholar
Google Scholar
[13] Shang S L, Wang Y, Kim D E, Zacherl C L, Du Y, Liu Z K 2011 Phys. Rev. B 83 144204
 Google Scholar
Google Scholar
[14] Pickering E J, Muñoz-Moreno R, Stone H J, Jones N G 2016 Scripta Mater. 113 106
 Google Scholar
Google Scholar
[15] Santodonato L J, Zhang Y, Feygenson M, Parish C M, Gao M C, Weber R J, Neuefeind J C, Tang Z, Liaw P K 2015 Nat. Commun. 6 5964
 Google Scholar
Google Scholar
[16] Singh S, Wanderka N, Murty B S, Glatzel U, Banhart J 2011 Acta Mater. 59 182
 Google Scholar
Google Scholar
[17] Kresse G, Furthmüller J 1996 Phys. Rev. B 54 11169
 Google Scholar
Google Scholar
[18] Feng W Q, Qi Y, Wang S Q 2017 Metals 7 482
 Google Scholar
Google Scholar
[19] Niu C, Windl W, Ghazisaeidi M 2017 Scripta Mater. 132 9
 Google Scholar
Google Scholar
[20] Ren X L, Shi P H, Zhang W W, Wu X Y, Xu Q, Wang Y X 2019 Acta Mater. 180 189
 Google Scholar
Google Scholar
[21] Cowley J M 1950 Phys. Rev. 77 669
 Google Scholar
Google Scholar
[22] Jiang D E, Carter E A 2004 Phys. Rev. B 70 064102
 Google Scholar
Google Scholar
[23] Brandes E A, Brook G B 1992 Smithells Metals Reference Book (7th Ed.) (Oxford: Reed Educational and Publishing Ltd.) pp129−136
[24] Takeuchi A, Inoue A 2005 Mater. Trans. 46 2817
 Google Scholar
Google Scholar
[25] Richard D 1993 J. Phys. Chem. 97 8617
 Google Scholar
Google Scholar
[26] 姚宝殿, 胡桂青, 于治水, 张慧芬, 施立群, 沈浩, 王月霞 2016 65 026202
 Google Scholar
Google Scholar
Yao B D, Hu G Q, Yu Z S, Zhang H F, Shi L Q, Shen H, Wang Y X 2016 Acta Phys. Sin. 65 026202
 Google Scholar
Google Scholar
[27] Ding Y C, Chen M, Gao X Y, Jiang M H 2012 Chin. Phys. B 21 067101
 Google Scholar
Google Scholar
[28] Feng J 2014 APL Mater. 2 081801
 Google Scholar
Google Scholar
[29] Ding Y, Chen M, Wu W 2014 Phys. B: Condens. Matter 433 48
 Google Scholar
Google Scholar
[30] 王浩玉, 农智学, 王继杰, 朱景川 2019 68 136401
Wang H Y, Nong Z S, Wang J J, Zhu J C 2019 Acta Phys. Sin. 68 136401
[31] Miao N, Sa B, Zhou J, Sun Z 2011 Comput. Mater. Sci. 50 1559
 Google Scholar
Google Scholar
[32] Gueler E, Gueler M 2015 Chin. J. Phys. 53 040807
-
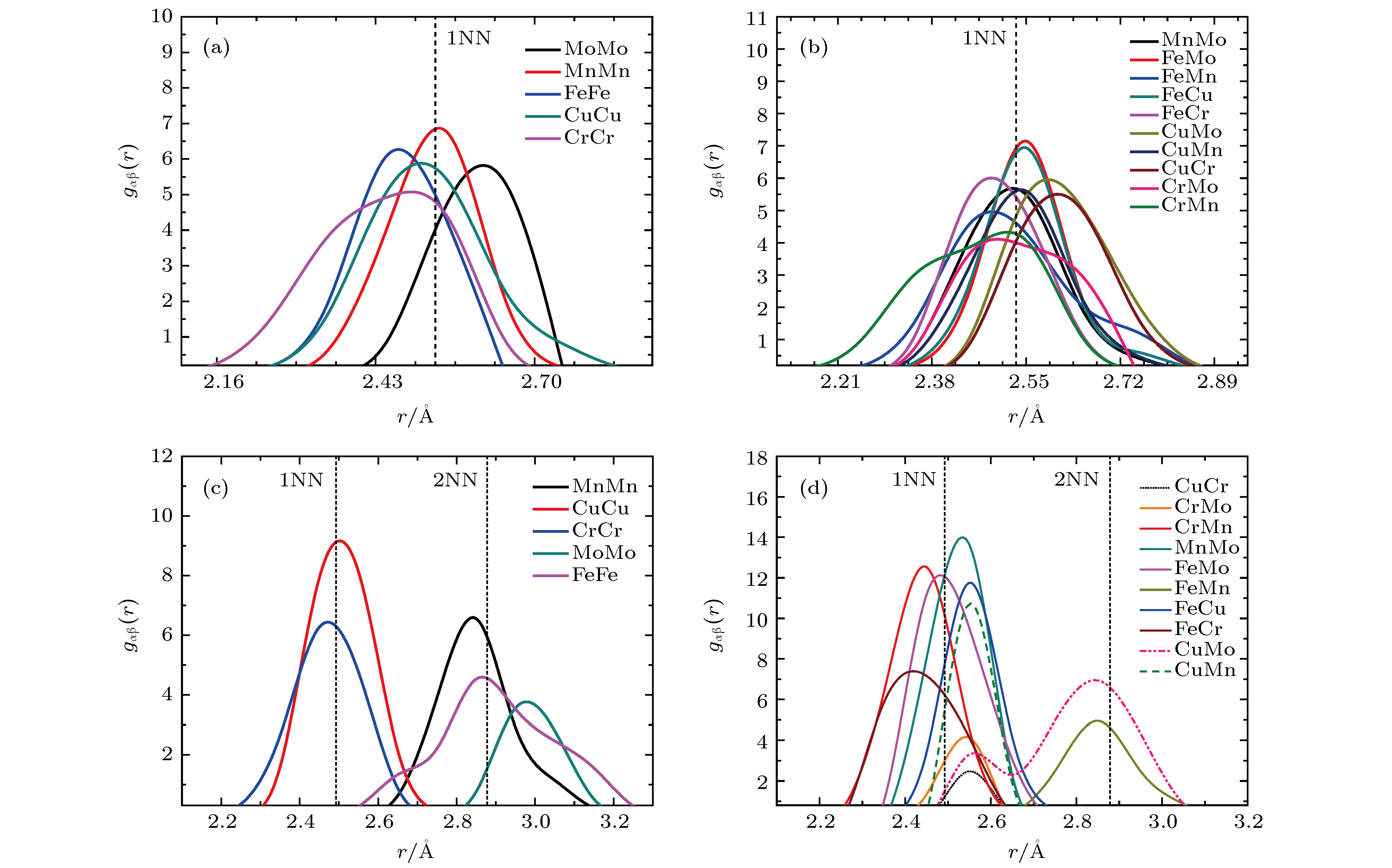
图 6 高熵合金结构优化后原子对间径向分布函数图 (a), (b) SQS结构; (c), (d) SRO结构. 虚线标明无畸变晶格中第一近邻(1NN)和第二近邻(2NN)的距离值
Figure 6. Partial pair distribution functions of FeCuCrMnMo alloy: (a), (b) SQS structure; (c), (d) SRO structure. Dotted lines show the distances of first nearest neighbor (1NN) and second nearest neighbor in unrelaxed lattice.
表 1 FeCuCrMnMo合金的SRO结构、SQS结构及相关纯金属的平均单个原子能、平均单个原子体积和晶格常数值. 其中FM, NM和AFM分别代表铁磁性、顺磁性和反铁磁性
Table 1. Cohesive energy per atom, structural volume per atom, and lattice parameters for SRO and SQS structures, and Fe, Cu, Cr, Mn, Mo, and W metals. FM, AFM, and NM denote ferromagnetism, antiferromagnetism, and nonmagnetic, respectively.
表 2 FeCuCrMnMo合金中不同原子对间的混合焓[24] (单位: KJ/mol)
Table 2. Enthalpy of mixing of binary systems containing the elements in FeCuCrMnMo alloy(Unit: KJ/mol).
混合焓 Cr Mn Mo Fe Cu 12 4 19 13 Cr 2 0 –1 Mn 5 0 Mo –2 表 3 FeCuCrMnMo合金中不同原子对的ICOHP平均值
Table 3. Mean values of ICOHPs for each atomic pair in FeCuCrMnMo alloy.
原子对 Cu-Fe Cu-Mn Cu-Mo Cu-Cr Cu-Cu ICOHP –0.54 –0.59 –0.88 –0.66 –0.39 原子对 Fe-Cr Fe-Mo Mn-Mo Cr-Mo Cr-Mn ICOHP –1.45 –1.68 –1.72 –1.78 –1.61 表 4 FeCuCrMnMo合金SRO结构和SQS结构的力学性质, 其中弹性模量的单位为GPa
Table 4. Calculated mechanical properties for SRO structure and SQS structure of FeCuCrMnMo alloy. The unit for the elastic moduli is GPa.
结构 C11 C12 C44 B BEOS G E ν G/B AZ SRO 263.4 187.5 100.5 212.8 188.7 88.2 232.5 0.318 2.415 2.65 SQS 156.1 139.3 85.1 144.9 154.4 43.4 118.4 0.364 3.323 10.1 -
[1] Yeh J W, Chen S K, Lin S J, Gan J Y, Chin T S, Shun T T, Tsau C H, Chang S Y 2004 Adv. Eng. Mater. 6 299
 Google Scholar
Google Scholar
[2] Zhang W, Liaw P K, Zhang Y 2018 Sci. China. Mater. 61 2
 Google Scholar
Google Scholar
[3] Cantor B, Chang I T H, Knight P, Vincent A J B 2004 Mater. Sci. Eng. A 213 375
[4] Granberg F, Nordlund K, Ullah M W, Jin K, Lu C, Bei H, Wang L M, Djurabekova F, Weber W J, Zhang Y 2016 Phys. Rev. Lett. 116 135504
 Google Scholar
Google Scholar
[5] Lu C, Niu L, Chen N, Jin K, Yang T, Xiu P, Zhang Y, Gao F, Bei H, Shi S, He M R, Robertson I M, Weber W J, Wang L 2016 Nat. Commun. 7 13564
 Google Scholar
Google Scholar
[6] Kumar N A P K, Li C, Leonard K J, Bei H, Zinkle S J 2016 Acta Mater. 113 230
 Google Scholar
Google Scholar
[7] Zhong Z H, Xu Q, Mori K, Tokitani M 2019 Philos. Mag. 99 1515
 Google Scholar
Google Scholar
[8] Tian F 2017 Front. Mater. 4
 Google Scholar
Google Scholar
[9] Soven P 1967 Phys. Rev. 156 809
 Google Scholar
Google Scholar
[10] Abrikosov I A, Simak S I, Johansson B, Ruban A V, Skriver H L 1997 Phys. Rev. B 56 9319
 Google Scholar
Google Scholar
[11] Zunger A, Wei S, Ferreira L G, Bernard J E 1990 Phys. Rev. Lett. 65 353
 Google Scholar
Google Scholar
[12] van de Walle A, Tiwary P, de Jong M, Olmsted D L, Asta M, Dick A, Shin D, Wang Y, Chen L Q, Liu Z K 2013 Calphad 42 13
 Google Scholar
Google Scholar
[13] Shang S L, Wang Y, Kim D E, Zacherl C L, Du Y, Liu Z K 2011 Phys. Rev. B 83 144204
 Google Scholar
Google Scholar
[14] Pickering E J, Muñoz-Moreno R, Stone H J, Jones N G 2016 Scripta Mater. 113 106
 Google Scholar
Google Scholar
[15] Santodonato L J, Zhang Y, Feygenson M, Parish C M, Gao M C, Weber R J, Neuefeind J C, Tang Z, Liaw P K 2015 Nat. Commun. 6 5964
 Google Scholar
Google Scholar
[16] Singh S, Wanderka N, Murty B S, Glatzel U, Banhart J 2011 Acta Mater. 59 182
 Google Scholar
Google Scholar
[17] Kresse G, Furthmüller J 1996 Phys. Rev. B 54 11169
 Google Scholar
Google Scholar
[18] Feng W Q, Qi Y, Wang S Q 2017 Metals 7 482
 Google Scholar
Google Scholar
[19] Niu C, Windl W, Ghazisaeidi M 2017 Scripta Mater. 132 9
 Google Scholar
Google Scholar
[20] Ren X L, Shi P H, Zhang W W, Wu X Y, Xu Q, Wang Y X 2019 Acta Mater. 180 189
 Google Scholar
Google Scholar
[21] Cowley J M 1950 Phys. Rev. 77 669
 Google Scholar
Google Scholar
[22] Jiang D E, Carter E A 2004 Phys. Rev. B 70 064102
 Google Scholar
Google Scholar
[23] Brandes E A, Brook G B 1992 Smithells Metals Reference Book (7th Ed.) (Oxford: Reed Educational and Publishing Ltd.) pp129−136
[24] Takeuchi A, Inoue A 2005 Mater. Trans. 46 2817
 Google Scholar
Google Scholar
[25] Richard D 1993 J. Phys. Chem. 97 8617
 Google Scholar
Google Scholar
[26] 姚宝殿, 胡桂青, 于治水, 张慧芬, 施立群, 沈浩, 王月霞 2016 65 026202
 Google Scholar
Google Scholar
Yao B D, Hu G Q, Yu Z S, Zhang H F, Shi L Q, Shen H, Wang Y X 2016 Acta Phys. Sin. 65 026202
 Google Scholar
Google Scholar
[27] Ding Y C, Chen M, Gao X Y, Jiang M H 2012 Chin. Phys. B 21 067101
 Google Scholar
Google Scholar
[28] Feng J 2014 APL Mater. 2 081801
 Google Scholar
Google Scholar
[29] Ding Y, Chen M, Wu W 2014 Phys. B: Condens. Matter 433 48
 Google Scholar
Google Scholar
[30] 王浩玉, 农智学, 王继杰, 朱景川 2019 68 136401
Wang H Y, Nong Z S, Wang J J, Zhu J C 2019 Acta Phys. Sin. 68 136401
[31] Miao N, Sa B, Zhou J, Sun Z 2011 Comput. Mater. Sci. 50 1559
 Google Scholar
Google Scholar
[32] Gueler E, Gueler M 2015 Chin. J. Phys. 53 040807
Catalog
Metrics
- Abstract views: 21858
- PDF Downloads: 857
- Cited By: 0















 DownLoad:
DownLoad: