-
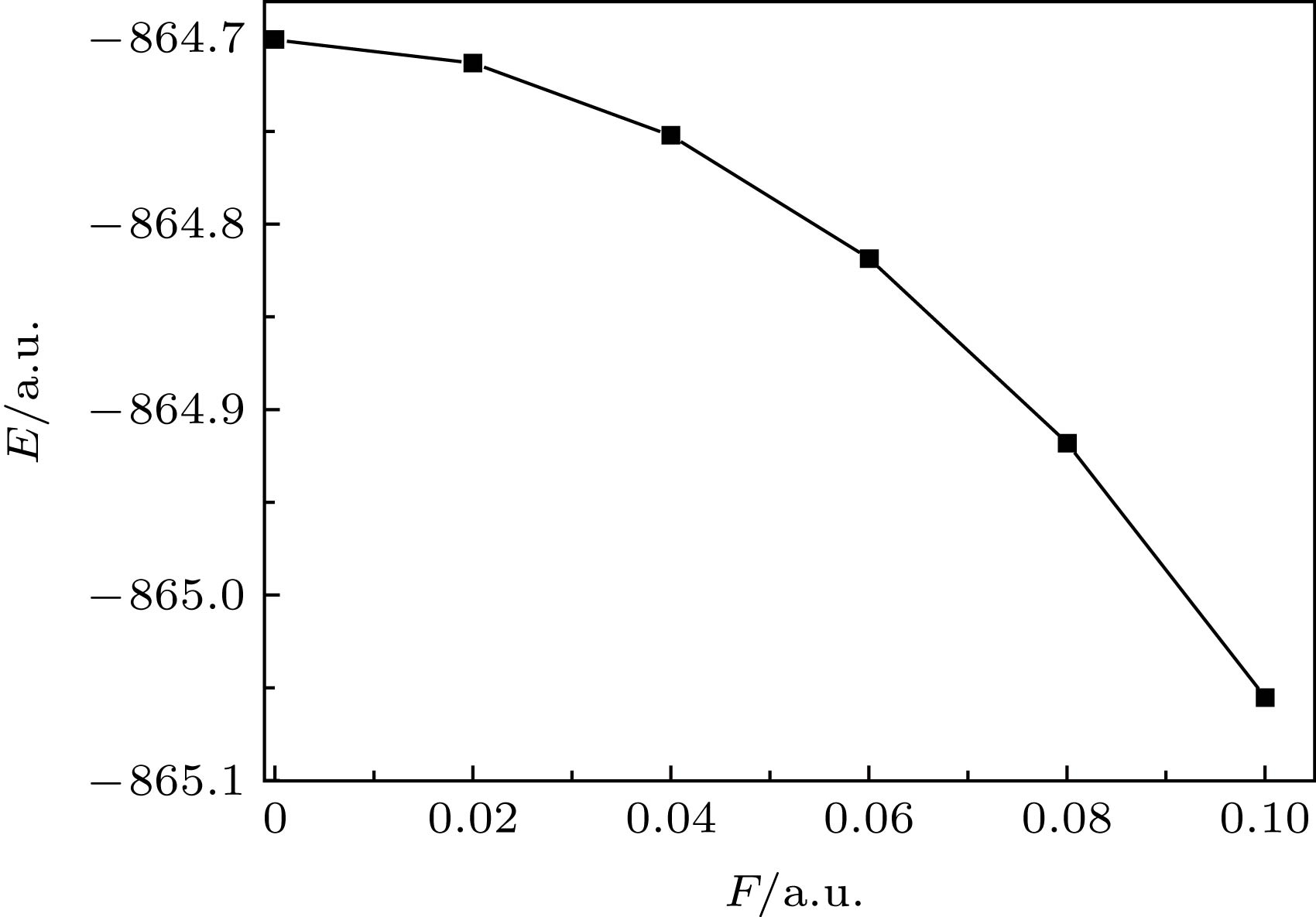
A variety of new materials have improved the production and life of human beings. Two-dimensional nano materials have become a research hotspot due to their unique physical and chemical properties. Molybdenum disulfide (MoS2) is representative of transition metal sulfide, with excellent mechanical properties and chemical stability. In order to study the influence of external electrical field on the molecular structure and spectrum, here in this work, the density functional theory with the hybrid B3LYP at Def2-TZVP level is employed to calculate the geometrical parameters of the ground state of MoS2 molecule under external electric fields ranging from 0 to 0.1 a.u. (0−5.1423 × 1010 V/m). Based on the optimized structures, the time-dependent density functional theory at the same level as the above is adopted to calculate the absorption wavelengths and the molar absorption coefficients for the first ten excited states of MoS2 molecule under external electric fields. The results show that the most strongest absorption band is located at 483 nm with a molar absorption coefficient of 461 L·mol–1·cm–1 in the UV-Vis absorption spectrum. The intramolecular charge transfers as a whole with the enhancement of the external electric field. The electric dipole moment increases with the external electric field rising, while the total molecular energy decreases with external electric field increasing. With the enhancement of the external electric field, the absorption peaks show a significant redshift. When the electric field increases to 0.1 a.u., the redshift is obvious. This can be explained as follows. When the external electric field is weaker, the electron transfer in the molecule is not significant. However, with the augment of the external electric field, the electron transfer in the molecule occurs as a whole. This makes the electron interaction between Mo and S weaker, thus the electron transition is more likely to occur. The energy required for excitation is reduced, and the wavelength of the excited state becomes longer, that is, the absorption peak takes a redshift. With the enhancement of the external electric field, the molar absorption coefficient increases obviously. This is because the overall transfer of the external electric field to the electron makes the electron cloud density of the MoS2 molecule increase and the number of electrons in transition augment. This work provides a theoretical basis for the utilization and improvement of MoS2 photoelectric properties, and also enlightens the application research of other photoelectric materials.
[1] Ayari A, Cobas E, Ogundadegbe O, Fuhrer M S 2007 J. Appl. Phys. 101 014507
 Google Scholar
Google Scholar
[2] Radisavljevic B, Radenovic A, Brivio J, Giacometti V, Kis A 2011 Nat. Nanotechnol. 6 147
 Google Scholar
Google Scholar
[3] 陈敏强 2017 硕士学位论文 (太原: 太原理工大学)
Chen M Q 2017 M. S. Thesis (Taiyuan: Taiyuan University of Technology) (in Chinese)
[4] Dominko R, Arcon D, Mrzel A, Zorko A, Cevc P, Venturini P, Gaberscek M, Remskar M, Mihailovic D 2002 Adv. Mater. 14 1531
 Google Scholar
Google Scholar
[5] 孙华婷 2018 硕士学位论文 (合肥: 安徽大学)
Sun H T 2018 M. S. Thesis (Hefei: Anhui University) (in Chinese)
[6] Mohammad H, Amir B F, Narayana R A 2015 Nat. Comms. 6 8616
 Google Scholar
Google Scholar
[7] Li W F, Yang Y M, Weber J K, Zhang G, Zhou R H 2016 ACS Nano 10 1829
 Google Scholar
Google Scholar
[8] Jafar A, Alireza K 2017 Comput. Mater. Sci. 137 201
 Google Scholar
Google Scholar
[9] Mateus H K, Jose R B, Marcia C B 2018 J. Chem. Phys. 148 222804
 Google Scholar
Google Scholar
[10] Wang P, Li W, Du C C, Zheng X, Sun X L, Yan Y G, Zhang J 2017 Comput. Mater. Sci. 140 284
 Google Scholar
Google Scholar
[11] Zhang Y D, Meng Z S, Shi Q, Gao H Q, Liu Y Z, Wang Y H, Rao D W, Deng K M, Lu R F 2017 J. Phys.: Condens. Matter 29 375201
 Google Scholar
Google Scholar
[12] Ellert C, Corkum P B 1999 Phys. Rev. A 59 R3170
 Google Scholar
Google Scholar
[13] 王藩侯, 黄多辉, 杨俊升 2013 62 073102
 Google Scholar
Google Scholar
Wang F H, Huang D H, Yang J S 2013 Acta Phys. Sin. 62 073102
 Google Scholar
Google Scholar
[14] Ledingham K W D, Singhal R P, Smith D J, McCanny T, Graham P, Kilic H S, Peng W X, Wang S L, Langley A J, Taday P F, Kosmidis C 1998 J. Phys. Chem. A 102 3002
 Google Scholar
Google Scholar
[15] Rai D, Joshi H, Kulkarni A D, Gejji S P, Pathak R K 2007 J. Phys. Chem. A 111 9111
 Google Scholar
Google Scholar
[16] Iwamae A, Hishikawa A, Yamanouchi K 2000 J. Phys. B: At. Mol. Opt. Phys. 33 223
 Google Scholar
Google Scholar
[17] Ellert C, Stapelfeldt H, Constant E 1998 Phil. Trans. R. Sol. Lond. A 356 329
 Google Scholar
Google Scholar
[18] 杜建宾, 冯志芳, 韩丽君, 唐延林, 武德起 2018 67 223101
 Google Scholar
Google Scholar
Du J B, Feng Z F, Han L J, Tang Y L, Wu D Q 2018 Acta Phys. Sin. 67 223101
 Google Scholar
Google Scholar
[19] 李世雄, 吴永刚, 令狐荣锋, 孙光宇, 张正平, 秦水介 2015 64 043101
 Google Scholar
Google Scholar
Li S X, Wu Y G, Linghu R F, Sun G Y Zhang Z P, Qin S J 2015 Acta Phys. Sin. 64 043101
 Google Scholar
Google Scholar
[20] 谢安东, 谢晶, 周玲玲, 伍冬兰, 阮文, 罗文浪 2016 原子与分子 33 989
Xie A D, Xie J, Zhou L L, Wu D L, Ruan W, Luo W L 2016 Chin. J. Atom. Mol. Phys. 33 989
[21] Liu Q H, Li L Z, Li Y F, Gao Z X, Chen Z F, Lu J 2012 J. Phys. Chem. C 116 21556
 Google Scholar
Google Scholar
[22] Gemming S, Seifert G, Götz M, Fischer T, Ganteför G 2010 Phys. Status Solidi B 247 1069
 Google Scholar
Google Scholar
[23] Peverati R, Truhlar D G 2012 Phys. Chem. Chem. Phys. 14 13171
 Google Scholar
Google Scholar
[24] Liang B Y, Andrews L 2002 J. Phys. Chem. A 106 6945
 Google Scholar
Google Scholar
[25] Mayhall N J, Becher E L, Chowdhury A, Raghavachari K 2011 J. Phys. Chem. A 115 2291
 Google Scholar
Google Scholar
[26] Wang B, Wu N, Zhang X B, Huang X, Zhang Y F, Chen W K, Ding K N 2013 J. Phys. Chem. A 117 5632
 Google Scholar
Google Scholar
[27] Wang Y Y, Deng J J, Wang X, Che J T, Ding X L 2018 Phys. Chem. Chem. Phys. 20 6365
 Google Scholar
Google Scholar
[28] Wu D L, Tan B, Wan H J, Zang X Q, Xie A D 2013 Chin. Phys. B 22 123101
 Google Scholar
Google Scholar
[29] Grozema F C, Telesca R, Joukman H T, Snijders J G 2001 J. Chem. Phys. 115 10014
 Google Scholar
Google Scholar
[30] 朱正和, 付依备, 高涛, 陈银亮, 陈晓军 2003 原子与分子 20 169
 Google Scholar
Google Scholar
Zhu Z H, Fu Y B, Gao T, Chen Y L, Chen X J 2003 Chin. J. Atom. Mol. Phys. 20 169
 Google Scholar
Google Scholar
[31] Gemming S, Tamuliene J, Seifert G, Bertram N, Kim Y D, Ganteför G, 2006 Appl. Phys. A: Mater. Sci. Process. 82 161
 Google Scholar
Google Scholar
[32] Murugan P, Kumar V, Kawazoe Y, Ota N 2005 Phys. Rev. A: At. Mol. Opt. Phys. 71 063203
 Google Scholar
Google Scholar
[33] Jelena P, Jasna V, Tijana T I, Marko S, Radoš G 2018 Opt. Quant. Electron. 50 291
 Google Scholar
Google Scholar
-
图 6 不同外电场下MoS2激发态的分子轨道(占据轨道, 填充了电子的分子轨道; 跃迁轨道, 分子被激发时, 电子从占据轨道跃迁到的空轨道; 轨道权重, 分子某一激发态, 构成轨道的各个组成部分的贡献, 图中给出的是所占比例最大的部分)
Figure 6. Excited state orbital diagram of MoS2 molecule under different external electric fields (occupied orbitals, molecular orbitals filled with electrons; a transition orbital is a vacant orbital that an electron jumps from an occupied orbital to when a molecule is excited; orbital weight, the contribution of each component of an excited state of a molecule to the composition of the orbital, shown in the figure is the one with the largest proportion).
表 1 不同电场下原子Mo和S的电荷Q/a.u.
Table 1. The charges of Mo and S (unit: a.u.) of the MoS2 molecule under different external electric fields.
F 0 0.02 0.04 0.06 0.08 0.10 1Mo 0.782 0.752 0.659 0.488 0.237 –0.069 2S –0.391 –0.376 –0.329 –0.244 –0.119 0.034 3S –0.391 –0.376 –0.329 –0.244 –0.119 0.034 -
[1] Ayari A, Cobas E, Ogundadegbe O, Fuhrer M S 2007 J. Appl. Phys. 101 014507
 Google Scholar
Google Scholar
[2] Radisavljevic B, Radenovic A, Brivio J, Giacometti V, Kis A 2011 Nat. Nanotechnol. 6 147
 Google Scholar
Google Scholar
[3] 陈敏强 2017 硕士学位论文 (太原: 太原理工大学)
Chen M Q 2017 M. S. Thesis (Taiyuan: Taiyuan University of Technology) (in Chinese)
[4] Dominko R, Arcon D, Mrzel A, Zorko A, Cevc P, Venturini P, Gaberscek M, Remskar M, Mihailovic D 2002 Adv. Mater. 14 1531
 Google Scholar
Google Scholar
[5] 孙华婷 2018 硕士学位论文 (合肥: 安徽大学)
Sun H T 2018 M. S. Thesis (Hefei: Anhui University) (in Chinese)
[6] Mohammad H, Amir B F, Narayana R A 2015 Nat. Comms. 6 8616
 Google Scholar
Google Scholar
[7] Li W F, Yang Y M, Weber J K, Zhang G, Zhou R H 2016 ACS Nano 10 1829
 Google Scholar
Google Scholar
[8] Jafar A, Alireza K 2017 Comput. Mater. Sci. 137 201
 Google Scholar
Google Scholar
[9] Mateus H K, Jose R B, Marcia C B 2018 J. Chem. Phys. 148 222804
 Google Scholar
Google Scholar
[10] Wang P, Li W, Du C C, Zheng X, Sun X L, Yan Y G, Zhang J 2017 Comput. Mater. Sci. 140 284
 Google Scholar
Google Scholar
[11] Zhang Y D, Meng Z S, Shi Q, Gao H Q, Liu Y Z, Wang Y H, Rao D W, Deng K M, Lu R F 2017 J. Phys.: Condens. Matter 29 375201
 Google Scholar
Google Scholar
[12] Ellert C, Corkum P B 1999 Phys. Rev. A 59 R3170
 Google Scholar
Google Scholar
[13] 王藩侯, 黄多辉, 杨俊升 2013 62 073102
 Google Scholar
Google Scholar
Wang F H, Huang D H, Yang J S 2013 Acta Phys. Sin. 62 073102
 Google Scholar
Google Scholar
[14] Ledingham K W D, Singhal R P, Smith D J, McCanny T, Graham P, Kilic H S, Peng W X, Wang S L, Langley A J, Taday P F, Kosmidis C 1998 J. Phys. Chem. A 102 3002
 Google Scholar
Google Scholar
[15] Rai D, Joshi H, Kulkarni A D, Gejji S P, Pathak R K 2007 J. Phys. Chem. A 111 9111
 Google Scholar
Google Scholar
[16] Iwamae A, Hishikawa A, Yamanouchi K 2000 J. Phys. B: At. Mol. Opt. Phys. 33 223
 Google Scholar
Google Scholar
[17] Ellert C, Stapelfeldt H, Constant E 1998 Phil. Trans. R. Sol. Lond. A 356 329
 Google Scholar
Google Scholar
[18] 杜建宾, 冯志芳, 韩丽君, 唐延林, 武德起 2018 67 223101
 Google Scholar
Google Scholar
Du J B, Feng Z F, Han L J, Tang Y L, Wu D Q 2018 Acta Phys. Sin. 67 223101
 Google Scholar
Google Scholar
[19] 李世雄, 吴永刚, 令狐荣锋, 孙光宇, 张正平, 秦水介 2015 64 043101
 Google Scholar
Google Scholar
Li S X, Wu Y G, Linghu R F, Sun G Y Zhang Z P, Qin S J 2015 Acta Phys. Sin. 64 043101
 Google Scholar
Google Scholar
[20] 谢安东, 谢晶, 周玲玲, 伍冬兰, 阮文, 罗文浪 2016 原子与分子 33 989
Xie A D, Xie J, Zhou L L, Wu D L, Ruan W, Luo W L 2016 Chin. J. Atom. Mol. Phys. 33 989
[21] Liu Q H, Li L Z, Li Y F, Gao Z X, Chen Z F, Lu J 2012 J. Phys. Chem. C 116 21556
 Google Scholar
Google Scholar
[22] Gemming S, Seifert G, Götz M, Fischer T, Ganteför G 2010 Phys. Status Solidi B 247 1069
 Google Scholar
Google Scholar
[23] Peverati R, Truhlar D G 2012 Phys. Chem. Chem. Phys. 14 13171
 Google Scholar
Google Scholar
[24] Liang B Y, Andrews L 2002 J. Phys. Chem. A 106 6945
 Google Scholar
Google Scholar
[25] Mayhall N J, Becher E L, Chowdhury A, Raghavachari K 2011 J. Phys. Chem. A 115 2291
 Google Scholar
Google Scholar
[26] Wang B, Wu N, Zhang X B, Huang X, Zhang Y F, Chen W K, Ding K N 2013 J. Phys. Chem. A 117 5632
 Google Scholar
Google Scholar
[27] Wang Y Y, Deng J J, Wang X, Che J T, Ding X L 2018 Phys. Chem. Chem. Phys. 20 6365
 Google Scholar
Google Scholar
[28] Wu D L, Tan B, Wan H J, Zang X Q, Xie A D 2013 Chin. Phys. B 22 123101
 Google Scholar
Google Scholar
[29] Grozema F C, Telesca R, Joukman H T, Snijders J G 2001 J. Chem. Phys. 115 10014
 Google Scholar
Google Scholar
[30] 朱正和, 付依备, 高涛, 陈银亮, 陈晓军 2003 原子与分子 20 169
 Google Scholar
Google Scholar
Zhu Z H, Fu Y B, Gao T, Chen Y L, Chen X J 2003 Chin. J. Atom. Mol. Phys. 20 169
 Google Scholar
Google Scholar
[31] Gemming S, Tamuliene J, Seifert G, Bertram N, Kim Y D, Ganteför G, 2006 Appl. Phys. A: Mater. Sci. Process. 82 161
 Google Scholar
Google Scholar
[32] Murugan P, Kumar V, Kawazoe Y, Ota N 2005 Phys. Rev. A: At. Mol. Opt. Phys. 71 063203
 Google Scholar
Google Scholar
[33] Jelena P, Jasna V, Tijana T I, Marko S, Radoš G 2018 Opt. Quant. Electron. 50 291
 Google Scholar
Google Scholar
Catalog
Metrics
- Abstract views: 14899
- PDF Downloads: 213
- Cited By: 0














 DownLoad:
DownLoad: