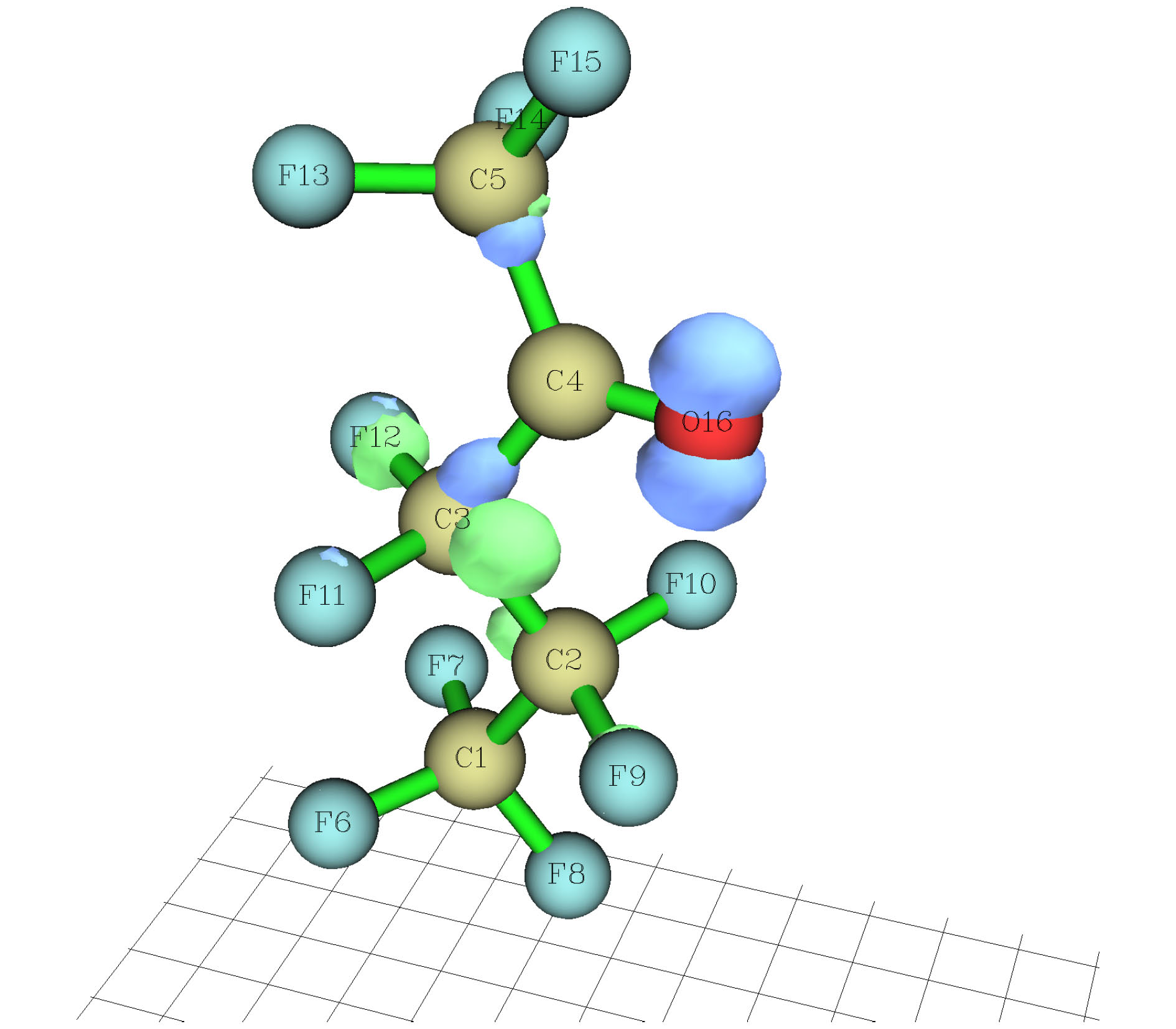
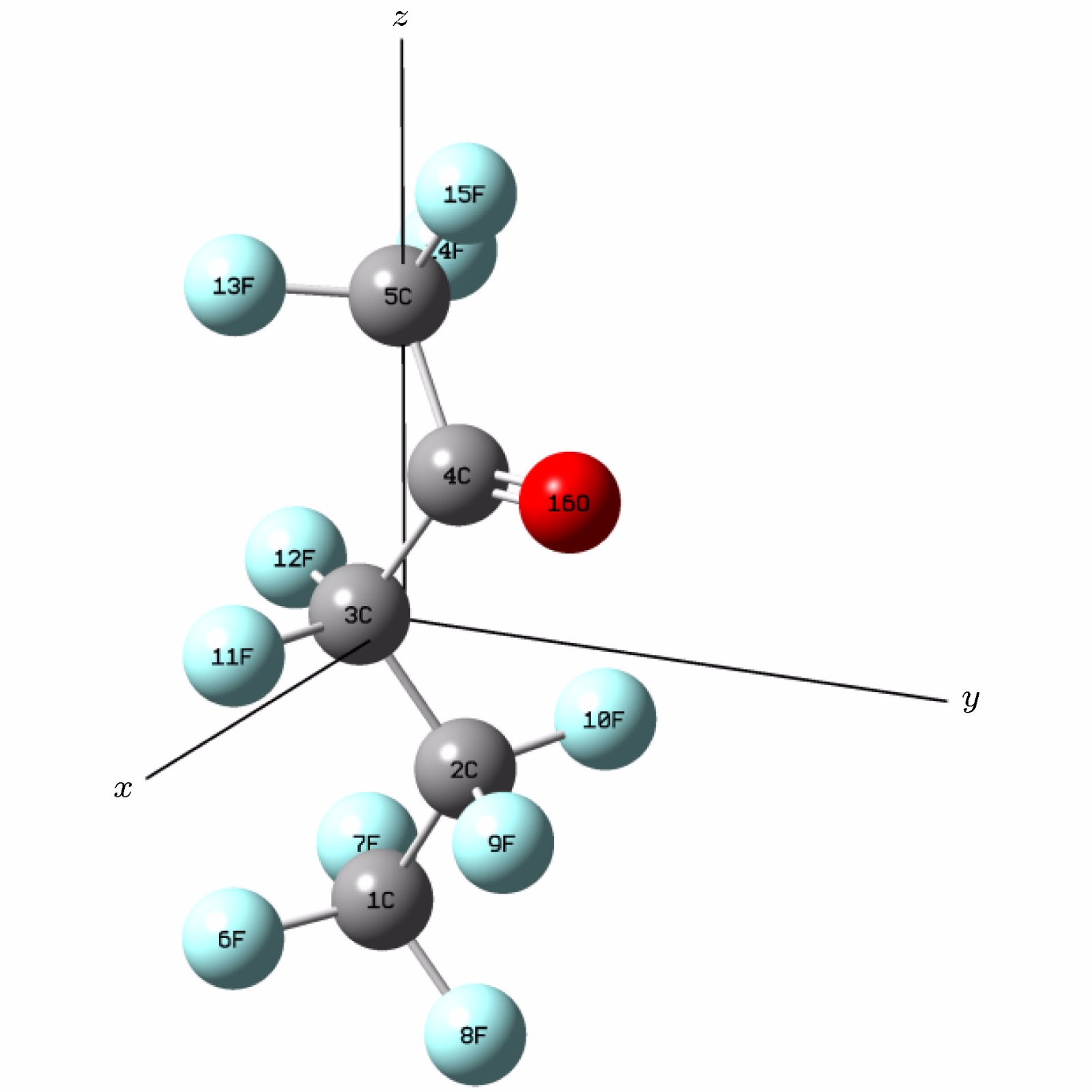
-
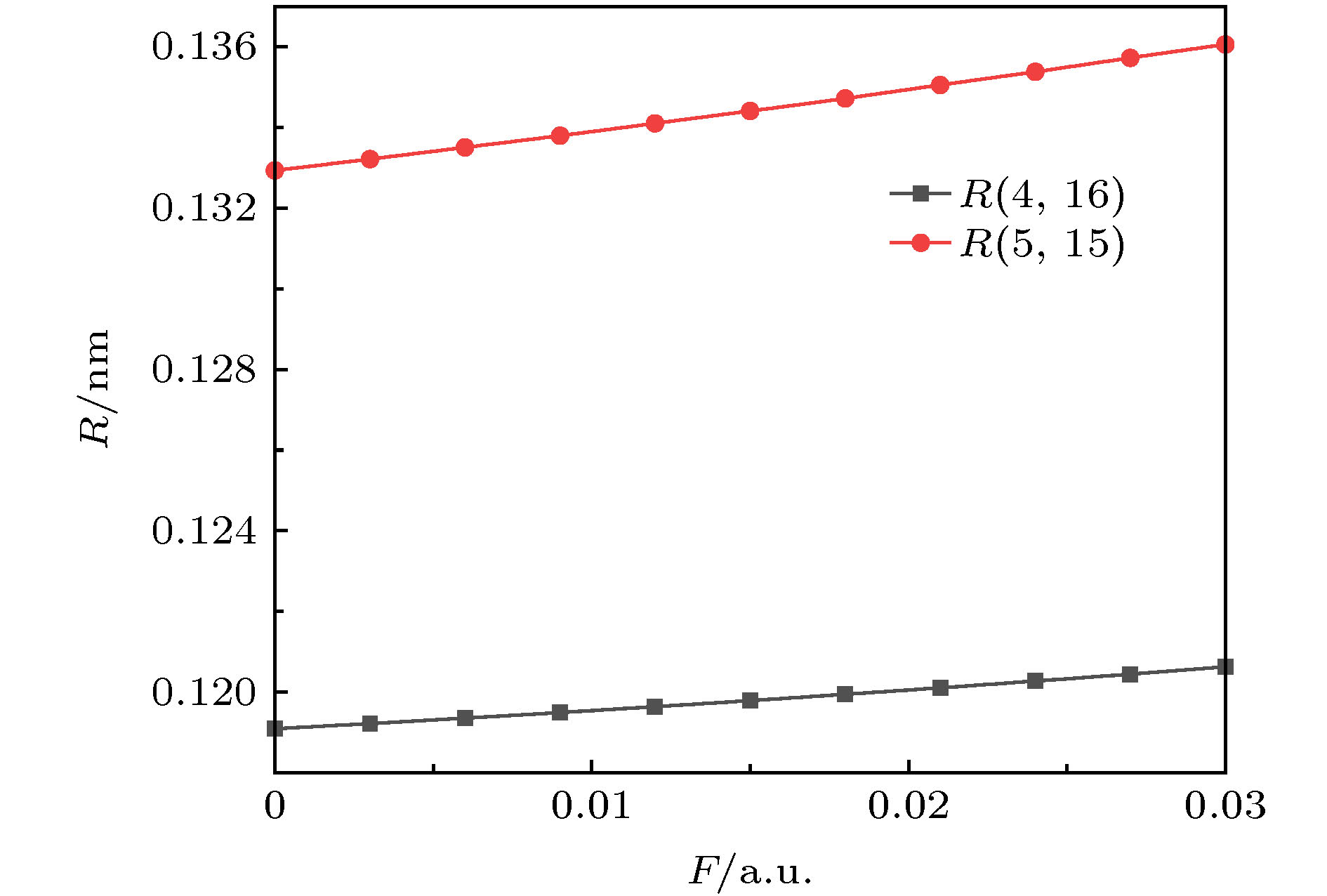
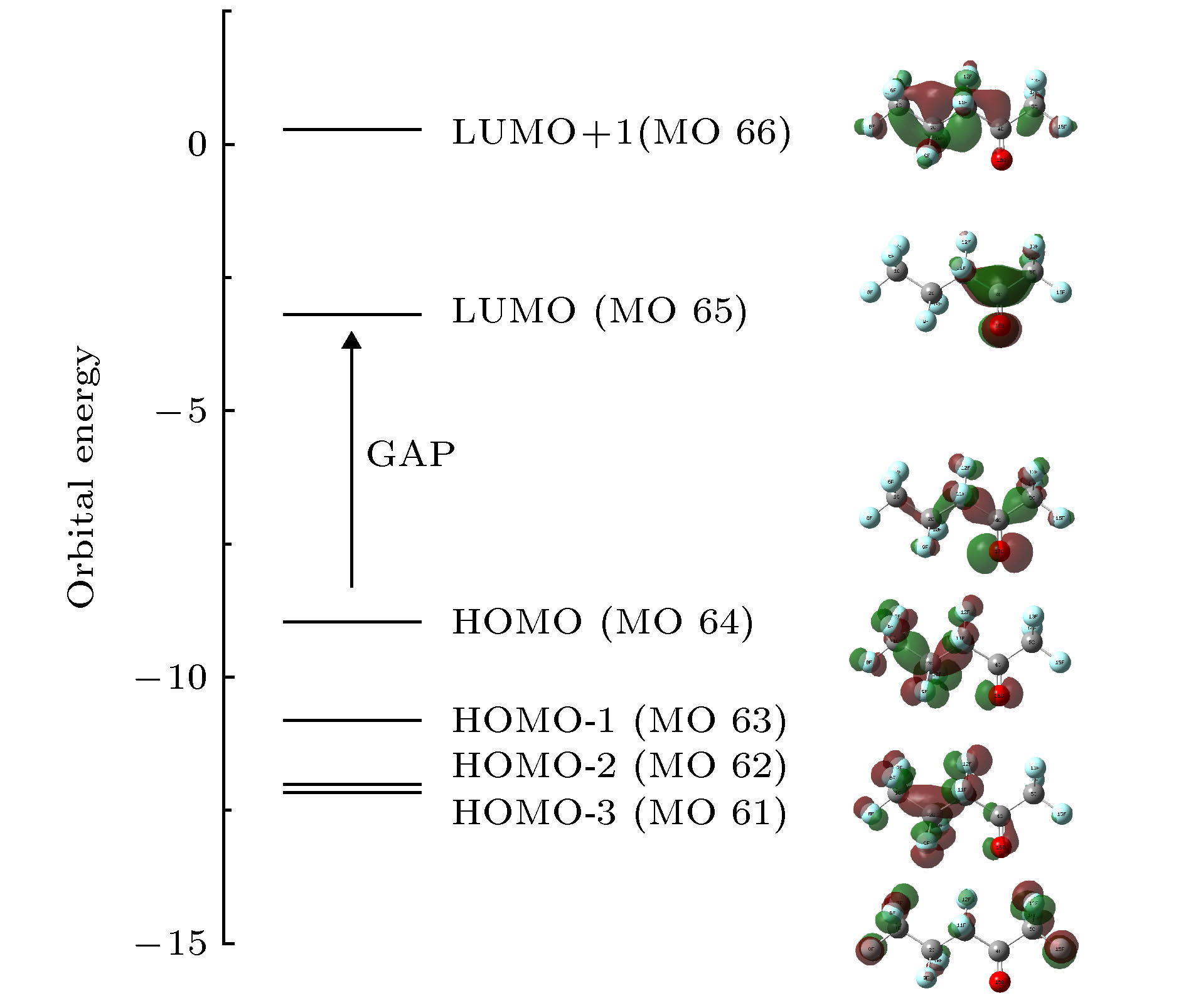
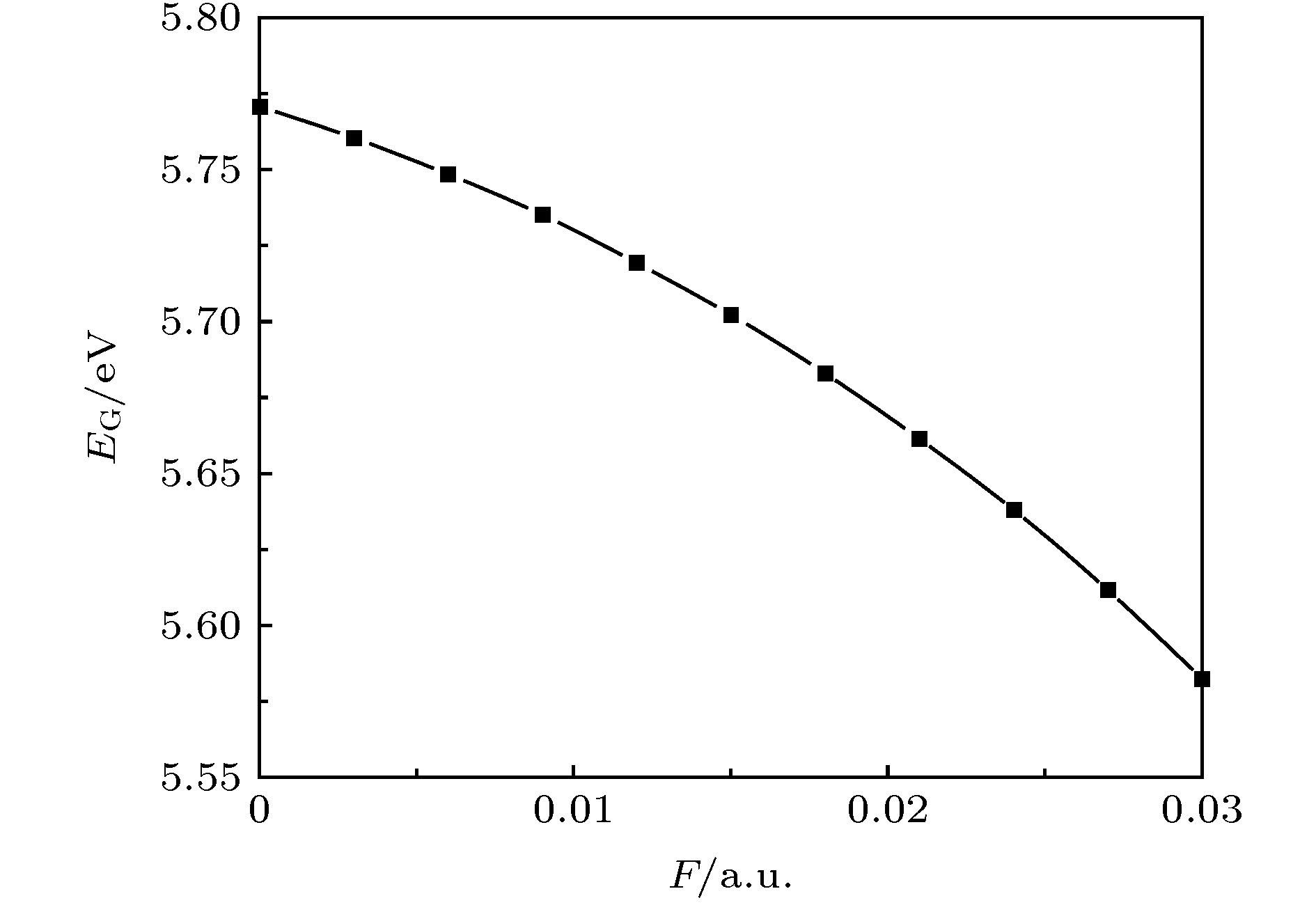
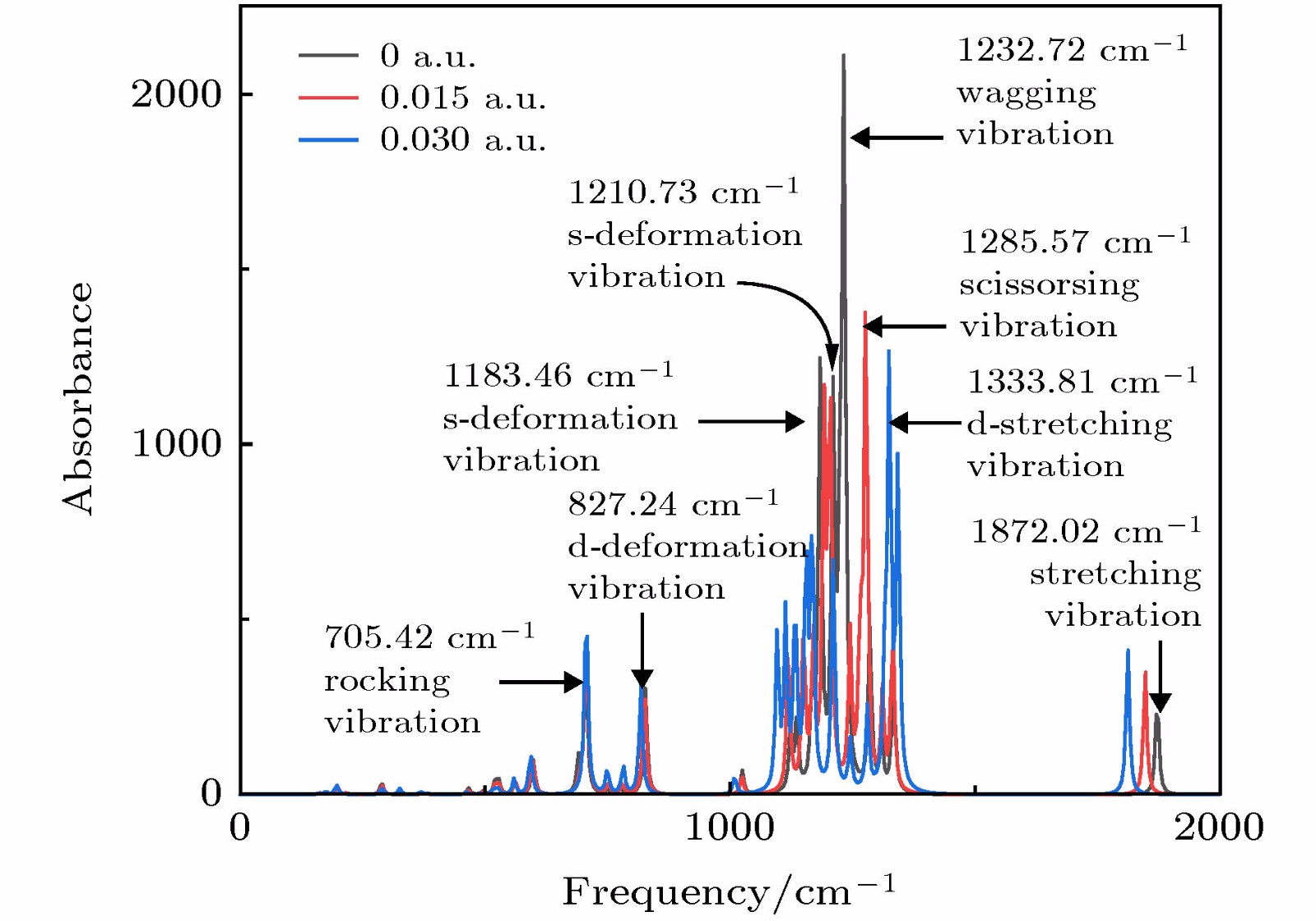
采用密度泛函(DFT)B3LYP/6-311g(d)对C5F10O分子进行几何结构优化, 研究外加电场(0-0.03 a.u., 1 a.u. = 5.142 × 1011 V/m)对分子的几何结构、能量、前线轨道能级、红外光谱的影响. 在相同基组下, 采用TD-DFT方法计算和分析C5F10O的轨道成分和激发特性. 研究表明: 随着电场增加, 5C—15F与4C=16O键能逐渐减小, 键长增大; 13F原子的电荷布居数变化最快, 更容易在外电场力的作用下失去电子; 分子体系势能不断增加, 稳定性逐渐减低; 能隙EG值不断减小, 分子更容易激发到激发态参与到化学反应中. 红外光谱中, 4个吸收峰发生蓝移, 4个吸收峰发生了红移. 使用空穴-电子分析法, 指认了C5F10O分子前8个单重激发态的激发特征. 第一激发态的激发能微小增长, 波长减小, 出现蓝移; 其余激发态的激发能均降低, 波长均变长, 发生红移, 导致C5F10O分子中的电子变得越来越容易激发, 体系的稳定程度减小.In this paper, we use the density functional theory (B3LYP) method with 6-311g(d) basis sets to optimize the molecular structure of C5F10O and obtain the stable structure of its ground state. On this basis, the geometric characteristics, energy, frontier orbital energy levels, and infrared spectra of C5F10O under the different external electric fields (from 0 to 0.03 a.u., 1 a.u. = 5.142 × 1011 V/m) are studied by the same method. Under the same basis sets, the orbital composition and excitation characteristics of C5F10O are calculated and analyzed by the TD-DFT method. The conclusions show that as the electric field increases, the bond energy of 5C—15F and 4C=16O gradually decrease, their bond lengths increase. The charge of 13F atoms changes fastest, and it is easier to lose electrons under the action of electric field force. The potential energy of the molecule increases, and the stability gradually decreases. The energy gap EG value continuously decreases, and the molecules are more likely to be excited to participate in the chemical reaction. In the infrared spectrum, four absorption peaks are blue-shifted, and four absorption peaks are red-shifted. The excitation characteristics of the first 8 singlet excited states of the C5F10O are identified by the hole-electron analysis method. The excitation energy of the first excited state increases slightly, and the wavelength decreases, and blue shift occurs. The excitation energy values of the other excited states decrease, their wavelengths increase, and red shifts occur. Because the electrons in C5F10O become easier to excite, the stability of the system is lower.
-
Keywords:
- C5F10O /
- density functional theory /
- external electric field /
- excited state
[1] 周安春, 高理迎, 冀肖彤, 张名 2018 电网技术 42 3429
Zhou A C, Gao L Y, Ji X T, Z M 2018 Power Syst Technol 42 3429
[2] Deng Y K, Xiao D M, Chen J 2013 High Voltage Eng 39 2288
[3] Chen X, Yang P Y, Ge G W, Wu Q L, Xie W 2019 Plasma Sci. Technol 21 83
[4] 高克利, 颜湘莲, 王浩, 何洁, 李志兵 2018 高电压技术 44 3105
Gao K L, Yan X L, Wang H, He J, Li B Z 2018 High Voltage Eng 44 3105
[5] Christophorou L G, Olthoff J K 2000 J Phys Chem Ref Data 29 267
 Google Scholar
Google Scholar
[6] 肖焓艳, 张晓星, 肖淞, 胡雄雄 2017 电工技术学报 32 20
Xiao H Y, Zhang X X, Xiao S, Hu X X, 2017 Trans. China Electrotechn. Soc 32 20
[7] 张晓星, 田双双, 肖淞, 李祎 2018 电工技术学报 33 2883
Zhang X X, Tian S S, Xiao S, Li W 2018 Trans. China Electrotechn. Soc 33 2883
[8] 肖登明 2016 高压技术 42 1035
Xiao D M 2016 High Voltage Eng 42 1035
[9] 汤峤永, 姚素梅 2018 有机氟工业 4 37
Tang Q Y, Yao S M 2018 Organo-Fluorine Industry 4 37
[10] Hyrenbach M, Hintzen T, Müller P, John O 2015 23rd International Conference on Electricity Distribution Lyon, June 15−18, 1
[11] Simka P, Ranjan N 2015 19 th International Symposium on High Voltage Engineering Pilsen Czech Republic, August 23-28
[12] Guo Z, Li X W, Li B X, Fu M L, Zhuo R, Wang D B 2019 IEEE Trans. Dielectr. Electr. Insul 26 129
 Google Scholar
Google Scholar
[13] Wada J, Ueta G, Okabe S 2016 IEEE Trans. Dielectr. Electr. Insul 23 838
 Google Scholar
Google Scholar
[14] 王小华, 傅熊雄, 韩国辉, 卢彦辉, 李旭旭, 高青青, 荣命哲 2017 高电压技术 43 715
Wang X H, Fu X X, Han G H, Lu Y H, Li X X, Gao Q Q, Rong M Z 2017 High Voltage Eng 43 715
[15] Märt A, Indrek J, Matti L, Peeter P, Jüri R 2018 J Phys D Appl Phys 51 135205
 Google Scholar
Google Scholar
[16] Stoller P C, Doiron C B, Tehlar D, Simka P, Ranjan N 2017 IEEE Trans. Dielectr. Electr. Insul 24 2712
 Google Scholar
Google Scholar
[17] 李兴文, 邓云坤, 姜旭, 赵虎, 卓然, 王邸博, 傅明利 2017 高电压技术 43 708
Li X W, Deng Y K, Jiang X, Zhao H, Zhuo R, Wang D B, Fu M L 2017 High Voltage Eng 43 708
[18] 邓云坤, 马仪, 王达达, 郭泽, 李兴文, 赵虎 2018 电器与能效管理技术 10 40
Deng Y K, Ma Y, Wang D D, Guo Z, Li X W, Zhao H 2018 Electr. Ene Manag Technol 10 40
[19] 马仪, 邓云坤 2018 广东电力 31 44
Ma Y, Deng Y K 2018 Guangdong Electr Power 31 44
[20] Lei Z C, Zeng F P, Tang J, Wan Z F, Zhang M X, Dai L J 2019 IEEE Access 7 92724
 Google Scholar
Google Scholar
[21] Zhang Y, Zhang X X, Li Y, Li Y L, Chen Q, Zhang G Z, Xiao S, Tang J 2019 RSC Adv 9 18963
 Google Scholar
Google Scholar
[22] Fu Y W, Wang X H, Yang A J, Rong M Z 2019 AIP Adv 9 015318
 Google Scholar
Google Scholar
[23] 李庆民, 黄旭炜, 刘涛, 闫江燕, 王兆东, 张颖, 鲁旭 2016 电工技术学报 31 1
 Google Scholar
Google Scholar
Li Q M, Huang X W, Liu T, Yan J Y, Wang Z D, Zhang Y, Lu X 2016 Trans. China Electrotechn. Soc 31 1
 Google Scholar
Google Scholar
[24] Frish M J, Trucks G W, Schlegal H B 2010 Gaussian 09, Revision B01. Walling ford: Gaussian Inc
[25] 段逸群, 刘玉柱, 李静, 张翔云, 秦朝朝, 布玛丽亚·阿布力米提 2018 原子与分子 35 719
 Google Scholar
Google Scholar
Duan Y Q, Liu Y Z, Li J, Zhang X Y, Qin C C, Abulimiti B 2018 J Atom Mol Phys 35 719
 Google Scholar
Google Scholar
[26] 吴永刚, 李世雄, 郝进欣, 徐梅, 孙光宇, 令狐荣锋 2015 64 153102
 Google Scholar
Google Scholar
Wu Y G, Li S X, Hao J X, Xu M, Sun G Y, Linghu R F 2015 Acta Phys. Sin 64 153102
 Google Scholar
Google Scholar
[27] 徐光宪, 黎乐民, 王德民 2007 量子化学: 基本原理和从头计算法(中册)(北京: 科学出版社)第3页
Xu G X, Li L M, Wang D M 2007 Quantum Chemistry: Basic Principle and ab Initio Calculation (Vol. 2) (Beijing: Science Press) P3 (in Chinese)
[28] Grozema F C, Telesca R, Joukman H T 2001 Chem. Phys. 115 10014
[29] Kjeellberg P, Zhi H, Tonu P J 2003 Phys. Chem. B 107 13737
 Google Scholar
Google Scholar
[30] 朱正和, 付依备, 高涛, 陈银亮, 陈晓军 2003 原子与分子 2 169
 Google Scholar
Google Scholar
Zhu Z H, Fu Y. B, Gao T, Chen Y L, Chen X J 2003 J Atom Mol Phys 2 169
 Google Scholar
Google Scholar
[31] 李祎, 张晓星, 肖淞, 黄立群, 唐焗, 邓载韬, 田双双 2018 中国电机工程学报 38 4298
Li Y, Zhang X X, Xiao S, Huang L Q, Tang J, Deng Z T, Tian S S 2018 Chin. Soc. Elec. Eng 38 4298
[32] 黄多辉, 王藩侯, 程晓洪, 万明杰, 蒋刚 2011 60 123101
Huang D H, Wang P H, Cheng X H, Wan M J, Jiang G 2011 Acta Phys. Sin 60 123101
[33] 卢天, 陈飞武 2012 物理化学学报 28 1
 Google Scholar
Google Scholar
Lu T, Chen F W 2012 Acta Phys-Chim. Sin. 28 1
 Google Scholar
Google Scholar
[34] 李鑫, 张梁, 羊梦诗, 储修祥, 徐灿, 陈亮, 王悦悦 2014 63 076102
Li X, Zhang L, Yang M S, Chu X X, Xu C, Chen L, Wang Y Y 2014 Acta Phys. Sin. 63 076102
[35] Lu T, Chen F W 2012 J. Comput. Chem 33 580
 Google Scholar
Google Scholar
[36] 段雪珂, 任娟娟, 郝赫, 张淇, 龚旗煌, 古英, 2019 68 144201
 Google Scholar
Google Scholar
Duan X K, Ren J J, Hao H, Zhang Q, Gong Q H, Gu Y 2019 Acta Phys. Sin. 68 144201
 Google Scholar
Google Scholar
[37] Lu T, Chen F W, 2013 J. Phys. Chem. A 117 3100
 Google Scholar
Google Scholar
-
表 1 C5F10O分子键长与文献值的对比
Table 1. The bond length of C5F10O compared with the reference
Contrast R(4, 16)/nm R(5, 15)/nm R(3, 4)/nm R(3, 12)/nm R(4, 5)/nm Reference 0.117000 0.130600 0.154000 0.132800 0.153900 Theoretical calculation 0.119086 0.132933 0.155896 0.135497 0.155892 Relative error/% 1.783 0.1786 1.231 2.030 1.294 表 2 不同电场强度下C5F10O的前线轨道能级
Table 2. Frontier orbital energy levels of C5F10O at different electric field.
F/a.u. EL/eV EH/eV EG/eV 0.000 –3.197 –8.968 5.771 0.003 –3.260 –9.021 5.760 0.006 –3.324 –9.073 5.748 0.009 –3.388 –9.123 5.735 0.012 –3.453 –9.172 5.719 0.015 –3.517 –9.219 5.702 0.018 –3.583 –9.265 5.683 0.021 –3.649 –9.310 5.661 0.024 –3.715 –9.353 5.638 0.027 –3.783 –9.395 5.612 0.030 –3.852 –9.435 5.582 表 3 C5F10O前8个单重激发态的激发特性
Table 3. Excitation characteristics of first 8 singlet-excited states of C5F10O.
Excited State D/Å t/Å EC/eV Orbital-Contribution (hole) Orbital-Contribution (electron) S(0) → S(1) 0.267 –0.700 9.775374 MO 64-95.39% MO 65-99.352% S(0) → S(2) 1.723 0.648 6.741638 MO 63-78.436% MO 65-98.828% S(0) → S(3) 0.570 –0.355 8.394609 MO 55-13.986% MO 57-44.649%, MO 65-99.139% S(0) → S(4) 1.135 –0.139 6.968679 MO 52-19.354% MO 55-17.810% MO 62-28.358%, MO 65-99.049% S(0) → S(5) 1.144 –0.300 7.015306 MO 64-86.457% MO 66-84.652% S(0) → S(6) 1.862 0.879 6.505353 MO 60-34.078% MO 61-55.521% MO 65-91.502% S(0) → S(7) 1.601 0.566 6.600581 MO 56-19.786% MO 58-16.594% MO 59-30.422% MO 65-96.849% S(0) → S(8) 0.948 –0.079 6.641575 MO 55-33.730% MO 57-21.883% MO 62-22.257% MO 65-99.078% 表 4 不同电场强度下C5F10O前8个单重激发态的激发能
Table 4. Excitation energy of first 8 singlet-excited states of C5F10O at different electric field.
F/a.u. Eex/eV 0.000 0.003 0.006 0.009 0.012 0.015 0.018 0.021 0.024 0.027 0.030 n = 1 4.041 4.052 4.063 4.072 4.078 4.086 4.091 4.095 4.097 4.097 4.095 n = 2 7.324 7.283 7.240 7.195 7.148 7.099 7.048 6.996 6.940 6.883 6.823 n = 3 8.427 8.400 8.368 8.329 8.283 8.210 8.089 7.963 7.833 7.700 7.565 n = 4 8.691 8.651 8.560 8.451 8.336 8.236 8.168 8.087 7.980 7.853 7.719 n = 5 8.715 8.673 8.618 8.567 8.499 8.397 8.277 8.154 8.041 7.935 7.823 n = 6 8.776 8.759 8.787 8.693 8.588 8.509 8.444 8.381 8.307 8.208 8.098 n = 7 9.019 8.919 8.811 8.814 8.770 8.663 8.553 8.446 8.351 8.283 8.225 n = 8 9.159 9.076 8.983 8.883 8.826 8.772 8.682 8.583 8.481 8.378 8.277 表 5 不同电场强度下C5F10O前8个单重激发态的波长
Table 5. Wavelength of first 8 singlet-excited states of C5F10O at different electric field.
F/a.u. λ/nm 0.000 0.003 0.006 0.009 0.012 0.015 0.018 0.021 0.024 0.027 0.030 n = 1 306.80 305.93 305.16 304.48 303.89 303.41 303.04 302.78 302.64 302.63 302.75 n = 2 169.28 170.24 171.25 172.32 173.45 174.64 175.90 177.23 178.64 180.13 181.71 n = 3 147.12 147.60 148.17 148.85 149.68 151.01 153.27 155.71 158.29 161.02 163.88 n = 4 142.65 143.31 144.84 146.72 148.73 150.54 151.79 153.31 155.36 157.87 160.62 n = 5 142.27 142.96 143.86 144.72 145.89 147.65 149.80 152.05 154.18 156.26 158.48 n = 6 141.28 141.55 141.10 142.63 144.37 145.72 146.83 147.94 149.25 151.05 153.10 n = 7 137.48 139.02 140.72 140.66 141.37 143.12 144.96 146.80 148.47 149.69 150.75 n = 8 135.37 136.61 138.03 139.57 140.47 141.34 142.80 144.45 146.19 147.98 149.79 表 6 不同电场强度下C5F10O前8个单重激发态的振子强度
Table 6. Oscillator strength of first 8 singlet-excited states of C5F10O at different electric field.
F/a.u. f 0.000 0.003 0.006 0.009 0.012 0.015 0.018 0.021 0.024 0.027 0.030 n = 1 0.0002 0.0002 0.0002 0.0002 0.0002 0.0001 0.0001 0.0001 0.0001 0.0001 0.0001 n = 2 0.0033 0.0032 0.0030 0.0029 0.0028 0.0026 0.0026 0.0025 0.0024 0.0024 0.0024 n = 3 0.0015 0.0020 0.0025 0.0030 0.0035 0.0039 0.0044 0.0045 0.0045 0.0045 0.0044 n = 4 0.0014 0.0012 0.0024 0.0035 0.0042 0.0048 0.0053 0.0055 0.0052 0.0052 0.0055 n = 5 0.0591 0.0027 0.0039 0.0049 0.0073 0.0083 0.0080 0.0086 0.0102 0.0117 0.0131 n = 6 0.0162 0.0738 0.0641 0.0066 0.0024 0.0005 0.0004 0.0004 0.0023 0.0047 0.0047 n = 7 0.0180 0.0127 0.0148 0.0570 0.0098 0.0101 0.0107 0.0104 0.0078 0.0048 0.0054 n = 8 0.0013 0.0008 0.0039 0.0094 0.0276 0.0078 0.0126 0.0170 0.0203 0.0228 0.0236 -
[1] 周安春, 高理迎, 冀肖彤, 张名 2018 电网技术 42 3429
Zhou A C, Gao L Y, Ji X T, Z M 2018 Power Syst Technol 42 3429
[2] Deng Y K, Xiao D M, Chen J 2013 High Voltage Eng 39 2288
[3] Chen X, Yang P Y, Ge G W, Wu Q L, Xie W 2019 Plasma Sci. Technol 21 83
[4] 高克利, 颜湘莲, 王浩, 何洁, 李志兵 2018 高电压技术 44 3105
Gao K L, Yan X L, Wang H, He J, Li B Z 2018 High Voltage Eng 44 3105
[5] Christophorou L G, Olthoff J K 2000 J Phys Chem Ref Data 29 267
 Google Scholar
Google Scholar
[6] 肖焓艳, 张晓星, 肖淞, 胡雄雄 2017 电工技术学报 32 20
Xiao H Y, Zhang X X, Xiao S, Hu X X, 2017 Trans. China Electrotechn. Soc 32 20
[7] 张晓星, 田双双, 肖淞, 李祎 2018 电工技术学报 33 2883
Zhang X X, Tian S S, Xiao S, Li W 2018 Trans. China Electrotechn. Soc 33 2883
[8] 肖登明 2016 高压技术 42 1035
Xiao D M 2016 High Voltage Eng 42 1035
[9] 汤峤永, 姚素梅 2018 有机氟工业 4 37
Tang Q Y, Yao S M 2018 Organo-Fluorine Industry 4 37
[10] Hyrenbach M, Hintzen T, Müller P, John O 2015 23rd International Conference on Electricity Distribution Lyon, June 15−18, 1
[11] Simka P, Ranjan N 2015 19 th International Symposium on High Voltage Engineering Pilsen Czech Republic, August 23-28
[12] Guo Z, Li X W, Li B X, Fu M L, Zhuo R, Wang D B 2019 IEEE Trans. Dielectr. Electr. Insul 26 129
 Google Scholar
Google Scholar
[13] Wada J, Ueta G, Okabe S 2016 IEEE Trans. Dielectr. Electr. Insul 23 838
 Google Scholar
Google Scholar
[14] 王小华, 傅熊雄, 韩国辉, 卢彦辉, 李旭旭, 高青青, 荣命哲 2017 高电压技术 43 715
Wang X H, Fu X X, Han G H, Lu Y H, Li X X, Gao Q Q, Rong M Z 2017 High Voltage Eng 43 715
[15] Märt A, Indrek J, Matti L, Peeter P, Jüri R 2018 J Phys D Appl Phys 51 135205
 Google Scholar
Google Scholar
[16] Stoller P C, Doiron C B, Tehlar D, Simka P, Ranjan N 2017 IEEE Trans. Dielectr. Electr. Insul 24 2712
 Google Scholar
Google Scholar
[17] 李兴文, 邓云坤, 姜旭, 赵虎, 卓然, 王邸博, 傅明利 2017 高电压技术 43 708
Li X W, Deng Y K, Jiang X, Zhao H, Zhuo R, Wang D B, Fu M L 2017 High Voltage Eng 43 708
[18] 邓云坤, 马仪, 王达达, 郭泽, 李兴文, 赵虎 2018 电器与能效管理技术 10 40
Deng Y K, Ma Y, Wang D D, Guo Z, Li X W, Zhao H 2018 Electr. Ene Manag Technol 10 40
[19] 马仪, 邓云坤 2018 广东电力 31 44
Ma Y, Deng Y K 2018 Guangdong Electr Power 31 44
[20] Lei Z C, Zeng F P, Tang J, Wan Z F, Zhang M X, Dai L J 2019 IEEE Access 7 92724
 Google Scholar
Google Scholar
[21] Zhang Y, Zhang X X, Li Y, Li Y L, Chen Q, Zhang G Z, Xiao S, Tang J 2019 RSC Adv 9 18963
 Google Scholar
Google Scholar
[22] Fu Y W, Wang X H, Yang A J, Rong M Z 2019 AIP Adv 9 015318
 Google Scholar
Google Scholar
[23] 李庆民, 黄旭炜, 刘涛, 闫江燕, 王兆东, 张颖, 鲁旭 2016 电工技术学报 31 1
 Google Scholar
Google Scholar
Li Q M, Huang X W, Liu T, Yan J Y, Wang Z D, Zhang Y, Lu X 2016 Trans. China Electrotechn. Soc 31 1
 Google Scholar
Google Scholar
[24] Frish M J, Trucks G W, Schlegal H B 2010 Gaussian 09, Revision B01. Walling ford: Gaussian Inc
[25] 段逸群, 刘玉柱, 李静, 张翔云, 秦朝朝, 布玛丽亚·阿布力米提 2018 原子与分子 35 719
 Google Scholar
Google Scholar
Duan Y Q, Liu Y Z, Li J, Zhang X Y, Qin C C, Abulimiti B 2018 J Atom Mol Phys 35 719
 Google Scholar
Google Scholar
[26] 吴永刚, 李世雄, 郝进欣, 徐梅, 孙光宇, 令狐荣锋 2015 64 153102
 Google Scholar
Google Scholar
Wu Y G, Li S X, Hao J X, Xu M, Sun G Y, Linghu R F 2015 Acta Phys. Sin 64 153102
 Google Scholar
Google Scholar
[27] 徐光宪, 黎乐民, 王德民 2007 量子化学: 基本原理和从头计算法(中册)(北京: 科学出版社)第3页
Xu G X, Li L M, Wang D M 2007 Quantum Chemistry: Basic Principle and ab Initio Calculation (Vol. 2) (Beijing: Science Press) P3 (in Chinese)
[28] Grozema F C, Telesca R, Joukman H T 2001 Chem. Phys. 115 10014
[29] Kjeellberg P, Zhi H, Tonu P J 2003 Phys. Chem. B 107 13737
 Google Scholar
Google Scholar
[30] 朱正和, 付依备, 高涛, 陈银亮, 陈晓军 2003 原子与分子 2 169
 Google Scholar
Google Scholar
Zhu Z H, Fu Y. B, Gao T, Chen Y L, Chen X J 2003 J Atom Mol Phys 2 169
 Google Scholar
Google Scholar
[31] 李祎, 张晓星, 肖淞, 黄立群, 唐焗, 邓载韬, 田双双 2018 中国电机工程学报 38 4298
Li Y, Zhang X X, Xiao S, Huang L Q, Tang J, Deng Z T, Tian S S 2018 Chin. Soc. Elec. Eng 38 4298
[32] 黄多辉, 王藩侯, 程晓洪, 万明杰, 蒋刚 2011 60 123101
Huang D H, Wang P H, Cheng X H, Wan M J, Jiang G 2011 Acta Phys. Sin 60 123101
[33] 卢天, 陈飞武 2012 物理化学学报 28 1
 Google Scholar
Google Scholar
Lu T, Chen F W 2012 Acta Phys-Chim. Sin. 28 1
 Google Scholar
Google Scholar
[34] 李鑫, 张梁, 羊梦诗, 储修祥, 徐灿, 陈亮, 王悦悦 2014 63 076102
Li X, Zhang L, Yang M S, Chu X X, Xu C, Chen L, Wang Y Y 2014 Acta Phys. Sin. 63 076102
[35] Lu T, Chen F W 2012 J. Comput. Chem 33 580
 Google Scholar
Google Scholar
[36] 段雪珂, 任娟娟, 郝赫, 张淇, 龚旗煌, 古英, 2019 68 144201
 Google Scholar
Google Scholar
Duan X K, Ren J J, Hao H, Zhang Q, Gong Q H, Gu Y 2019 Acta Phys. Sin. 68 144201
 Google Scholar
Google Scholar
[37] Lu T, Chen F W, 2013 J. Phys. Chem. A 117 3100
 Google Scholar
Google Scholar
计量
- 文章访问数: 11621
- PDF下载量: 115
- 被引次数: 0













 下载:
下载: