-
Phthalocyanine (Pc) is a kind of important photoelectric material, but a lot of questions remain to be clarified, like the relationship between the structure of Pc and its photoelectric property. High pressure is a powerful tool to study the structure transformation. High pressure study on piezochromic materials, which shows color change under high pressure due to structure changing, serves as an effectively way of studying the relationship between materials’ structure and photoelectric property. In this work Raman spectrum is employed to study the phase change of α phase metal-free Pc (α-H2Pc) under high pressure, meanwhile the effect of pressure on fluorescence (FL) is also studied to show how the Pc’s structure affects the photoelectric property. The diamond anvil cell is employed to achieve the high pressure condition, by using NaCl as a pressure transmitting medium. And Raman and FL measurements are performed by using a LabRam HR Evolution spectrometer equipped with a 473 nm laser. The Raman spectra of α-H2Pc show to slightly change during compression to 12.0 GPa. The main Raman peaks remain at highest pressure, including the Raman peak from macrocyclic of Pc molecules, which shows the stability of Pc molecules. Note that an enhancement of Raman peak at 623 cm–1 can be found with the pressure increasing, which appears only in the Raman spectrum of χ phase metal-free Pc (χ-H2Pc), showing that α-H2Pc is converted into χ-H2Pc under pressure. The curve for Raman frequency as a function of pressure shows that no obvious evidence related to bonding or structure transition can be observed, which means that α-H2Pc is transformed into χ-H2Pc gradually. For FL spectrum, only the FL of excimer can be found in α-H2Pc at atmosphere pressure. When the solid α-H2Pc is compressed, the FL intensity is found to decrease as pressure increases, and it is quenched at 3.0 GPa. The FL of Pc molecule, which is not found in α-H2Pc at ambient pressure, appears at 0.7 GPa. As the pressure increases, the FL intensity ratio between Pc molecule and excimer is enhanced. Considering the pressure induced phase transition from α-H2Pc to χ-H2Pc gradually, the change in FL spectrum should be due to the structure transformation. It is proved that the degree of overlapping between Pc molecules in α-H2Pc is larger than that in χ-H2Pc. We think, the degree of overlapping decreases under high pressure, which hinders the formation of excimer. It makes the excimer emission decrease and the FL of Pc molecules appear under high pressure. Our work can explain the relationship between Pc crystal structure and its fluorescence, reveals the kinetic behavior of macromolecules similar to Pc system under high pressure, and provides a new possibility of designing the photoelectric materials with excellent performances.
-
Keywords:
- phthalocyanine /
- high pressure /
- fluorescence
[1] Zugenmaier P, Bluhm T L, Deslandes Y, Orts W J, Hamer G K 1997 J. Mater. Sci. 32 5561
 Google Scholar
Google Scholar
[2] Sharma V B, Jain S L, Sain B 2003 Tetrahedron Lett. 44 383
 Google Scholar
Google Scholar
[3] Janczak J, Kubiak R 1992 J. Alloys Compd. 190 121
 Google Scholar
Google Scholar
[4] Kubiak R, Janczak J 1992 J. Alloys Compd. 190 117
 Google Scholar
Google Scholar
[5] Hammond R B, Roberts K J, Docherty R, Edmondson M, Gairns R 1996 J. Chem. Soc. Perkin Trans. 28 1527
[6] Takano S, Enokida T, Kakuta A, Mori Y 1984 Chem. Lett. 13 2037
 Google Scholar
Google Scholar
[7] 李战强, 李祥高, 李健, 胡雅琴 2013 有机化学 33 891
Li Z Q, Li G Q, Li J, Hu Y Q 2013 Chin. J. Org. Chem. 33 891
[8] Menzel E R, Jordan K J 1978 Chem. Phys. 32 223
 Google Scholar
Google Scholar
[9] Dong Y J, Xu B, Zhang J B, Tan X, Wang L J, Chen J L, Lv H G, Wen S P, Li B, Ye L, Zou B, Tian W J 2012 Angew. Chem. 124 10940
 Google Scholar
Google Scholar
[10] Meng X, Qi G Y, Zhang C, Wang K, Zou B, Ma Y G 2015 Chem. Commun. 51 9320
 Google Scholar
Google Scholar
[11] Yuan H S, Wang K, Yang K, Liu B B, Zou B 2014 J. Phys. Chem. Lett. 5 2968
 Google Scholar
Google Scholar
[12] 郭宏伟, 刘然, 王玲瑞, 崔金星, 宋波, 王凯, 刘冰冰, 邹勃 2017 66 030701
 Google Scholar
Google Scholar
Guo H W, Liu R, Wang L R, Cui J X, Song B, Wang K, Liu B B, Zou B 2017 Acta Phys. Sin. 66 030701
 Google Scholar
Google Scholar
[13] Grenoble D C, Drickamer H G 1971 J. Chem. Phys. 55 1624
 Google Scholar
Google Scholar
[14] Aroca R, Dilella D P, Loutfy R O 1982 J. Phys. Chem. Solids 43 707
 Google Scholar
Google Scholar
[15] Zhang X X, Bao M, Pan N, Zhang Y X, Jiang J Z 2004 Chin. J. Chem. 22 325
[16] Lehrer S S 1997 Methods Enzymol. 278 286
 Google Scholar
Google Scholar
[17] Birks J B, Kazzaz A A, King T A 1966 Proc. R. Soc. London Ser. A 291 556
 Google Scholar
Google Scholar
[18] Tanaka J 1963 Bull. Chem. Soc. Jpn. 36 1237
 Google Scholar
Google Scholar
[19] Chandross E A, Dempster C J 1970 J. Am. Chem. Soc. 92 3586
 Google Scholar
Google Scholar
[20] Jones P F, Nicol M 1965 J. Chem. Phys. 43 3759
 Google Scholar
Google Scholar
[21] Birks J B 1975 Rep. Prog. Phys. 38 903
 Google Scholar
Google Scholar
-
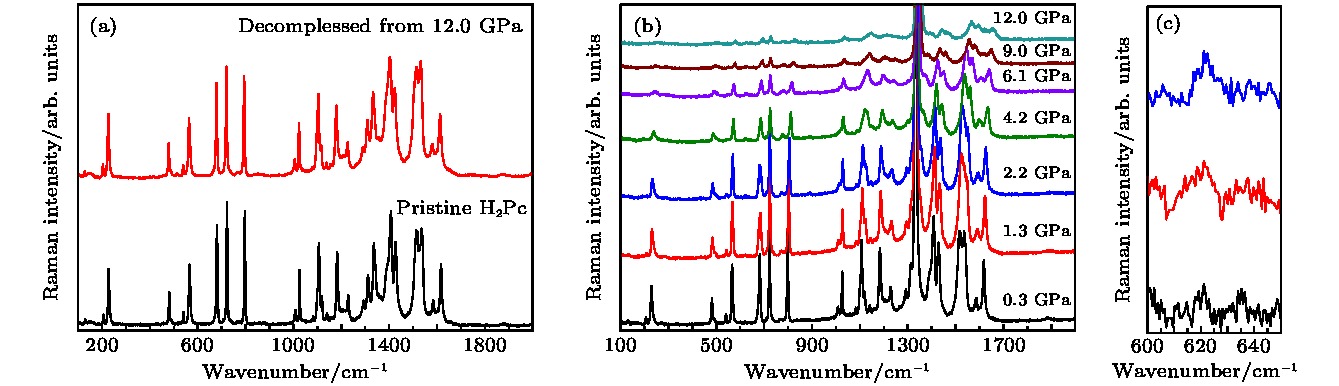
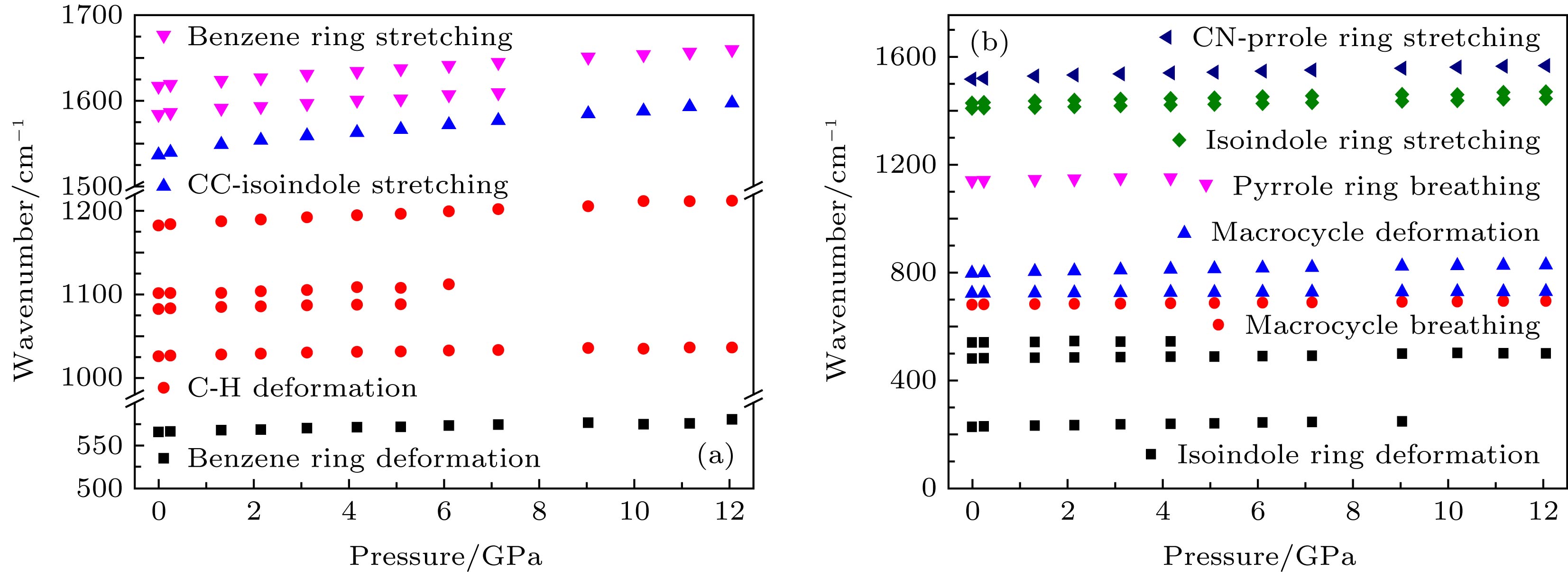
图 2 样品拉曼光谱测试 (a) 常压与卸压样品的拉曼光谱; (b) 酞菁的高压拉曼光谱; (c) 峰位为623 cm–1的拉曼峰随着压力升高峰强增强
Figure 2. The Raman spectra of α-H2Pc: (a) The Raman spectra of sample at ambient pressure and decompressed from 12.0 GPa; (b) the Raman spectra of α-H2Pc under high pressure; (c) the Raman peak around 623 cm–1 under high pressure
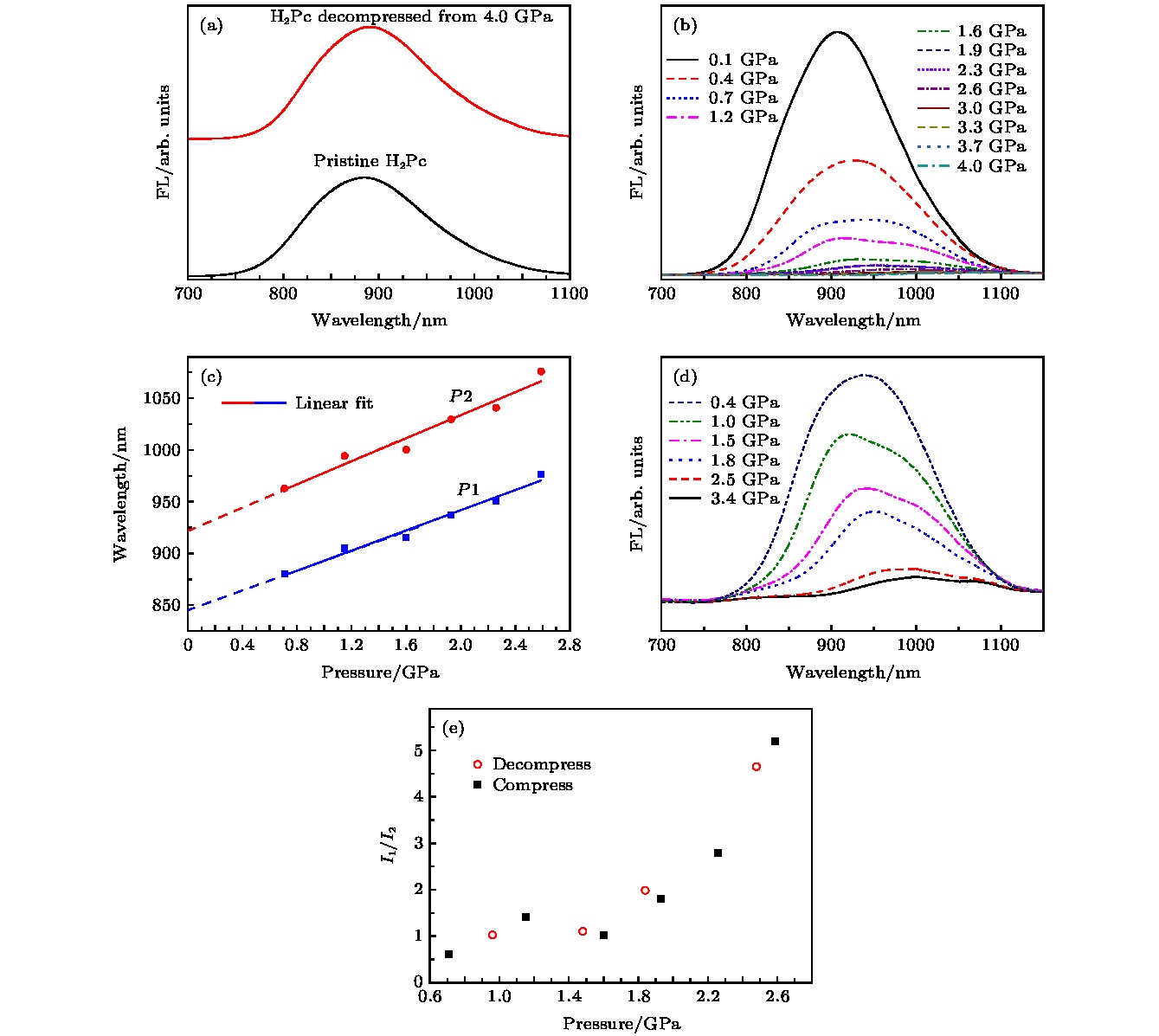
图 4 样品的荧光光谱 (a)样品的常压和卸压荧光光谱; (b) 样品的升压荧光光谱; (c) 样品的荧光中心随压力的变化及其线性拟合, 虚线为线性拟合所得直线的延长线; (d) 样品卸压过程中的荧光光谱; (e) 两个荧光中心的峰强比随压力的变化图
Figure 4. The fluorescence (FL) spectra of α-H2Pc: (a) The FL spectra of α-H2Pc at ambient pressure (black line) and decompressed from 4.0 GPa (red line); (b) the FL spectra of α-H2Pc under high pressure during compressing; (c) the blue and red dots are the position of P1 and P2 during compressing, the blue and red lines are the linear fitting between the position and pressure, and the dash lines are the extension of the blue and red line; (d) the FL spectra of α-H2Pc under high pressure during decompressing; (e) the intensity ratio of P1 and P2 under high pressure
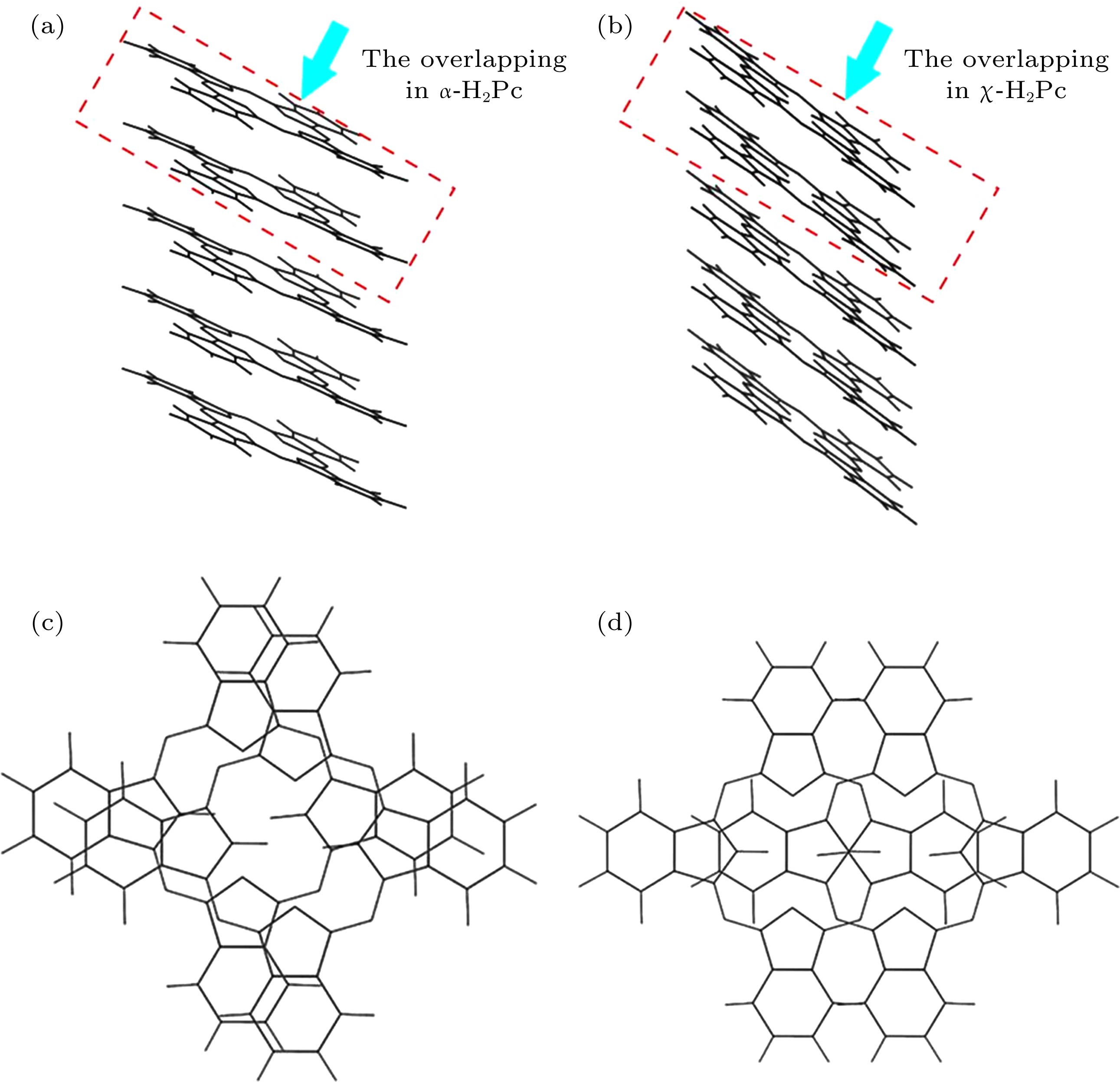
图 5 α相和χ相酞菁的分子排布和重叠程度示意图 (a) α相酞菁的分子排布; (b) χ相酞菁的分子排布; (c) α相酞菁的分子重叠程度; (d) χ相酞菁的分子重叠程度
Figure 5. The arrangement and overlapping of PC molecules in α-H2Pc and χ-H2Pc: (a) The arrangement of Pc molecules in α-H2Pc; (b) the arrangement of Pc molecules in χ-H2Pc; (c) the overlapping of Pc molecules in α-H2Pc; (d) the overlapping of Pc molecules in χ-H2Pc [1,3].
-
[1] Zugenmaier P, Bluhm T L, Deslandes Y, Orts W J, Hamer G K 1997 J. Mater. Sci. 32 5561
 Google Scholar
Google Scholar
[2] Sharma V B, Jain S L, Sain B 2003 Tetrahedron Lett. 44 383
 Google Scholar
Google Scholar
[3] Janczak J, Kubiak R 1992 J. Alloys Compd. 190 121
 Google Scholar
Google Scholar
[4] Kubiak R, Janczak J 1992 J. Alloys Compd. 190 117
 Google Scholar
Google Scholar
[5] Hammond R B, Roberts K J, Docherty R, Edmondson M, Gairns R 1996 J. Chem. Soc. Perkin Trans. 28 1527
[6] Takano S, Enokida T, Kakuta A, Mori Y 1984 Chem. Lett. 13 2037
 Google Scholar
Google Scholar
[7] 李战强, 李祥高, 李健, 胡雅琴 2013 有机化学 33 891
Li Z Q, Li G Q, Li J, Hu Y Q 2013 Chin. J. Org. Chem. 33 891
[8] Menzel E R, Jordan K J 1978 Chem. Phys. 32 223
 Google Scholar
Google Scholar
[9] Dong Y J, Xu B, Zhang J B, Tan X, Wang L J, Chen J L, Lv H G, Wen S P, Li B, Ye L, Zou B, Tian W J 2012 Angew. Chem. 124 10940
 Google Scholar
Google Scholar
[10] Meng X, Qi G Y, Zhang C, Wang K, Zou B, Ma Y G 2015 Chem. Commun. 51 9320
 Google Scholar
Google Scholar
[11] Yuan H S, Wang K, Yang K, Liu B B, Zou B 2014 J. Phys. Chem. Lett. 5 2968
 Google Scholar
Google Scholar
[12] 郭宏伟, 刘然, 王玲瑞, 崔金星, 宋波, 王凯, 刘冰冰, 邹勃 2017 66 030701
 Google Scholar
Google Scholar
Guo H W, Liu R, Wang L R, Cui J X, Song B, Wang K, Liu B B, Zou B 2017 Acta Phys. Sin. 66 030701
 Google Scholar
Google Scholar
[13] Grenoble D C, Drickamer H G 1971 J. Chem. Phys. 55 1624
 Google Scholar
Google Scholar
[14] Aroca R, Dilella D P, Loutfy R O 1982 J. Phys. Chem. Solids 43 707
 Google Scholar
Google Scholar
[15] Zhang X X, Bao M, Pan N, Zhang Y X, Jiang J Z 2004 Chin. J. Chem. 22 325
[16] Lehrer S S 1997 Methods Enzymol. 278 286
 Google Scholar
Google Scholar
[17] Birks J B, Kazzaz A A, King T A 1966 Proc. R. Soc. London Ser. A 291 556
 Google Scholar
Google Scholar
[18] Tanaka J 1963 Bull. Chem. Soc. Jpn. 36 1237
 Google Scholar
Google Scholar
[19] Chandross E A, Dempster C J 1970 J. Am. Chem. Soc. 92 3586
 Google Scholar
Google Scholar
[20] Jones P F, Nicol M 1965 J. Chem. Phys. 43 3759
 Google Scholar
Google Scholar
[21] Birks J B 1975 Rep. Prog. Phys. 38 903
 Google Scholar
Google Scholar
Catalog
Metrics
- Abstract views: 11248
- PDF Downloads: 126
- Cited By: 0














 DownLoad:
DownLoad: