-
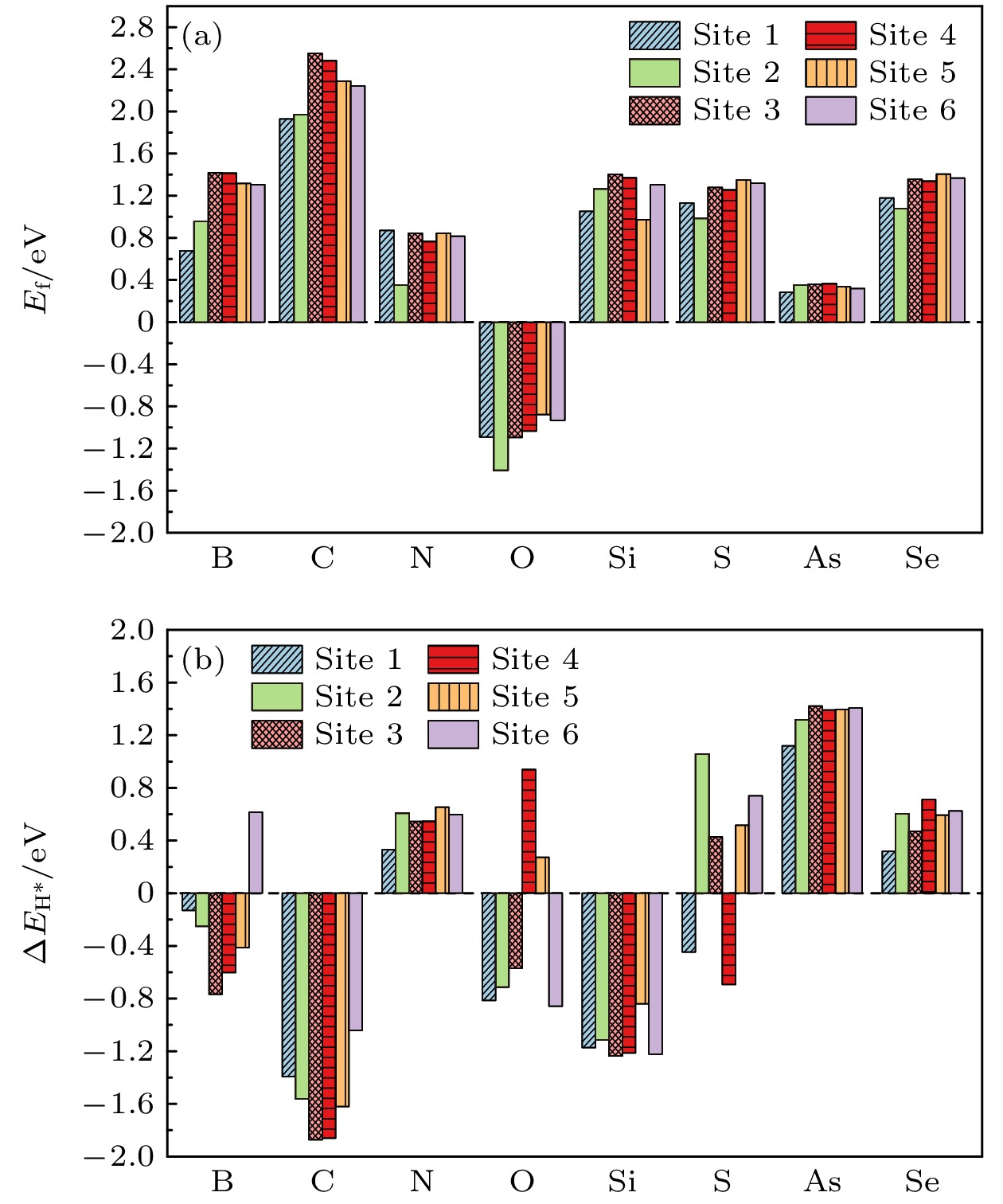
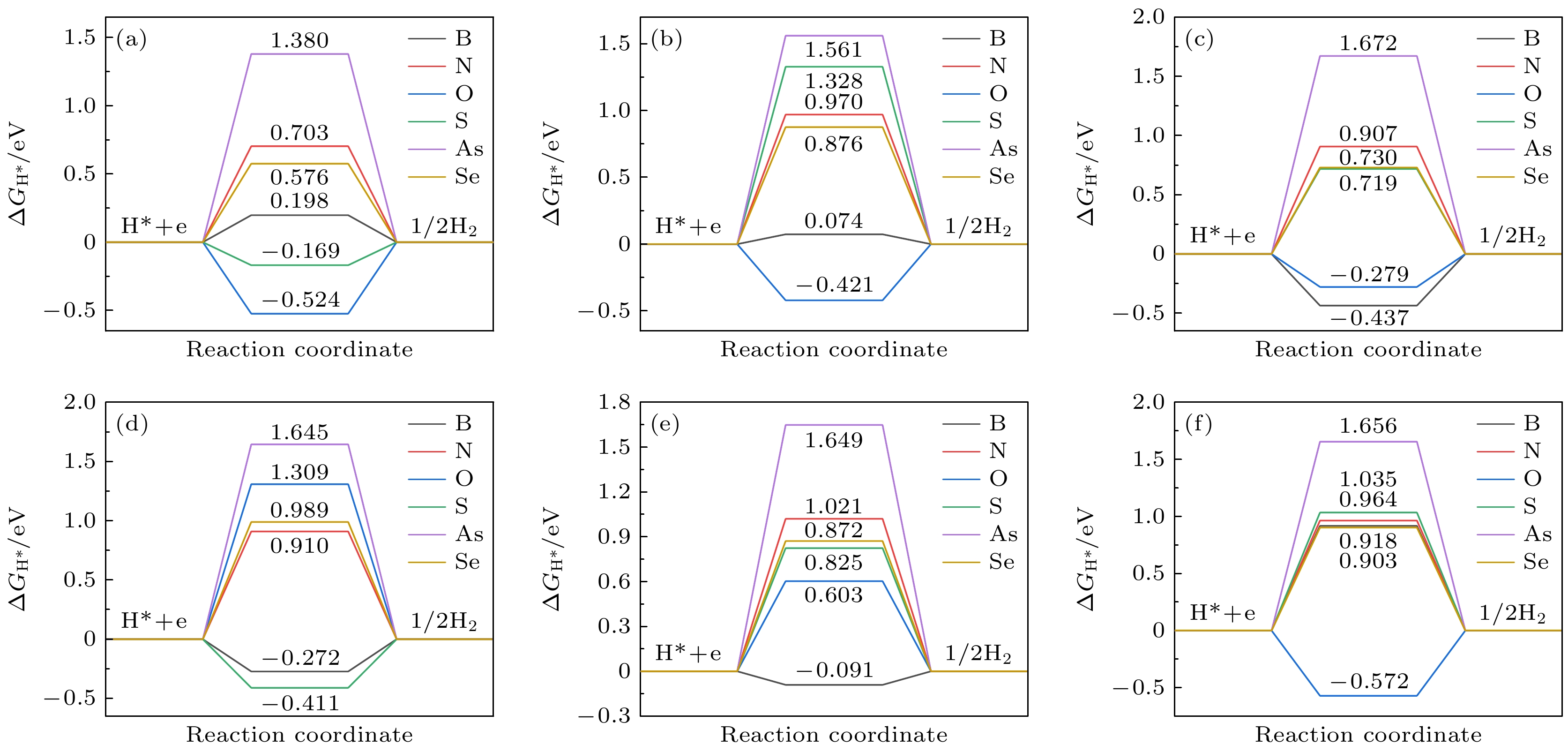
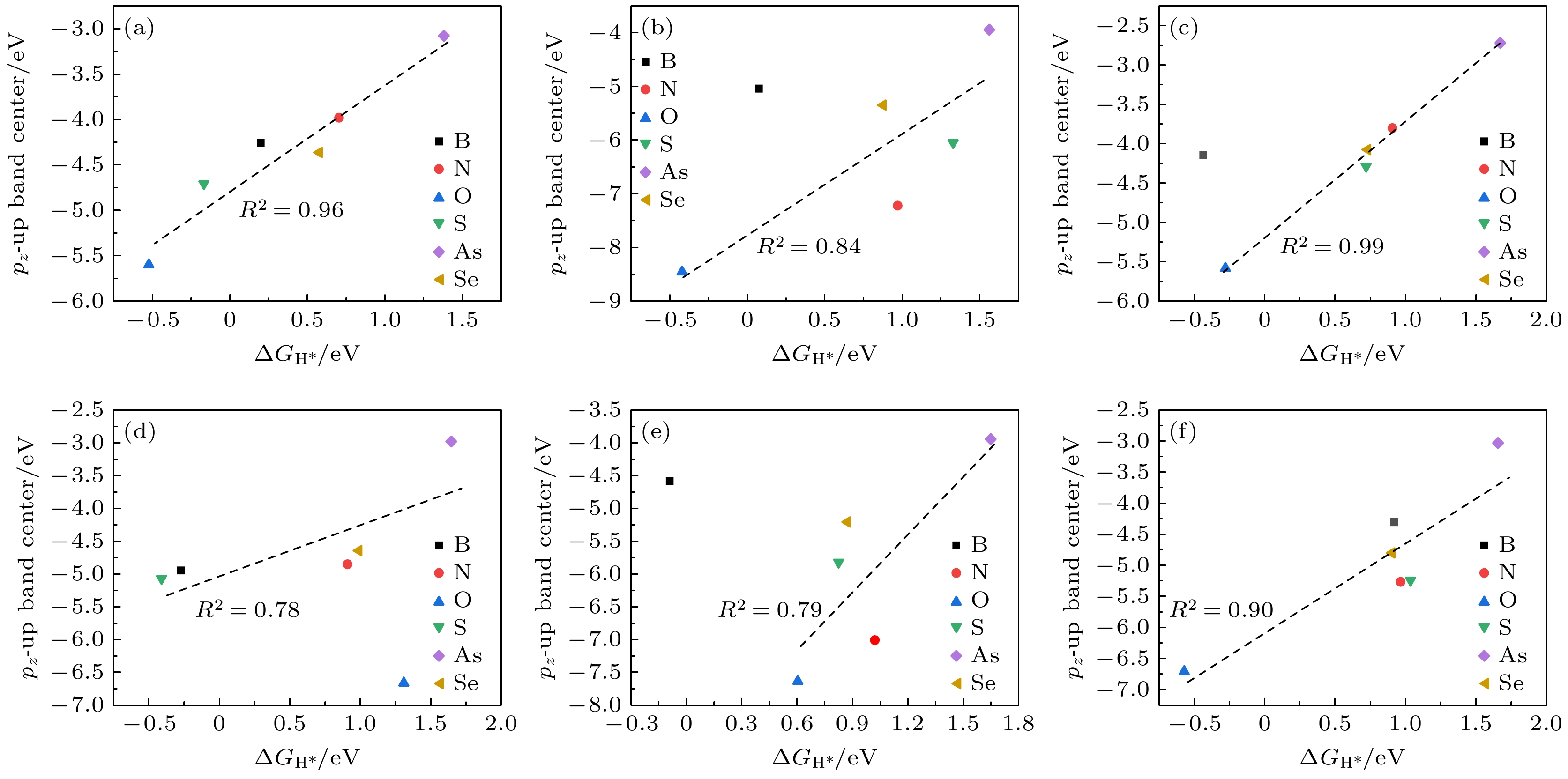
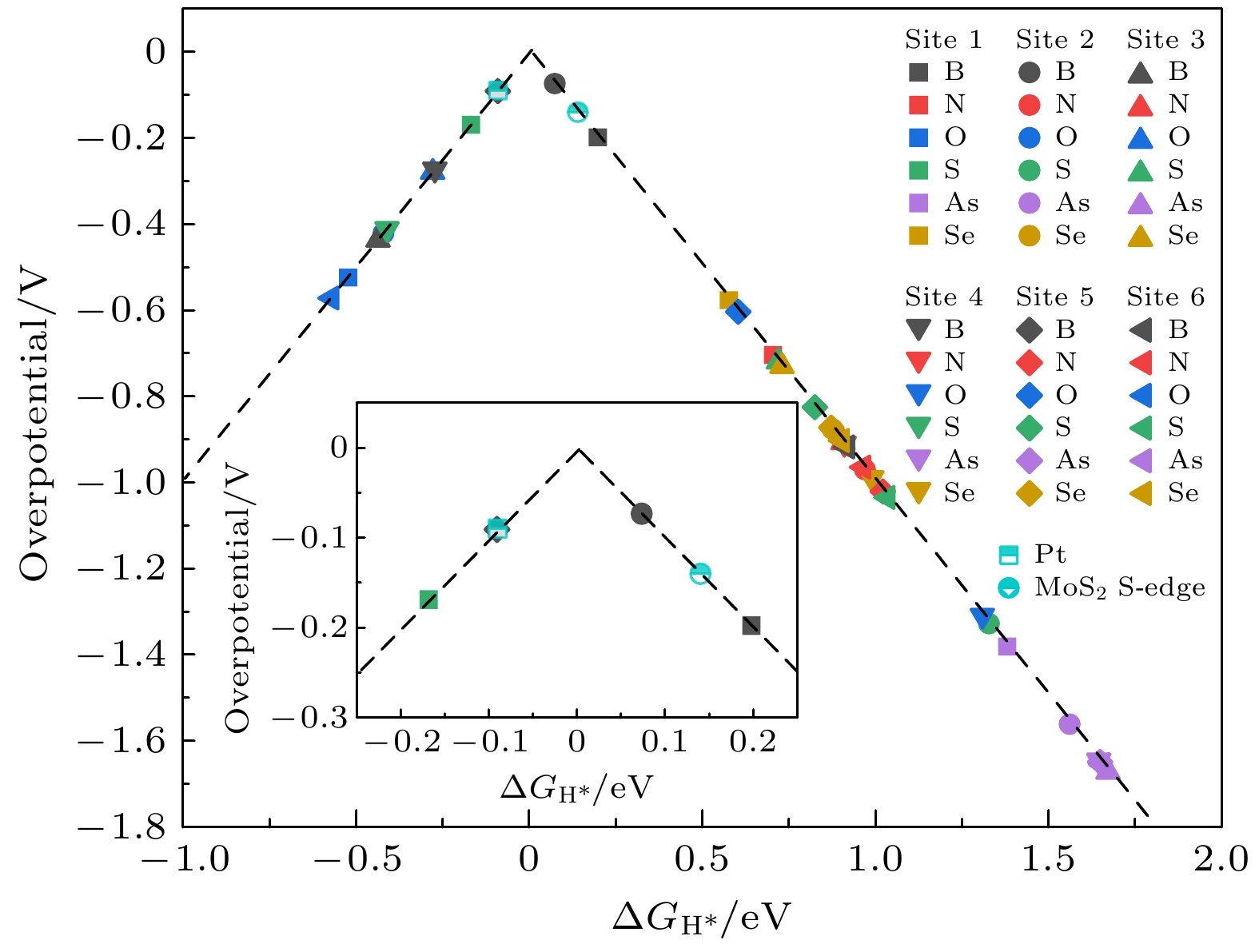
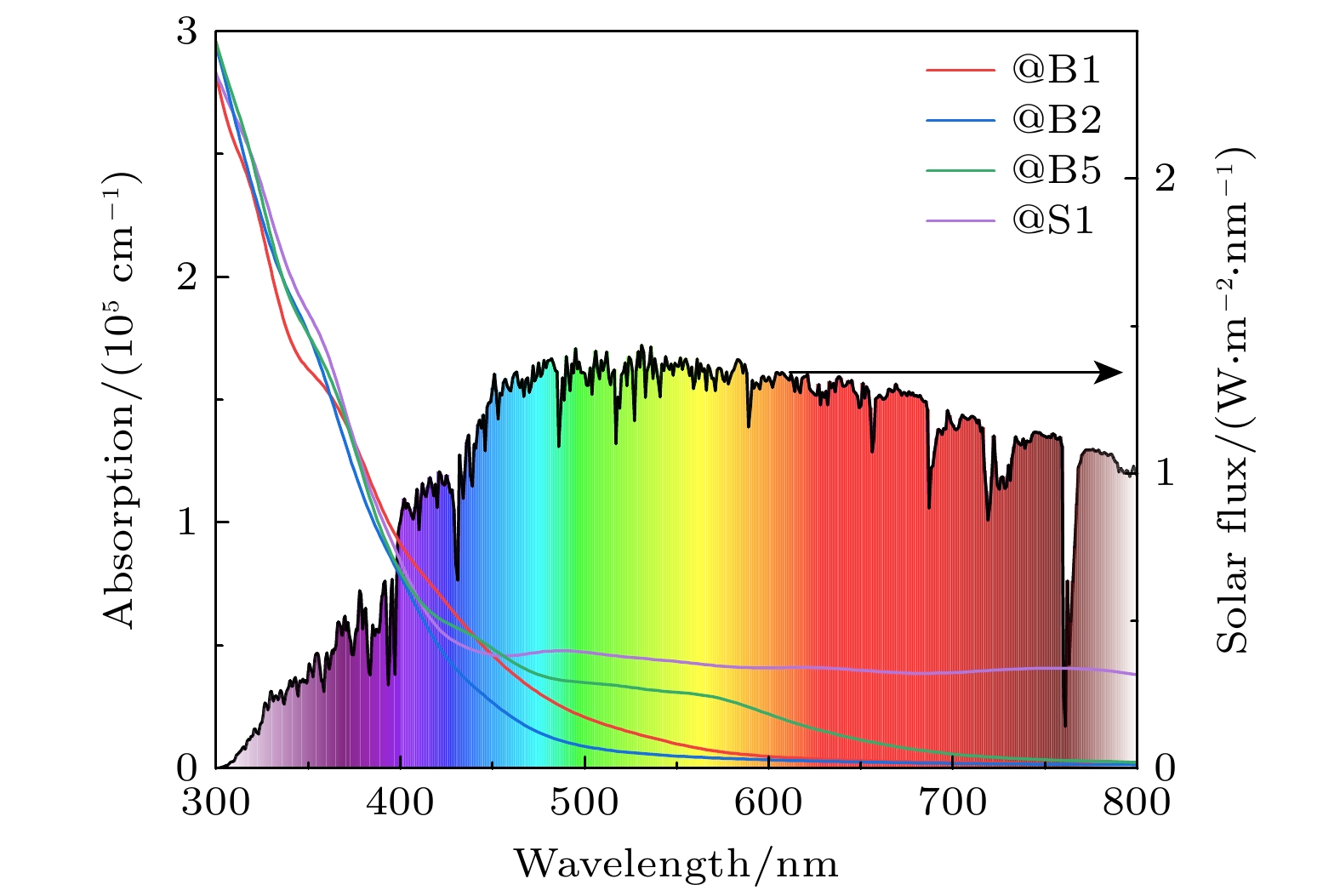
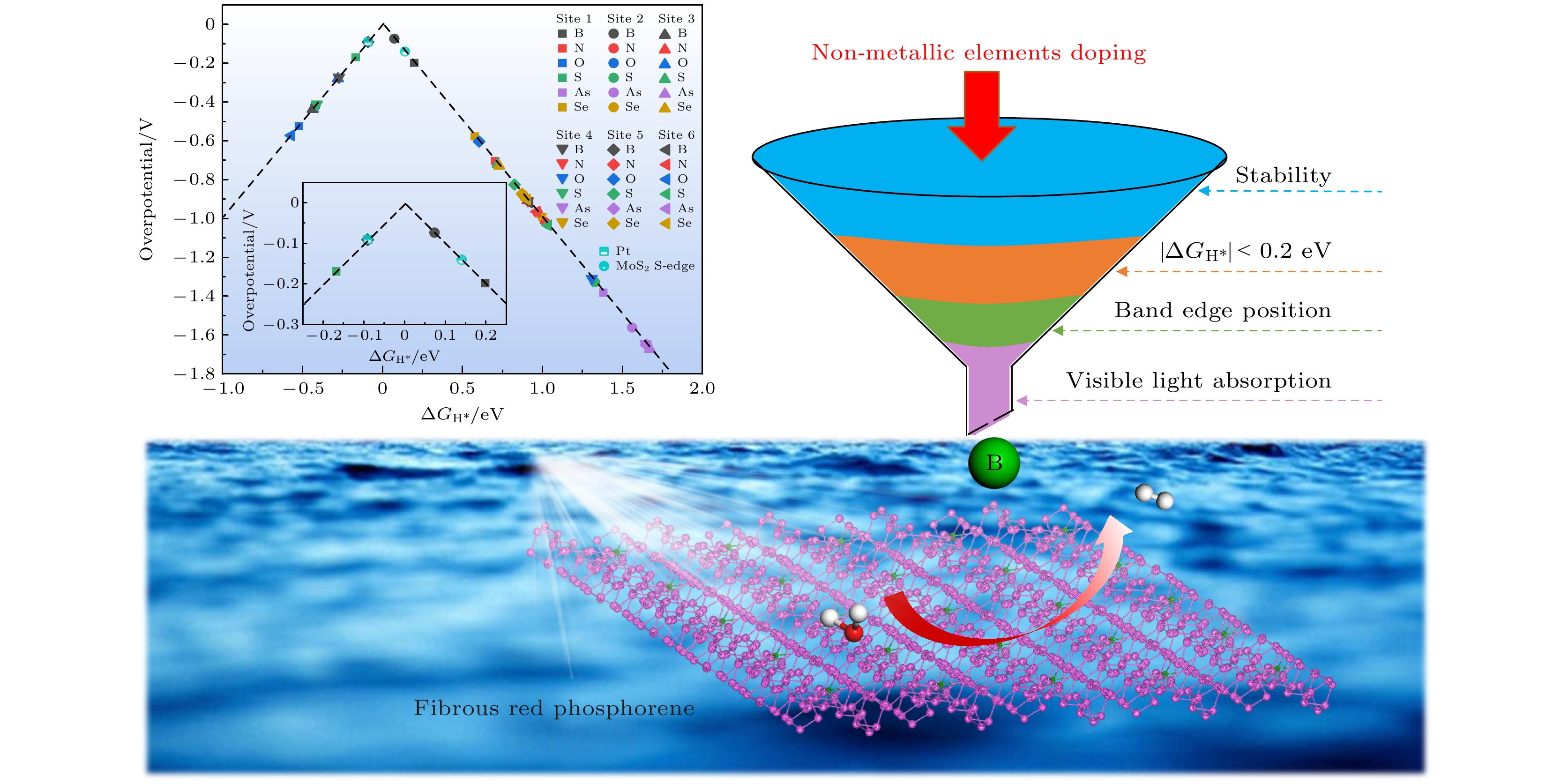
在能源危机与环境污染的双重挑战下, 光催化分解水制氢技术因其绿色可持续特性成为清洁能源领域的研究热点. 纤维红磷(FRP)作为一种新型准一维半导体材料, 凭借其适中的带隙、高载流子迁移率及优异的空气稳定性, 展现出显著的光催化析氢潜力. 基于第一性原理计算, 本文系统探究了一系列非金属元素X (X = B, C, N, O, Si, S, As和Se)掺杂对单层FRP电子结构及催化性能的调控机制. 结果表明, 杂质X能有效提升单层FRP的析氢反应(HER)活性. 其中, 4种掺杂体系(S掺杂位点1、B掺杂位点1/2/5)表现出优异的HER催化活性. 尤其是B掺杂位点2体系, 具有最理想的氢吸附自由能, 其过电位与贵金属Pt催化剂相当. 电子结构分析发现, HER催化活性的增强与吸附位点X pz带中心的下移密切相关, 氢吸附自由能与X pz带中心呈现正相关性, 表明X pz带中心可作为调控HER活性的关键电子描述符. 杂化泛函计算进一步证实, B掺杂体系的带边位置能够横跨水的氧化还原电势两侧, 且光吸收范围覆盖可见光区域, 表明了该体系在光催化全解水应用中的热力学可行性与光谱响应优势. 该研究为基于非金属掺杂策略设计高效非金属基光催化材料提供了重要理论指导.Under the dual challenges of the energy crisis and environmental pollution, the technology of photocatalytic water splitting for hydrogen production has become a research hotspot for clean energy due to its green and sustainable characteristics. Fibrous red phosphorus (FRP), as a novel quasi-one-dimensional semiconductor material, exhibits remarkable photocatalytic hydrogen evolution potential because of its moderate bandgap, high carrier mobility, and excellent air stability. Based on the first-principles calculations, the regulatory mechanisms of electronic structure and catalytic performance of single-layer FRP doped by a series of non-metallic elements X (X = B, C, N, O, Si, S, As, and Se) are systematically investigated in this work. The results show that the element X can effectively enhance the hydrogen evolution reaction (HER) activity of single-layer FRP. Among those doped systems, four specific systems (S-doped at site 1, B-doped at sites 1/2/5) exhibit excellent catalytic activity for HER. Especially, the B-doped system at site 2 has the most ideal free energy of hydrogen adsorption (ΔGH*), and its overpotential (η = –0.074 V) is comparable to that of the noble metal Pt catalyst. The analysis of the electronic structure indicates that the enhancement of the HER catalytic activity is closely related to the downward shift of the X pz-band center at the adsorption site. There is a direct proportional relationship between ΔGH* and the X pz-band center (R2 ≥ 0.78), indicating that the X pz-band center can serve as a key electronic descriptor for regulating the HER activity. Further verification by calculations using the HSE06 hybrid functional shows that the band edge positions of the B-doped system can span both sides of the redox potential of water, and the light absorption range covers the visible light region, indicating the thermodynamic feasibility and spectral response advantages of this system in the application of photocatalytic overall water splitting. This study provides important theoretical guidance for designing efficient FRP-based photocatalytic materials based on the non-metallic doping strategy.
-
Keywords:
- fibrous red phosphorene /
- doping /
- photocatalysis /
- first principles
[1] Fujishima A, Honda K 1972 Nature 238 37
 Google Scholar
Google Scholar
[2] Yin W J, Tang H, Wei S H, Al-Jassim W M, Turner J, Yan Y F 2010 Phys. Rev. B 82 045106
 Google Scholar
Google Scholar
[3] Wei W, Dai Y, Guo M, Yu L, Huang B B 2009 J. Phys. Chem. C 113 15046
 Google Scholar
Google Scholar
[4] 陈钱, 匡勤, 谢兆雄 2021 化学学报 79 10
 Google Scholar
Google Scholar
Chen Q, Kuang Q, Xie Z X 2021 Acta Chim. Sin. 79 10
 Google Scholar
Google Scholar
[5] Zou J, Liao G D, Jiang J Z, Xiong Z G, Bai S S, Wang H T, Wu P X, Zhang P, Li X 2022 Chin. J. Struct. Chem. 41 2201025
 Google Scholar
Google Scholar
[6] Zhang Z J, Zhu Y F, Chen X J, Zhang H J, Wang J 2019 Adv. Mater. 31 1806626
 Google Scholar
Google Scholar
[7] Wang X, Ma M, Zhao X W, Jiang P, Wang Y, Wang J H, Zhang J Y, Zhang F X 2023 Small Struct. 4 2300123
 Google Scholar
Google Scholar
[8] Zhu Y K, Ren J, Zhang X L, Yang D J 2020 Nanoscale 12 13297
 Google Scholar
Google Scholar
[9] Chen Z Y, Zhu Y B, Wang Q M, Liu W Y, Cui Y T, Tao X Y, Zhang D K 2019 Electrochim. Acta 295 230
 Google Scholar
Google Scholar
[10] Bachhuber F, Appen J, Dronskowski R, Schmidt P, Nilges T, Pfitzner A, Weihrich R 2014 Angew. Chem. Int. Ed. 53 11629
 Google Scholar
Google Scholar
[11] Smith J B, Hagaman D, DiGuiseppi D, Schweitzer-Stenner R, Ji H F 2016 Angew. Chem. Int. Ed. 55 11829
 Google Scholar
Google Scholar
[12] Amaral P E M, Nieman G P, Schwenk G R, Jing H, Zhang R, Cerkez E B, Strongin D, Ji H F 2019 Angew. Chem. Int. Ed. 58 6766
 Google Scholar
Google Scholar
[13] Thurn H, Kerbs H 2010 Angew. Chem. Int. Ed. 5 1047
 Google Scholar
Google Scholar
[14] Thurn H, Krebs H 1966 Angew. Chem. Int. Ed. Engl. 5 1047
 Google Scholar
Google Scholar
[15] Tsai H S, Lai C C, Hsiao C H, Medina H, Su T Y, Ouyang H, Chen T H, Liang J H, Chueh Y L 2015 ACS Appl. Mater. Interfaces 7 13723
 Google Scholar
Google Scholar
[16] Shen Z R, Hu Z F, Wang W J, Lee S F, Chan D K L, Li Y C, Gu T, Yu J C 2014 Nanoscale 6 14163
 Google Scholar
Google Scholar
[17] Sun Z J, Chen W J, Zhang B W, Gao L, Tao K Z, Li Q, Sun J L, Yan Q F 2023 Nat. Commun. 14 4398
 Google Scholar
Google Scholar
[18] He S, Liu D M, Zhang G Q, Chu F H, Xu G L, Li G L, Liu J F, Yang Y H, Zhang Y Z 2024 ACS Omega 9 43368
 Google Scholar
Google Scholar
[19] Du L J, Zhao Y C, Wu L L, Hu X R, Yao L D, Wang Y D, Bai X Y, Dai Y Y, Qiao J S, Uddin M G, Li X M, Lahtinen J, Bai X D, Zhang G Y, Ji W, Sun Z P 2021 Nat. Commun. 12 4822
 Google Scholar
Google Scholar
[20] Chu F H, Zhou W C, Zhou R K, Li S Y, Liu D M, Zheng Z L, Li J Z, Zhang Y Z 2022 J. Phys. Chem. Lett. 13 10778
 Google Scholar
Google Scholar
[21] Lu Y L, Dong S J, Li J S, Wu Y Q, Wang L, Zhao H 2020 Phys. Chem. Chem. Phys. 22 13713
 Google Scholar
Google Scholar
[22] Hu Z F, Guo W Q 2021 Small 17 2008004
 Google Scholar
Google Scholar
[23] Wang X H, An C Y, Zhang S J, Wang S S, Li J Z, Zhu Y K 2024 Sep. Purif. Technol. 340 126733
 Google Scholar
Google Scholar
[24] Dai S H, Zhou W, Liu Y Y, Lu Y L, Sun L L, Wu P 2018 Appl. Surf. Sci. 448 281
 Google Scholar
Google Scholar
[25] 韩佳凝, 黄俊铭, 曹胜果, 李占海, 张振华 2023 72 197101
 Google Scholar
Google Scholar
Han J N, Huang J M, Cao S G, Li Z H, Zhang Z H 2023 Acta Phys. Sin. 72 197101
 Google Scholar
Google Scholar
[26] Zhang L, Liu Y Q, Xu Z Y, Gao G Y 2023 2D Mater. 10 045005
[27] Zhang L, Liu Y Q, Wu M H, Gao G Y 2025 Adv. Funct. Mater. 35 2417857
 Google Scholar
Google Scholar
[28] 黄广峥, 李坤玮, 罗艳楠, 张强, 潘远龙, 高洪 2024 化学学报 82 314
 Google Scholar
Google Scholar
Huang G Z, Li K W, Luo Y N, Zhang Q, Pan Y L, Gao H 2024 Acta Chim. Sin. 82 314
 Google Scholar
Google Scholar
[29] Liu H H, Cao X R, Ding L X, Wang H H 2022 Adv. Funct. Mater. 32 2111161
 Google Scholar
Google Scholar
[30] Hu H G, Shi Z, Khan K, Cao R, Liang W Y, Tareen A K, Zhang Y, Huang W C, Guo Z N, Luo X L, Zhang H 2020 J. Mater. Chem. A 8 5421
 Google Scholar
Google Scholar
[31] Lu Y L, Dong S J, He H Y, Li J S, Wang X Y, Zhao H, Wu P 2019 Comput. Mater. Sci. 163 209
 Google Scholar
Google Scholar
[32] 卢一林, 董盛杰, 崔方超, 张开成, 刘春梅, 李杰森, 毛卓 2024 73 016301
 Google Scholar
Google Scholar
Lu Y L, Dong S J, Cui F C, Zhang K C, Liu C M, Li J S, Mao Z 2024 Acta Phys. Sin. 73 016301
 Google Scholar
Google Scholar
[33] Lu Y L, Dong S J, Cui F C, Zhang K C, Liu C M, Li J S, Mao Z 2025 Int. J. Hydrogen Energy 101 222
 Google Scholar
Google Scholar
[34] Lu Y L, Dong S J, He H Y, Li J S, Wu X Y, Zhao H 2022 Physica E 138 115068
 Google Scholar
Google Scholar
[35] He Q Y, Wang D D, Qiu H X, Si N, Yuan Q L, Wang R, Liu S Y, Wang Y M 2024 ACS Nano 19 427
 Google Scholar
Google Scholar
[36] Zhao X W, Gu M Y, Zhai R, Zhang Y H, Jin M T, Wang Y H, Li J F, Cheng Y H, Xiao B, Zhang J Y 2023 Small 19 2302859
 Google Scholar
Google Scholar
[37] Kresse G, Hafner J 1993 Phys. Rev. B 47 R558
 Google Scholar
Google Scholar
[38] Kresse G, Hafner J 1994 Phys. Rev. B 49 14251
 Google Scholar
Google Scholar
[39] Blochl P E 1994 Phys. Rev. B 50 17953
 Google Scholar
Google Scholar
[40] Perdew J P, Burke K, Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[41] Monkhorst H J, Pack J D 1976 Phys. Rev. B 13 5188
 Google Scholar
Google Scholar
[42] Grimme S 2006 J. Comput. Chem. 27 1787
 Google Scholar
Google Scholar
[43] Heyd J, Scuseria G E, Ernzerhof M 2003 J. Chem. Phys. 118 8207
 Google Scholar
Google Scholar
[44] Ruck M, Hoppe D, Wahl B, Simon P, Wang Y, Seifert G 2005 Angew. Chem. Int Ed. 44 7616
 Google Scholar
Google Scholar
[45] Zhang B B, Mao Z, Wu P 2021 Appl. Surf. Sci. 565 150546
 Google Scholar
Google Scholar
[46] Casolo S, Lovvik O M, Martinazzo R, Tantardini G F 2009 J. Chem. Phys. 130 10
 Google Scholar
Google Scholar
[47] Kulish V V, Malyi O I, Persson C, Wu P 2015 Phys. Chem. Chem. Phys. 17 992
 Google Scholar
Google Scholar
[48] Eftekhari A 2017 Int. J. Hydrogen Energy 42 11053
 Google Scholar
Google Scholar
[49] Nørskov J K, Bligaard T, Logadottir A, Kitchin J R, Chen J G, Pandelov S, Stimming U 2005 J. Electrochem. Soc. 152 J23
 Google Scholar
Google Scholar
[50] Zhao Y M, Ma D W, Zhang J, Lu Z S, Wang Y X 2019 Phys. Chem. Chem. Phys. 21 20432
 Google Scholar
Google Scholar
[51] Yuan J, Wang C, Liu Y Y, Wu P, Zhou W 2018 J. Phys. Chem. C 123 526
 Google Scholar
Google Scholar
[52] Zhou S, Yang X W, Pei W, Liu N S, Zhao J J 2018 Nanoscale 10 10876
 Google Scholar
Google Scholar
[53] Pei W, Zhou S, Bai Y Z, Zhao J J 2018 Carbon 133 260
 Google Scholar
Google Scholar
[54] Tsai C, Abild-Pedersen F, Nørskov J K 2014 Nano Lett. 14 1381
 Google Scholar
Google Scholar
[55] 卢一林, 董盛杰, 崔方超, 薄婷婷, 毛卓 2025 化学学报 83 377
 Google Scholar
Google Scholar
Lu Y L, Dong S J, Cui F C, Bo T T, Mao Z 2025 Acta Chim. Sin. 83 377
 Google Scholar
Google Scholar
[56] Chen Y X, Shi T T, Liu P Y, Ma X G, Shui L L, Shang C Q, Chen Z H, Wang X, Kempa K, Zhou G F 2018 J. Mater. Chem. A 6 19167
 Google Scholar
Google Scholar
[57] Liao J M, Sa B S, Zhou J, Ahuja R, Sun Z M 2014 J. Phys. Chem. C 118 17594
 Google Scholar
Google Scholar
[58] Liu J J, Cheng B, Yu J G 2016 Phys. Chem. Chem. Phys. 18 31175
 Google Scholar
Google Scholar
[59] Zhang Y, Qiang Z B, Ding J X, Xie K X, Duan L, Ni L 2024 CrystEngComm 26 2621
 Google Scholar
Google Scholar
-
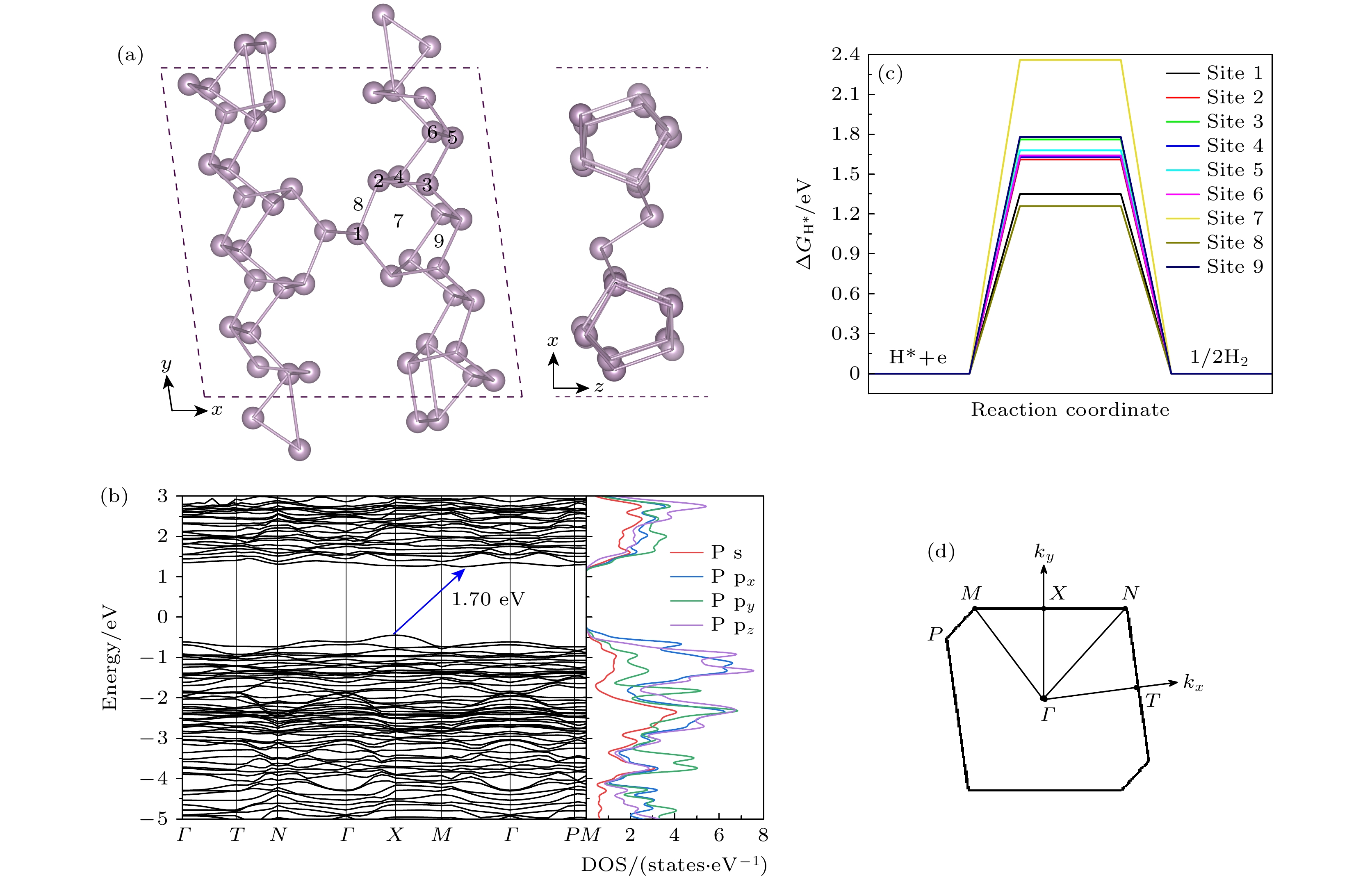
图 1 GGA-PBE泛函计算得到的单层FRP的(a)优化结构俯视图和侧视图, 其中数字1—6表示H吸附的顶位, 7表示H吸附的空心位, 8和9为H吸附的桥位; (b)能带结构和态密度; (c)析氢反应的吉布斯自由能图; (d)第一布里渊区
Fig. 1. (a) Top and side views of the optimized structure, where the numbers 1–6 represent the top sites for hydrogen adsorption, 7 represents the hollow site for hydrogen adsorption, and 8 and 9 are the bridge sites for hydrogen adsorption; (b) band structure and density of states; (c) Gibbs free energy illustration of hydrogen evolution reaction (HER); (d) the first Brillouin zone of FRP monolayer at the GGA-PBE level.
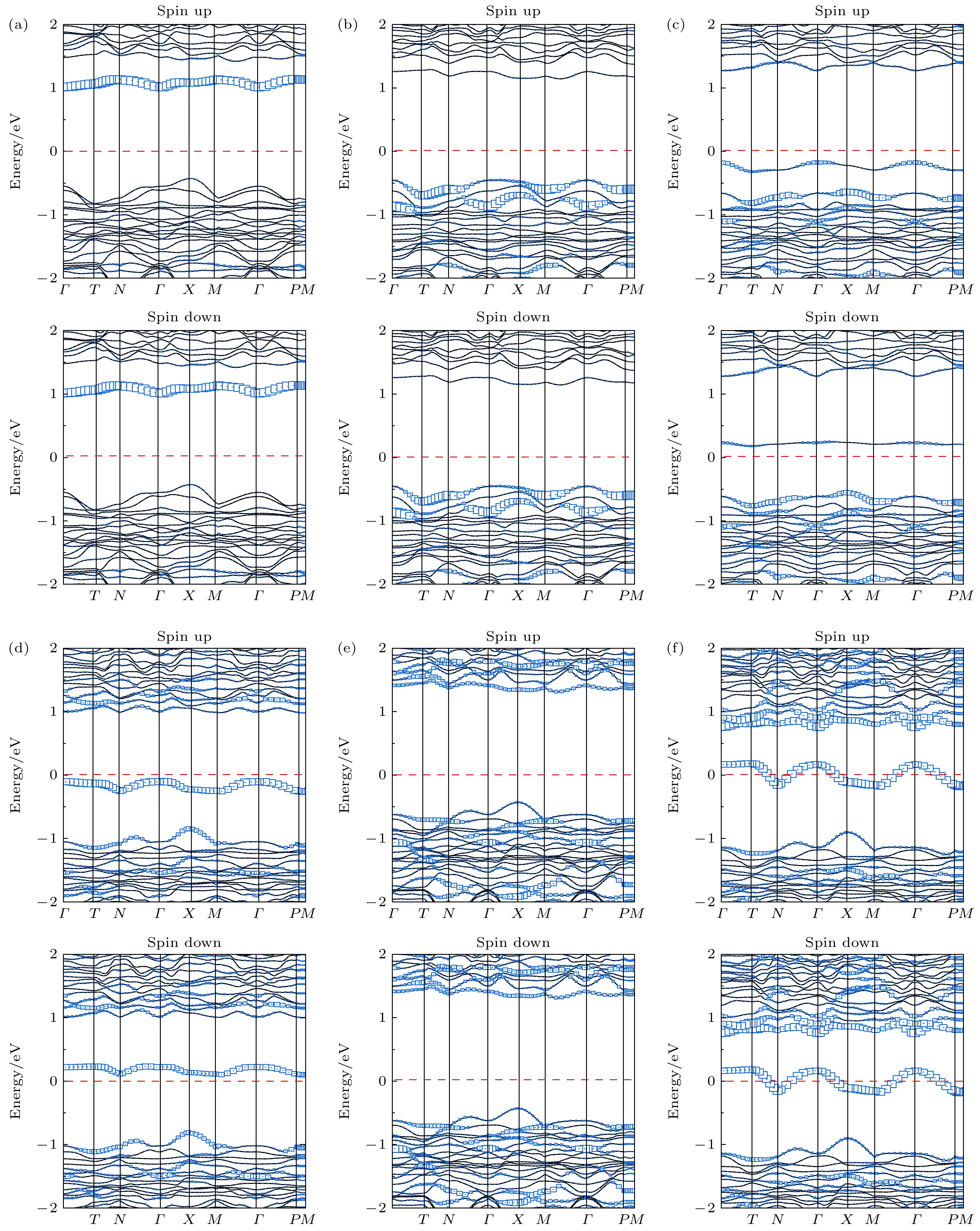
图 5 GGA-PBE泛函计算得到的掺杂体系在位点1下的投影能带结构图 (a) B掺杂; (b) N掺杂; (c) O掺杂; (d) S掺杂; (e) As掺杂; (f) Se掺杂, 其中蓝色方形的大小代表X p轨道的权重
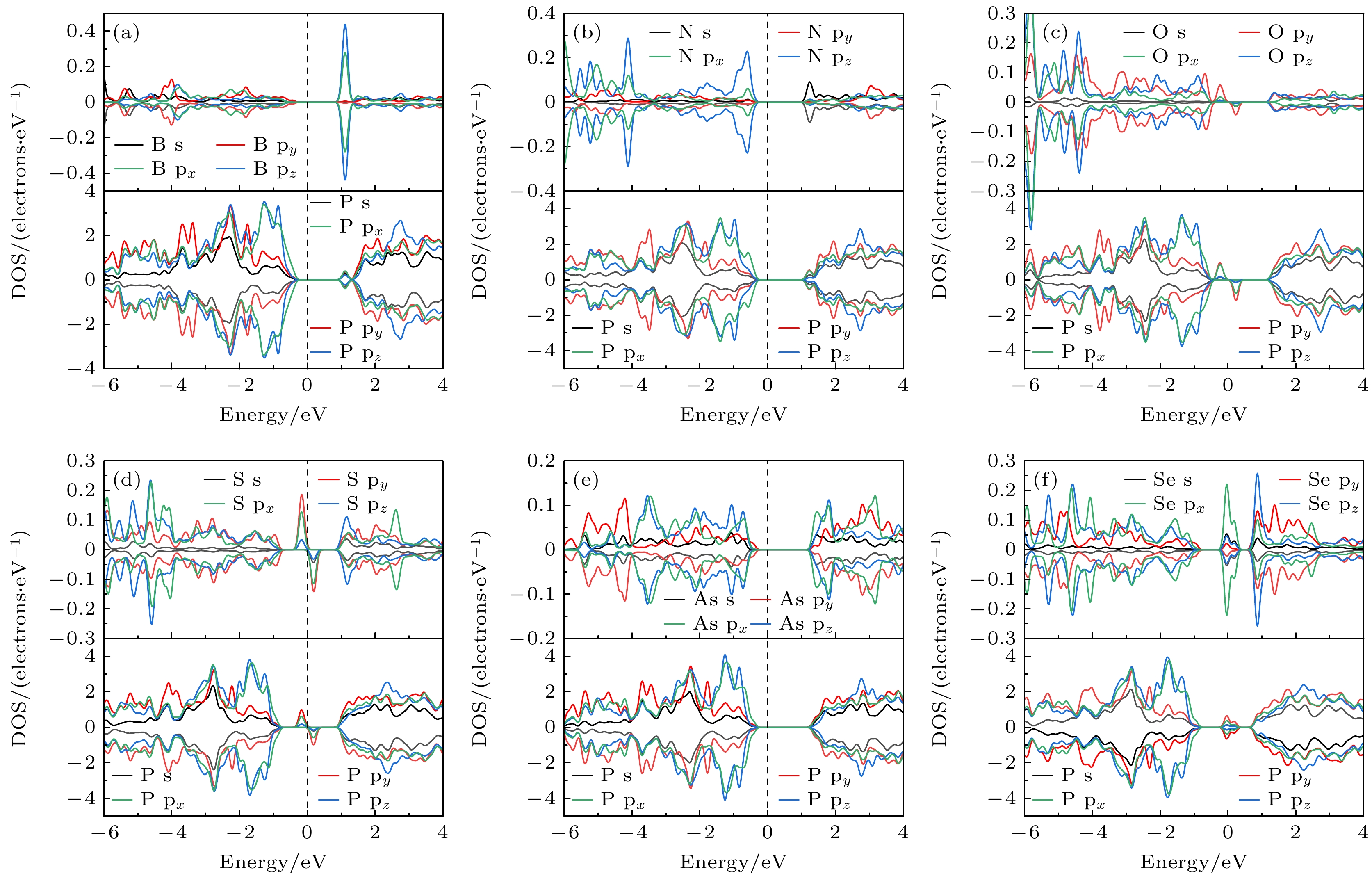
Fig. 5. At the GGA-PBE level, orbital-resolved energy band structures of the doped systems at site 1: (a) B doping; (b) N doping; (c) O doping; (d) S doping; (e) As doping; (f) Se doping, the size of the blue square represents the weight of the X p orbital projection.
图 8 (a) GGA-PBE泛函计算得到的氢分别吸附于活性位点3、位点2和位点6时掺杂体系的投影态密度图; (b) 掺杂体系与吸附物H (Ads.)之间成键的示意图
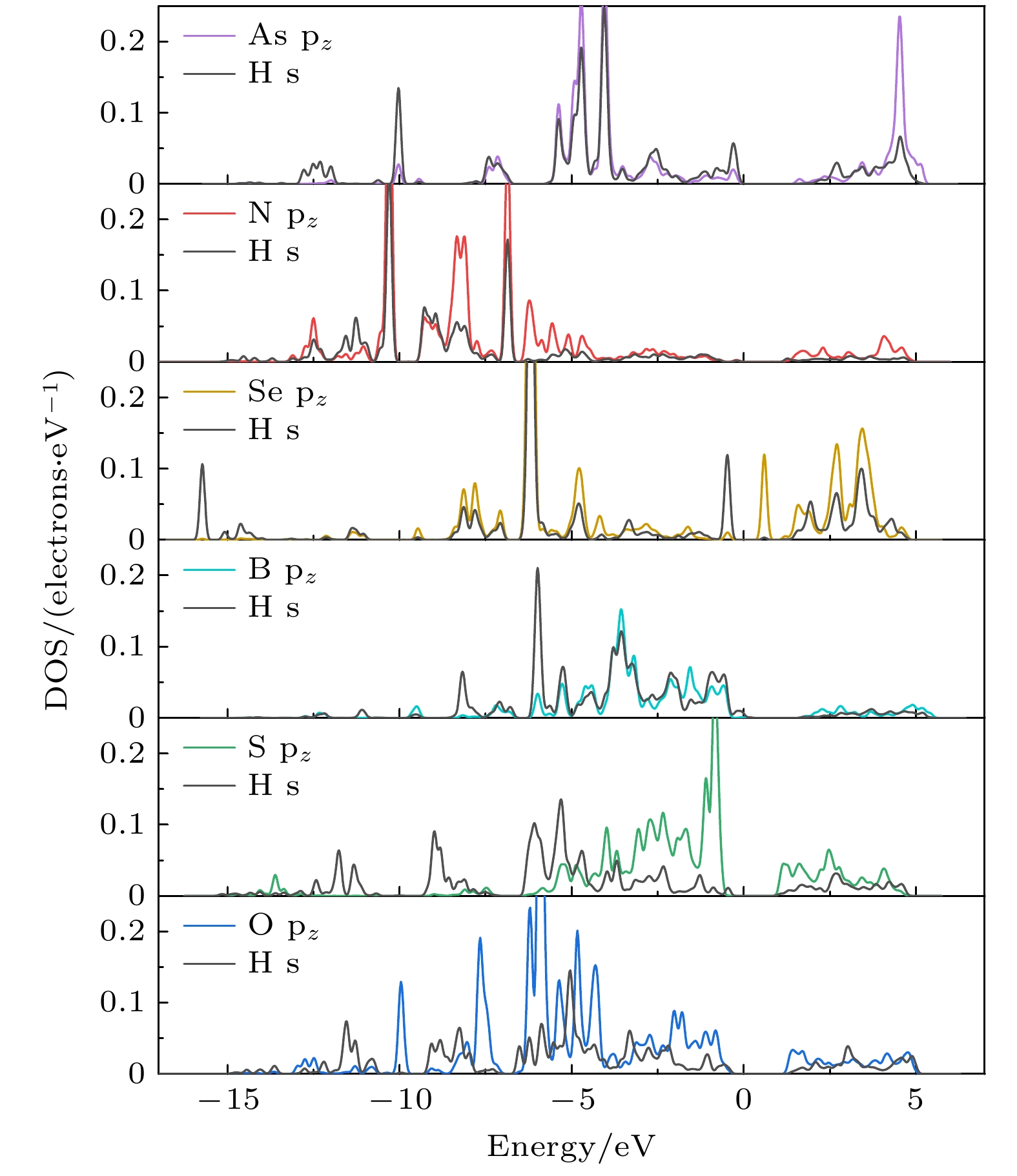
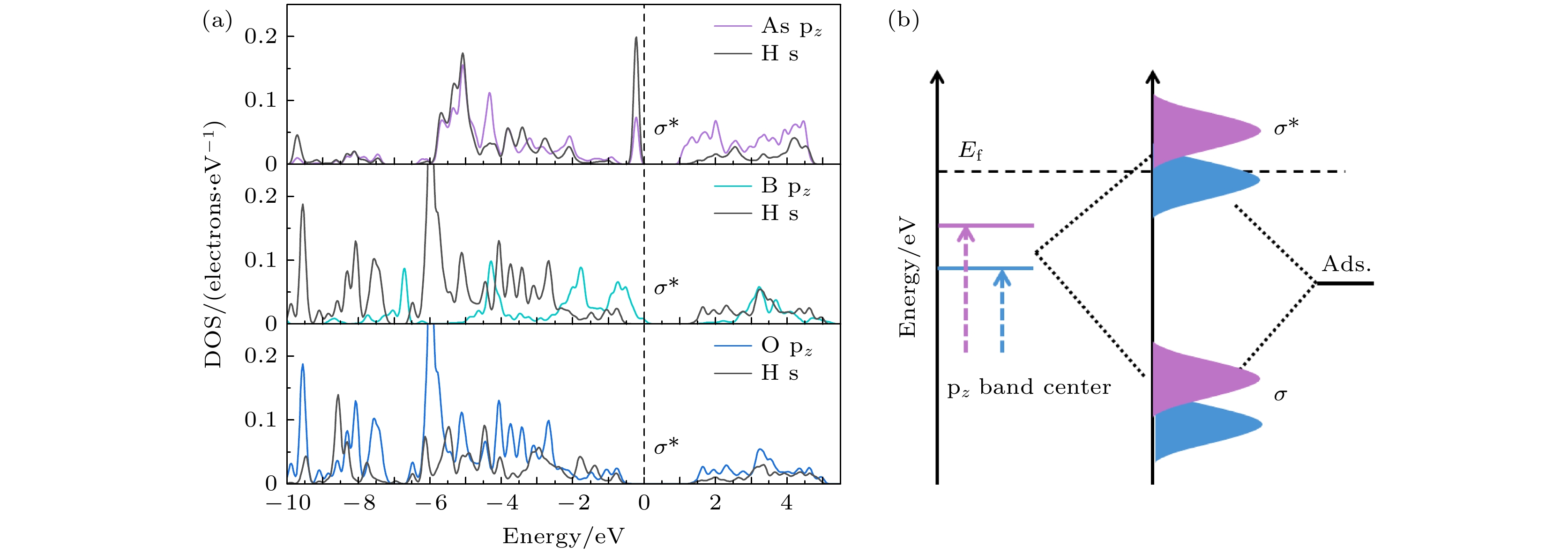
Fig. 8. (a) At the GGA-PBE level, the projected DOS of an H atom adsorbed on the active site 3, site 2, and site 6 of the doped systems; (b) schematic illustration of bond formation between the doped systems and the adsorbate H (Ads.).
图 10 HSE06杂化泛函计算得到的火山顶4种掺杂体系与单层磷烯(FRP, VP[55]和BP[56])、单层二硫化物(SnS2[55]和MoS2[57])、单层g-C3N4[58]及BiVO4(001)[56]表面的导带和价带的带边位置与水分解氧化还原电位的相对关系, 图中灰色区域表示杂质带
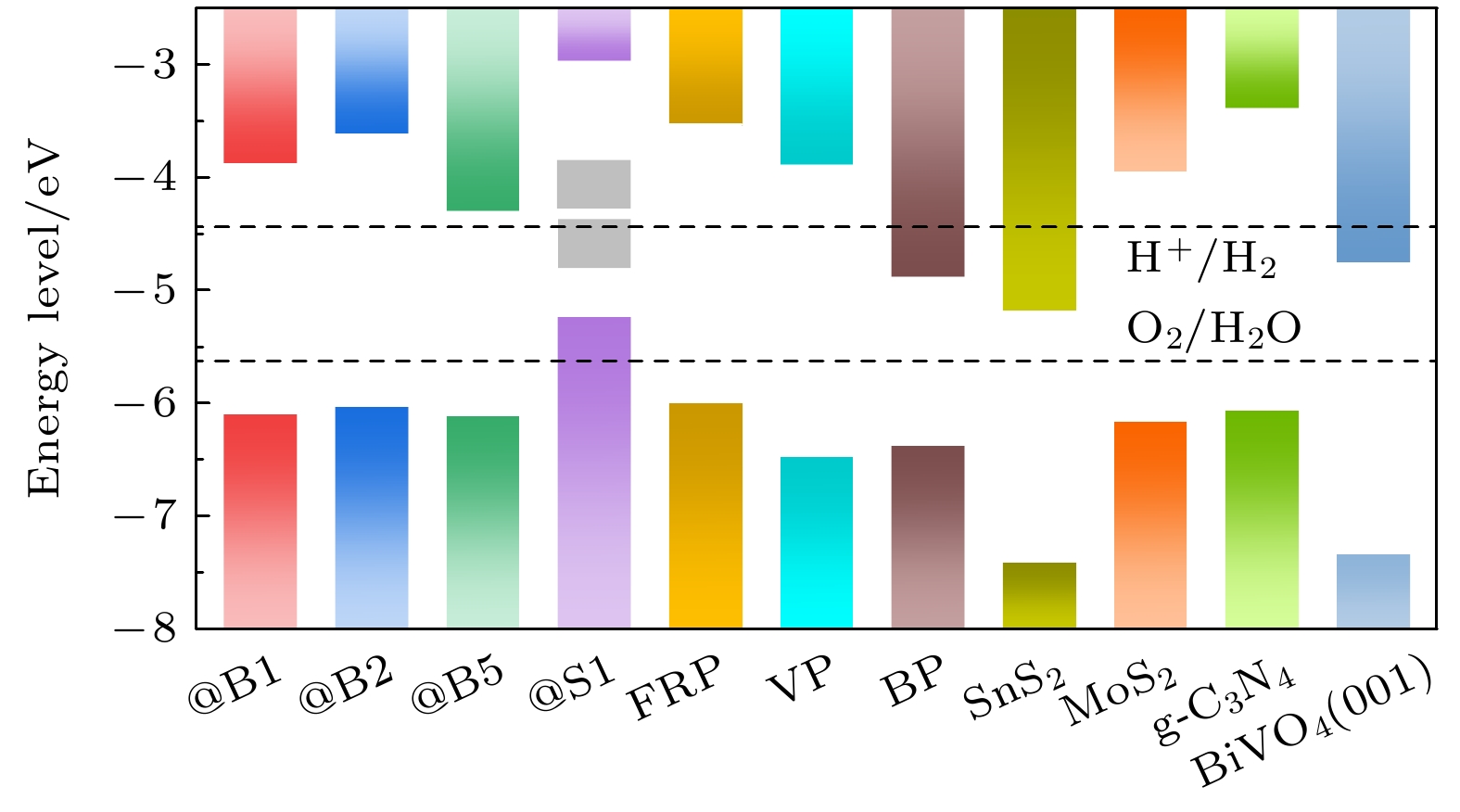
Fig. 10. At the HSE06 level, the conduction and valence band edge positions and the redox potential of water splitting of the four doped systems at the volcano peak, single-layer phosphorene (FRP, VP[55], and BP[56]), single-layer disulfides (SnS2[55] and MoS2[57]), single-layer g-C3N4[58], and BiVO4 (001)[56] surface. The gray area represents the impurity band.
-
[1] Fujishima A, Honda K 1972 Nature 238 37
 Google Scholar
Google Scholar
[2] Yin W J, Tang H, Wei S H, Al-Jassim W M, Turner J, Yan Y F 2010 Phys. Rev. B 82 045106
 Google Scholar
Google Scholar
[3] Wei W, Dai Y, Guo M, Yu L, Huang B B 2009 J. Phys. Chem. C 113 15046
 Google Scholar
Google Scholar
[4] 陈钱, 匡勤, 谢兆雄 2021 化学学报 79 10
 Google Scholar
Google Scholar
Chen Q, Kuang Q, Xie Z X 2021 Acta Chim. Sin. 79 10
 Google Scholar
Google Scholar
[5] Zou J, Liao G D, Jiang J Z, Xiong Z G, Bai S S, Wang H T, Wu P X, Zhang P, Li X 2022 Chin. J. Struct. Chem. 41 2201025
 Google Scholar
Google Scholar
[6] Zhang Z J, Zhu Y F, Chen X J, Zhang H J, Wang J 2019 Adv. Mater. 31 1806626
 Google Scholar
Google Scholar
[7] Wang X, Ma M, Zhao X W, Jiang P, Wang Y, Wang J H, Zhang J Y, Zhang F X 2023 Small Struct. 4 2300123
 Google Scholar
Google Scholar
[8] Zhu Y K, Ren J, Zhang X L, Yang D J 2020 Nanoscale 12 13297
 Google Scholar
Google Scholar
[9] Chen Z Y, Zhu Y B, Wang Q M, Liu W Y, Cui Y T, Tao X Y, Zhang D K 2019 Electrochim. Acta 295 230
 Google Scholar
Google Scholar
[10] Bachhuber F, Appen J, Dronskowski R, Schmidt P, Nilges T, Pfitzner A, Weihrich R 2014 Angew. Chem. Int. Ed. 53 11629
 Google Scholar
Google Scholar
[11] Smith J B, Hagaman D, DiGuiseppi D, Schweitzer-Stenner R, Ji H F 2016 Angew. Chem. Int. Ed. 55 11829
 Google Scholar
Google Scholar
[12] Amaral P E M, Nieman G P, Schwenk G R, Jing H, Zhang R, Cerkez E B, Strongin D, Ji H F 2019 Angew. Chem. Int. Ed. 58 6766
 Google Scholar
Google Scholar
[13] Thurn H, Kerbs H 2010 Angew. Chem. Int. Ed. 5 1047
 Google Scholar
Google Scholar
[14] Thurn H, Krebs H 1966 Angew. Chem. Int. Ed. Engl. 5 1047
 Google Scholar
Google Scholar
[15] Tsai H S, Lai C C, Hsiao C H, Medina H, Su T Y, Ouyang H, Chen T H, Liang J H, Chueh Y L 2015 ACS Appl. Mater. Interfaces 7 13723
 Google Scholar
Google Scholar
[16] Shen Z R, Hu Z F, Wang W J, Lee S F, Chan D K L, Li Y C, Gu T, Yu J C 2014 Nanoscale 6 14163
 Google Scholar
Google Scholar
[17] Sun Z J, Chen W J, Zhang B W, Gao L, Tao K Z, Li Q, Sun J L, Yan Q F 2023 Nat. Commun. 14 4398
 Google Scholar
Google Scholar
[18] He S, Liu D M, Zhang G Q, Chu F H, Xu G L, Li G L, Liu J F, Yang Y H, Zhang Y Z 2024 ACS Omega 9 43368
 Google Scholar
Google Scholar
[19] Du L J, Zhao Y C, Wu L L, Hu X R, Yao L D, Wang Y D, Bai X Y, Dai Y Y, Qiao J S, Uddin M G, Li X M, Lahtinen J, Bai X D, Zhang G Y, Ji W, Sun Z P 2021 Nat. Commun. 12 4822
 Google Scholar
Google Scholar
[20] Chu F H, Zhou W C, Zhou R K, Li S Y, Liu D M, Zheng Z L, Li J Z, Zhang Y Z 2022 J. Phys. Chem. Lett. 13 10778
 Google Scholar
Google Scholar
[21] Lu Y L, Dong S J, Li J S, Wu Y Q, Wang L, Zhao H 2020 Phys. Chem. Chem. Phys. 22 13713
 Google Scholar
Google Scholar
[22] Hu Z F, Guo W Q 2021 Small 17 2008004
 Google Scholar
Google Scholar
[23] Wang X H, An C Y, Zhang S J, Wang S S, Li J Z, Zhu Y K 2024 Sep. Purif. Technol. 340 126733
 Google Scholar
Google Scholar
[24] Dai S H, Zhou W, Liu Y Y, Lu Y L, Sun L L, Wu P 2018 Appl. Surf. Sci. 448 281
 Google Scholar
Google Scholar
[25] 韩佳凝, 黄俊铭, 曹胜果, 李占海, 张振华 2023 72 197101
 Google Scholar
Google Scholar
Han J N, Huang J M, Cao S G, Li Z H, Zhang Z H 2023 Acta Phys. Sin. 72 197101
 Google Scholar
Google Scholar
[26] Zhang L, Liu Y Q, Xu Z Y, Gao G Y 2023 2D Mater. 10 045005
[27] Zhang L, Liu Y Q, Wu M H, Gao G Y 2025 Adv. Funct. Mater. 35 2417857
 Google Scholar
Google Scholar
[28] 黄广峥, 李坤玮, 罗艳楠, 张强, 潘远龙, 高洪 2024 化学学报 82 314
 Google Scholar
Google Scholar
Huang G Z, Li K W, Luo Y N, Zhang Q, Pan Y L, Gao H 2024 Acta Chim. Sin. 82 314
 Google Scholar
Google Scholar
[29] Liu H H, Cao X R, Ding L X, Wang H H 2022 Adv. Funct. Mater. 32 2111161
 Google Scholar
Google Scholar
[30] Hu H G, Shi Z, Khan K, Cao R, Liang W Y, Tareen A K, Zhang Y, Huang W C, Guo Z N, Luo X L, Zhang H 2020 J. Mater. Chem. A 8 5421
 Google Scholar
Google Scholar
[31] Lu Y L, Dong S J, He H Y, Li J S, Wang X Y, Zhao H, Wu P 2019 Comput. Mater. Sci. 163 209
 Google Scholar
Google Scholar
[32] 卢一林, 董盛杰, 崔方超, 张开成, 刘春梅, 李杰森, 毛卓 2024 73 016301
 Google Scholar
Google Scholar
Lu Y L, Dong S J, Cui F C, Zhang K C, Liu C M, Li J S, Mao Z 2024 Acta Phys. Sin. 73 016301
 Google Scholar
Google Scholar
[33] Lu Y L, Dong S J, Cui F C, Zhang K C, Liu C M, Li J S, Mao Z 2025 Int. J. Hydrogen Energy 101 222
 Google Scholar
Google Scholar
[34] Lu Y L, Dong S J, He H Y, Li J S, Wu X Y, Zhao H 2022 Physica E 138 115068
 Google Scholar
Google Scholar
[35] He Q Y, Wang D D, Qiu H X, Si N, Yuan Q L, Wang R, Liu S Y, Wang Y M 2024 ACS Nano 19 427
 Google Scholar
Google Scholar
[36] Zhao X W, Gu M Y, Zhai R, Zhang Y H, Jin M T, Wang Y H, Li J F, Cheng Y H, Xiao B, Zhang J Y 2023 Small 19 2302859
 Google Scholar
Google Scholar
[37] Kresse G, Hafner J 1993 Phys. Rev. B 47 R558
 Google Scholar
Google Scholar
[38] Kresse G, Hafner J 1994 Phys. Rev. B 49 14251
 Google Scholar
Google Scholar
[39] Blochl P E 1994 Phys. Rev. B 50 17953
 Google Scholar
Google Scholar
[40] Perdew J P, Burke K, Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[41] Monkhorst H J, Pack J D 1976 Phys. Rev. B 13 5188
 Google Scholar
Google Scholar
[42] Grimme S 2006 J. Comput. Chem. 27 1787
 Google Scholar
Google Scholar
[43] Heyd J, Scuseria G E, Ernzerhof M 2003 J. Chem. Phys. 118 8207
 Google Scholar
Google Scholar
[44] Ruck M, Hoppe D, Wahl B, Simon P, Wang Y, Seifert G 2005 Angew. Chem. Int Ed. 44 7616
 Google Scholar
Google Scholar
[45] Zhang B B, Mao Z, Wu P 2021 Appl. Surf. Sci. 565 150546
 Google Scholar
Google Scholar
[46] Casolo S, Lovvik O M, Martinazzo R, Tantardini G F 2009 J. Chem. Phys. 130 10
 Google Scholar
Google Scholar
[47] Kulish V V, Malyi O I, Persson C, Wu P 2015 Phys. Chem. Chem. Phys. 17 992
 Google Scholar
Google Scholar
[48] Eftekhari A 2017 Int. J. Hydrogen Energy 42 11053
 Google Scholar
Google Scholar
[49] Nørskov J K, Bligaard T, Logadottir A, Kitchin J R, Chen J G, Pandelov S, Stimming U 2005 J. Electrochem. Soc. 152 J23
 Google Scholar
Google Scholar
[50] Zhao Y M, Ma D W, Zhang J, Lu Z S, Wang Y X 2019 Phys. Chem. Chem. Phys. 21 20432
 Google Scholar
Google Scholar
[51] Yuan J, Wang C, Liu Y Y, Wu P, Zhou W 2018 J. Phys. Chem. C 123 526
 Google Scholar
Google Scholar
[52] Zhou S, Yang X W, Pei W, Liu N S, Zhao J J 2018 Nanoscale 10 10876
 Google Scholar
Google Scholar
[53] Pei W, Zhou S, Bai Y Z, Zhao J J 2018 Carbon 133 260
 Google Scholar
Google Scholar
[54] Tsai C, Abild-Pedersen F, Nørskov J K 2014 Nano Lett. 14 1381
 Google Scholar
Google Scholar
[55] 卢一林, 董盛杰, 崔方超, 薄婷婷, 毛卓 2025 化学学报 83 377
 Google Scholar
Google Scholar
Lu Y L, Dong S J, Cui F C, Bo T T, Mao Z 2025 Acta Chim. Sin. 83 377
 Google Scholar
Google Scholar
[56] Chen Y X, Shi T T, Liu P Y, Ma X G, Shui L L, Shang C Q, Chen Z H, Wang X, Kempa K, Zhou G F 2018 J. Mater. Chem. A 6 19167
 Google Scholar
Google Scholar
[57] Liao J M, Sa B S, Zhou J, Ahuja R, Sun Z M 2014 J. Phys. Chem. C 118 17594
 Google Scholar
Google Scholar
[58] Liu J J, Cheng B, Yu J G 2016 Phys. Chem. Chem. Phys. 18 31175
 Google Scholar
Google Scholar
[59] Zhang Y, Qiang Z B, Ding J X, Xie K X, Duan L, Ni L 2024 CrystEngComm 26 2621
 Google Scholar
Google Scholar
计量
- 文章访问数: 548
- PDF下载量: 9
- 被引次数: 0













 下载:
下载: