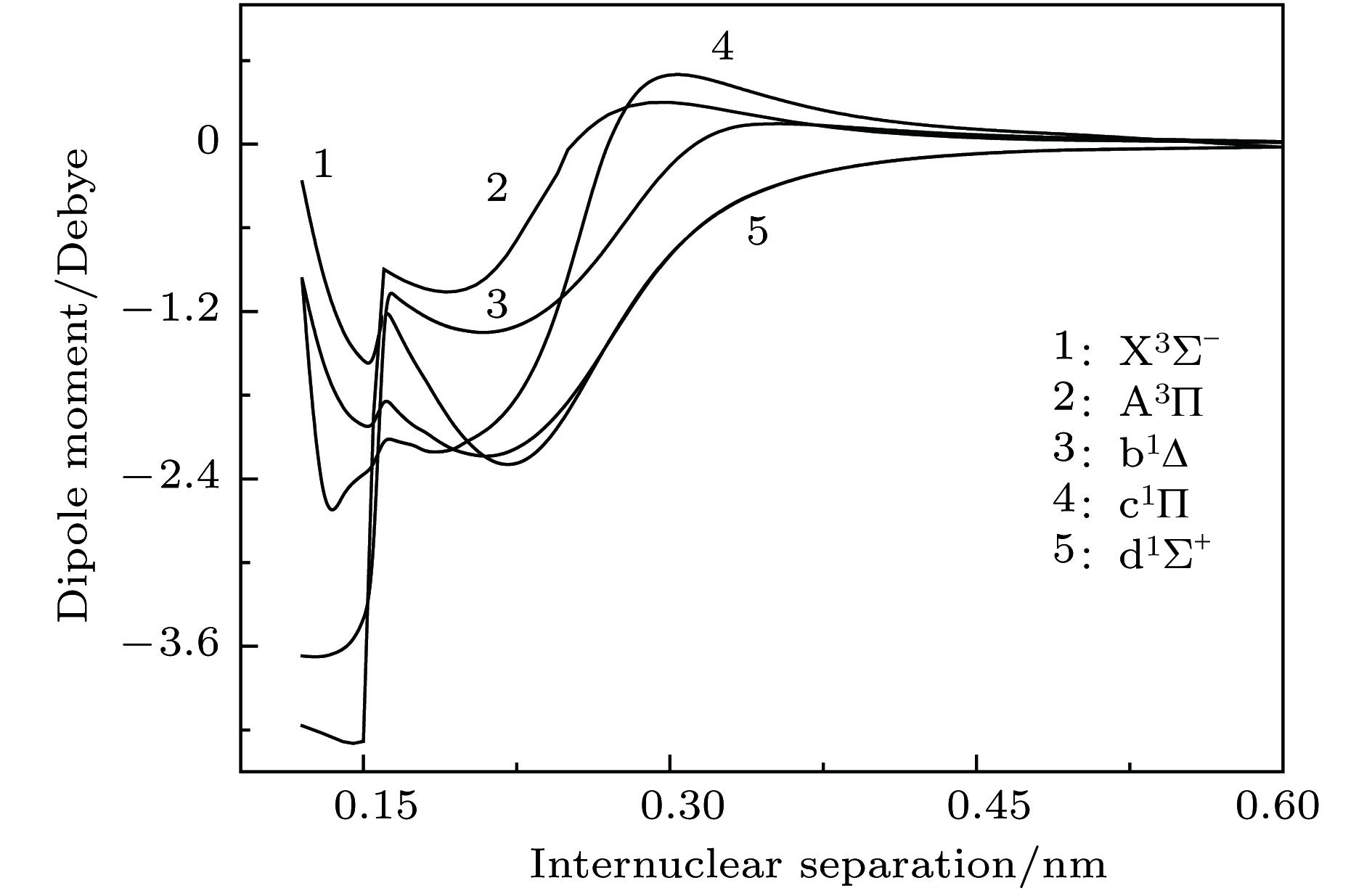
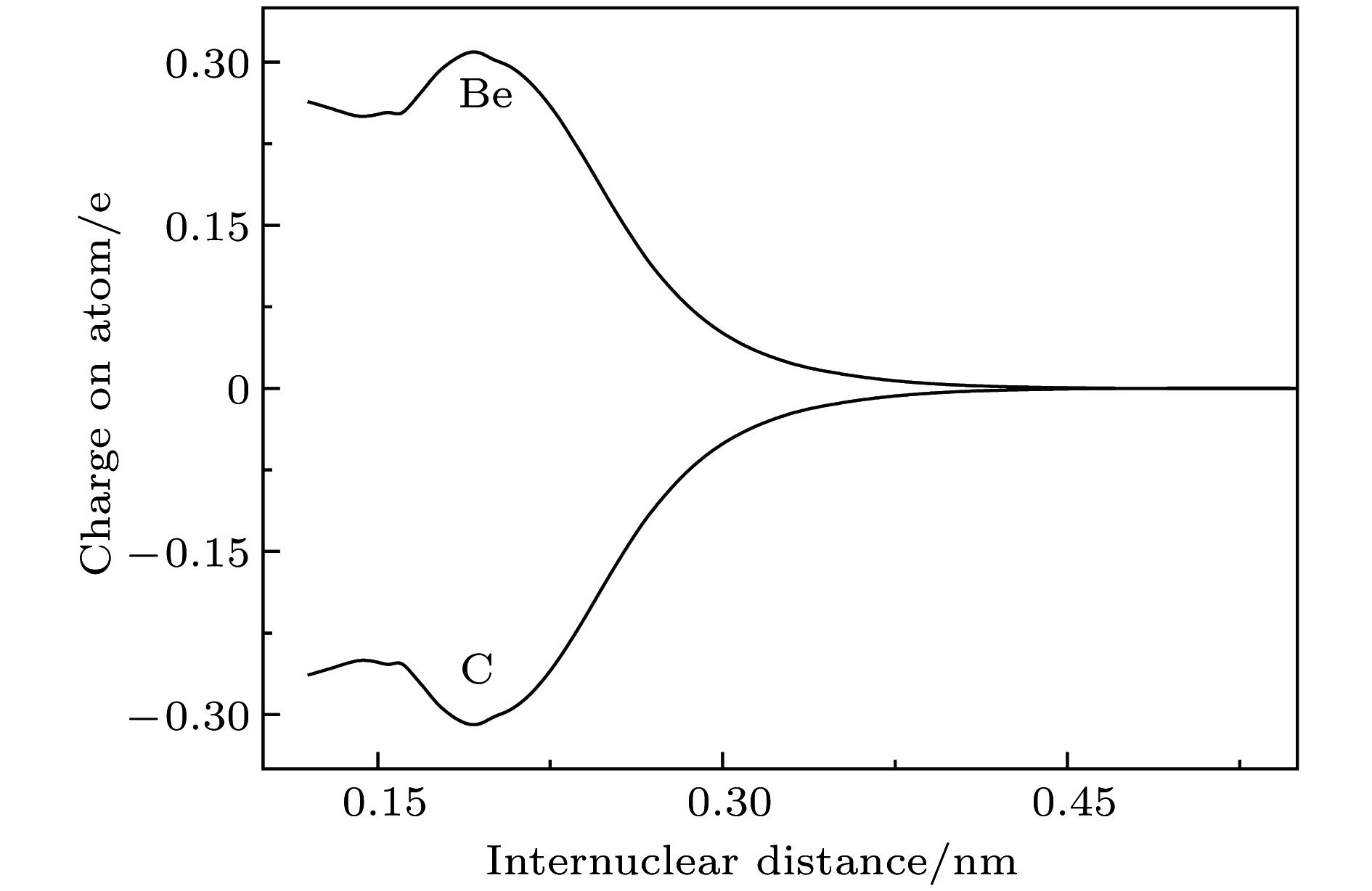
-
BeC是一个具有丰富低激发电子态的分子, 本文基于动态权重完全活性空间自冾场方法获得的参考波函数, 采用多参考组态相互作用方法对BeC分子进行高精度的从头计算, 获得了BeC分子
$ {{\rm{X}}^3} {{\text{Σ}} ^ - } $ ,${\rm{A}}^3 {\text{Π}}$ ,$ {{\rm{b}}^1} {{\text{Δ}} } $ ,${{\rm{c}}^1} {\text{Π}}$ 和$ {{\rm{d}}^1}{{\text{Σ}} ^ + } $ 共5个电子态的势能曲线. 为了获得精确的光谱结果, 在计算中考虑了标量相对论效应修正, 并把相互作用能外推至完全基组极限. 在此基础上获得了这些态的光谱常数和偶极距, 以及一些允许跃迁的跃迁偶极距、弗兰克-康登因子和辐射寿命. 最后, 通过扩展的Rydberg函数拟合获得了基态势能曲线精确的解析表达式.Diatomic molecule BeC has a complex electronic structure with a large number of low-lying excited states that are all strongly bound electronic states. Thus, the BeC molecule has the abundant spectral information. In this work, the potential energy curves and wavefunctions of$ {{\rm{X}}^3} {{\text{Σ}} ^ - } $ ,${\rm{A}}^3 {\text{Π}}$ ,$ {{\rm{b}}^1} {{\text{Δ}} } $ ,${{\rm{c}}^1} {\text{Π}}$ and$ {{\rm{d}}^1}{{\text{Σ}} ^ + } $ states of the BeC molecule are calculated by using the internally contracted multi-reference configuration interaction (MRCI) approach, which is based on the use of a dynamically weighted complete active space self-consistent field (DW-CASSCF) procedure. To improve the reliability and accuracy of calculation, the scalar relativistic corrections and the extrapolation of potential energy to the complete basis set limit are taken into account. On the basis of the calculated potential energy curves and wavefunctions, the spectroscopic constants (Te, Re,${\omega _{\rm{e}}}$ ,${\omega _{\rm{e}}}{x_{\rm{e}}}$ ,${\omega _{\rm{e}}}{y_{\rm{e}}}$ , Be,${\alpha _{\rm{e}}}$ , and De) and permanent dipole moments of those states are determined, the results of which are in good agreement with the existing available experimental and theoretical values. The obtained permanent dipole moments indicate that the electrons transfer from Be to C and the polarity for molecule is$ {\rm{B}}{{\rm{e}}^{{\text{δ}} + }}{{\rm{C}}^{{\text{δ}} - }}$ . The transition properties of the spin-allowed${\rm{A}}^3 {\text{Π}}$ −$ {{\rm{X}}^3} {{\text{Σ}} ^ - } $ ,${{\rm{c}}^1} {\text{Π}}$ −$ {{\rm{b}}^1} {{\text{Δ}} } $ ,${{\rm{c}}^1} {\text{Π}}$ −$ {{\rm{d}}^1}{{\text{Σ}} ^ + } $ transitions are predicted, including the transition dipole moments, Franck-Condon factors, and radiative lifetimes. The radiative lifetimes for the${\rm{A}}^3 {\text{Π}}$ −$ {{\rm{X}}^3} {{\text{Σ}} ^ - } $ transitions are predicated to be at a$ {{\text{µ}}\rm{ s}}$ level, and the good agreement with previous theoretical values is found. Radiative lifetimes for${{\rm{c}}^1} {\text{Π}}$ −$ {{\rm{b}}^1} {{\text{Δ}} } $ and${{\rm{c}}^1} {\text{Π}}$ −$ {{\rm{d}}^1}{{\text{Σ}} ^ + } $ transitions are also evaluated at the levels of$ {{\text{µ}}\rm{ s}}$ and ms, respectively. The PEC for the ground state is fitted into accurate analytical potential energy functions by using the extended-Rydberg potential function.-
Keywords:
- dynamically weighted /
- spectroscopic constants /
- transition properties /
- BeC
[1] Borodin D, Doerner R, Nishijima D, Kirschner A, Kreter A, Matveev D, Galonska A, Philipps V 2011 J. Nucl. Mater. 415 219
 Google Scholar
Google Scholar
[2] Conn R W, Doerner R P, Won J 1997 Fusion Eng. Des. 37 481
[3] Chen M D, Li X B, Yang J, Zhang Q E, Au C T 2006 Int. J. Mass Spectrom. 253 30
 Google Scholar
Google Scholar
[4] Chen M D, Li X B, Yang J, Zhang Q E 2006 J. Phys. Chem. A 110 4502
[5] Zhang C J 2006 J. Mol. Struc.: Theochem. 759 201
 Google Scholar
Google Scholar
[6] Barker B J, Antonov I O, Merritt J M, Bondybey V E, Heaven M C, Dawes R 2012 J. Chem. Phys. 137 214313
 Google Scholar
Google Scholar
[7] Wright J S, Kolbuszewski M 1993 J. Chem. Phys. 98 9725
 Google Scholar
Google Scholar
[8] Borin A C, Ornellas F R 1993 J. Chem. Phys. 98 8761
 Google Scholar
Google Scholar
[9] da Silva C O, Teixeira F E C, Azevedo, J A T, Da Silva E C, Nascimento M A C 1996 Int. J. Quant. Chem. 60 433
 Google Scholar
Google Scholar
[10] Pelegrini M, Roberto-Neto O, Ornellas F R, Machado F B C 2004 Chem. Phys. Lett. 383 143
 Google Scholar
Google Scholar
[11] Wells N, Lane I C 2011 Phys. Chem. Chem. Phys. 13 19036
 Google Scholar
Google Scholar
[12] Koch W, Frenking G, Gauss J, Cremer D, Sawaryn A, Schleyer P V R 1986 J. Am. Chem. Soc. 108 5732
 Google Scholar
Google Scholar
[13] Fioressi S E, Binning Jr R C, Bacelo D E 2014 Chem. Phys. 443 76
 Google Scholar
Google Scholar
[14] Patrick A D, Williams P, Blaisten-Barojas E 2007 J. Mol. Struc.: Theochem. 824 39
 Google Scholar
Google Scholar
[15] Ghouri M M, Yareeda L, Mainardi D S 2007 J. Phys. Chem. A 111 13133
 Google Scholar
Google Scholar
[16] Midda S, Das A K 2004 J. Mol. Spectrosc. 224 1
 Google Scholar
Google Scholar
[17] Borin A C, Ornellas F R 1995 Chem. Phys. 190 43
 Google Scholar
Google Scholar
[18] Borin A C, Ornellas F R A 1995 J. Mol. Struc.: Theochem 335 107
 Google Scholar
Google Scholar
[19] Teberekidis V I, Kerkines I S K, Carsky P, Tsipis C A, Mavridis A 2005 Int. J. Quant. Chem. 102 762
 Google Scholar
Google Scholar
[20] Deskevich M P, Nesbitt D J, Werner H J 2004 J. Chem. Phys. 120 7281
 Google Scholar
Google Scholar
[21] Dawes R, Jasper A W, Tao C, Richmond C, Mukarakate C, Kable S H, Reid S A 2010 J. Phys. Chem. Lett. 1 641
 Google Scholar
Google Scholar
[22] Werner H J, Knowles P J 1988 J. Chem. Phys. 89 5803
 Google Scholar
Google Scholar
[23] Knowles P J, Werner H J 1988 Chem. Phys. Lett. 145 514
 Google Scholar
Google Scholar
[24] Werner H J, Knowles P, Knizia G, Manby F R, Schütz M, Celani P, Korona T, Lindh R, Mitrushenkov A, Rauhut G 2010 Molpro Version 2010.1: A Package of ab initio Programs
[25] Peterson K A, Wilson A K, Woon D E, Dunning Jr T H 1997 Theor. Chem. Acc. 97 251
 Google Scholar
Google Scholar
[26] Woon D E, Dunning Jr T H 1995 J. Chem. Phys. 103 4572
 Google Scholar
Google Scholar
[27] Prascher B P, Woon D E, Peterson K A, Dunning T H, Wilson A K 2011 Theor. Chem. Acc. 128 69
 Google Scholar
Google Scholar
[28] Karton A, Martin J M L. 2006 Theor. Chem. Acc. 115 330
 Google Scholar
Google Scholar
[29] Halkier A, Helgaker T, Jørgesen P, Klopper W, Koch H, Olsen J, Wilson A K 1998 Chem. Phys. Lett. 286 243
 Google Scholar
Google Scholar
[30] Nakajima T, Hirao K 2000 J. Chem. Phys. 113 7786
 Google Scholar
Google Scholar
[31] Fedorov D G, Nakajima T, Hirao K 2003 J. Chem. Phys. 118 4970
 Google Scholar
Google Scholar
[32] Paulovic J, Nakajima T, Hirao K, Lindh R, Malmqvist P Å 2003 J. Chem. Phys. 119 798
 Google Scholar
Google Scholar
[33] NIST, Atomic Spectra Database, see http://www.nist.gov/pml/data/asd.cfm (2012)
[34] Haris K, Kramida A, 2017 Astrophys. J. Suppl. S. 233 16
 Google Scholar
Google Scholar
[35] LeRoy R J 2010 LEVEL 8.0: A Computer Program for Solving the Radial Schrödinger Equation for Bound and Quasibound Levels, Chemical Physics: Research Report CP-663 (Ontario: University of Waterloo)
[36] Herzberg G. 1950 Molecular Spectra and Molecular Structure I. Spectra of Diatomic Molecules(New York: Van Nostrand Reinhold) p658
[37] Murrel J N, Carter S, Farantos S C, Huxley P, Varandas A J C 1984 Molecular Potential Energy Functions (Wiley: Chichester, U. K.) p9
[38] 7D-Soft High Technology, Inc. 1st Opt User Manual (V.6.0) Beijing, People’s Republic of China, 2014
-
表 1 BeC分子6个态的离解极限关系
Table 1. Dissociation relationship of six electronic states of BeC molecule.
原子态 $ {\text{Λ}}{\text{-}}{\rm S}$态 相对能量/cm–1 本文 (无Q) 本文 (+Q) 实验值[34] Be(1Sg)+C(3Pg) $ {{\rm{X}}^3} {{\text{Σ}} ^ - } $, ${\rm{A}}^3 {\text{Π}}$ 0 0 Be(1Sg)+C(1Dg) $ {{\rm{b}}^1} {{\text{Δ}} } $, ${{\rm{c}}^1} {\text{Π}}$, $ {{\rm{d}}^1}{{\text{Σ}} ^ + } $ 10124.12 10169.70 10192.66 Be(1Sg)+C(1Sg) ${2^1}{{\text{Σ}} ^ + } $ 21679.58 21581.62 21648.03 表 2 BeC分子
$ {{\rm{X}}^3}{\text{Σ}} $ ,${{\rm{A}}^3}{\text{Π}}$ ,$ {{\rm{b}}^1} {{\text{Δ}} } $ ,${{\rm{c}}^1} {\text{Π}}$ 和$ {{\rm{d}}^1}{{\text{Σ}} ^ + } $ 等5个态的光谱参数Table 2. Spectroscopic constants of the five states of BeC molecule.
Te/cm–1 Re/nm ${\omega _{\rm{e}}}$/cm–1 $ {\omega _{\rm{e}}}{x_{\rm{e}}} $/cm–1 102${\omega _{\rm{e}}}$уe/cm–1 Be/cm–1 $ {10^3}{\alpha _{\rm{e}}}$/cm–1 De/eV ${{{\rm{X}}^3}{\text{Σ}}^- } $ 0 0.1673 918.08 7.350 17.87 1.1783 15.644 2.1873 Cal. [6] 0 0.1661 937.9 9.6 — 1.19 — — Cal. [7] 0 0.1693 905 — — — — 2.04 Cal. [8] 0 0.1667 951 8.42 — 1.183 — 2.39 Cal. [10] 0 0.1683 925 11.25 — — — 2.14 Cal. [19] 0 0.1680 — — — — — 2.04 ${{\rm{A}}^3}{\text{Π}}$ 8916.35 0.1771 772.74 8.692 199.89 1.0518 28.367 1.0777 Cal. [7] 9033.41 0.1799 — — — — — 0.92 Cal. [8] 9466 0.1756 764 14.69 — 1.0752 — 1.16 Cal. [10] 8961 0.1791 874 26.26 — — — 1.03 $ {{\rm{b}}^1}{\text{Δ}} $ 7823.39 0.1675 933.50 8.301 7.56 1.1714 16.700 2.4408 Cal. [7] 8872.10 0.1693 904 — — — — 2.27 Cal. [8] 8732 0.1668 956 7.6 — 1.1757 — 2.63 $ {{\rm{c}}^1}{\text{Π}} $ 10909.21 0.1760 834.80 7.138 9.20 1.0551 14.365 2.0933 Cal. [7] 11291.76 0.1778 818 — — — — 1.97 Cal. [8] 11618 0.1758 847 6.94 — 1.0612 — 2.24 $ {{\rm{d}}^1}{{\text{Σ}} ^ + } $ 12139.14 0.16698 936.21 17.553 126.98 1.1764 16.000 1.9060 Cal. [7] 12582.24 0.1693 905 — — — — 1.81 Cal. [8] 13579 0.167 955 7.3 — 1.1732 — 2.02 注: Cal. 为理论计算值. 表 3
${{\rm{A}}^3}{\text{Π}}$ —$ {{\rm{X}}^3} {{\text{Σ}} ^ - } $ ,${{\rm{c}}^1}{\text{Π}}$ —$ {{\rm{b}}^1} {{\text{Δ}} } $ ,$ {{\rm{d}}^1}{{\text{Σ}} ^ + } $ —${{\rm{c}}^1}{\text{Π}}$ 跃迁的弗兰克-康登因子Table 3. Franck-condon factor for
${{\rm{A}}^3}{\text{Π}}$ −$ {{\rm{X}}^3} {{\text{Σ}} ^ - } $ ,${{\rm{c}}^1}{\text{Π}}$ −$ {{\rm{b}}^1}{{\text{Δ}} } $ ,$ {{\rm{d}}^1}{{\text{Σ}} ^ + } $ −${{\rm{c}}^1}{\text{Π}}$ transitions.$\nu '$—$ \nu ''$ FC $\nu '$—$ \nu ''$ FC $\nu '$—$ \nu ''$ FC $\nu '$—$ \nu ''$ FC $\nu '$—$ \nu ''$ FC $\nu '$— $ \nu ''$ FC ${\rm{A}}^3 {\text{Π}}$—${\rm{X}}^3 {\text{Σ}}^-$ 0—0 0.5439 0—1 0.3653 1—0 0.3049 1—2 0.4143 1—3 0.1670 2—0 0.1080 2—1 0.2660 2—3 0.3601 2—4 0.2276 3—2 0.1654 3—4 0.2908 3—5 0.2691 4—2 0.1763 4—5 0.2255 4—6 0.2993 5—3 0.1561 5—6 0.1639 5—7 0.3216 6—3 0.1070 6—4 0.1273 6—6 0.1339 6—7 0.1052 6—8 0.3348 6—9 0.1183 7—4 0.1095 7—5 0.1000 7—7 0.1618 7—9 0.3342 7—10 0.1531 ${{\rm{c}}^1}{\text{Π}}$ —${{\rm{b}}^1}{\text{Δ}}$ 0—0 0.6237 0—1 0.3153 1—0 0.2701 1—1 0.1740 1—2 0.3999 1—3 0.1368 2—1 0.2689 2—3 0.3470 2—4 0.2322 3—1 0.1518 3—2 0.1803 3—4 0.2122 3—5 0.2964 4—2 0.1894 4—4 0.1027 4—5 0.1045 4—6 0.3042 4—7 0.1238 5—3 0.1634 5—5 0.1435 5—7 0.3138 6—3 0.1236 6—4 0.1268 6—6 0.1567 6—8 0.2968 7—4 0.1289 7—7 0.1495 7—9 0.2690 8—5 0.1219 8—8 0.1212 8—10 0.2380 9—11 0.2025 10—12 0.1699 ${{\rm{d}}^1}{\text{Σ}}$—${{\rm{c}}^1}{\text{Π}}$ 0—0 0.6164 0—1 0.2717 1—0 0.3232 1—1 0.1693 1—2 0.2617 2—1 0.4016 2—3 0.1699 2—4 0.1850 3—1 0.3901 3—2 0.3513 3—5 0.1510 4—2 0.2362 4—3 0.2156 4—4 0.1073 4—6 0.1068 5—3 0.3014 5—4 0.1011 5—5 0.1501 6—4 0.3109 6—6 0.1613 7—4 0.1292 7—5 0.3175 7—7 0.1442 8—5 0.1801 8—6 0.2945 8—8 0.1054 9—6 0.2192 9—7 0.2612 10—7 0.2563 10—8 0.2265 表 4
${\rm{A}}^3 {\text{Π}}$ ,$ {{\rm{b}}^1} {{\text{Δ}} } $ ,${{\rm{c}}^1}{\text{Π}}$ 和$ {{\rm{d}}^1}{{\text{Σ}} ^ + } $ 态几个振动能级的辐射寿命Table 4. Radiative lifetime of the vibrational energy levels for
${\rm{A}}^3 {\text{Π}}$ ,$ {{\rm{b}}^1} {{\text{Δ}} } $ ,${{\rm{c}}^1} {\text{Π}}$ and$ {{\rm{d}}^1}{{\text{Σ}} ^ + } $ states.跃迁 辐射寿命$/{{\text{μ}}{\rm{ s}}}$ $\nu '$ = 0 $\nu '$ = 1 $\nu '$ = 2 $\nu '$ = 3 $\nu '$ = 4 $\nu '$ = 5 $\nu '$ = 6 $\nu '$ = 7 ${\rm{A}}^3 {\text{Π}}$—${{\rm{X}}^3}{{\text{Σ}} ^ - }$ 14.66 14.64 14.46 13.82 12.99 12.16 11.65 11.31 Cal.[10] 14.0 14.5 15.6 ${{\rm{c}}^1} {\text{Π}}$—$ {{\rm{b}}^1} {{\text{Δ}} } $ 191.10 166.59 161.38 159.48 151.88 176.09 197.32 239.85 ${{\rm{c}}^1} {\text{Π}}$—$ {{\rm{d}}^1}{{\text{Σ}} ^ + } $ 2730.00 1010.00 630.00 460.00 380.00 330 280.00 230.00 表 5 BeC分子基态解析势能函数参数值
Table 5. The values for the analytic parameters for the ground state of BeC molecule.
参数 C1 C2 C3 C4 C5 参数值 4.584720688 6.825173375 4.979780662 2.012372055 1.900852427 参数 C6 C7 C8 C9 C10 参数值 –5.415648763 –6.033517345 12.82526525 –6.086434893 0.939009741 -
[1] Borodin D, Doerner R, Nishijima D, Kirschner A, Kreter A, Matveev D, Galonska A, Philipps V 2011 J. Nucl. Mater. 415 219
 Google Scholar
Google Scholar
[2] Conn R W, Doerner R P, Won J 1997 Fusion Eng. Des. 37 481
[3] Chen M D, Li X B, Yang J, Zhang Q E, Au C T 2006 Int. J. Mass Spectrom. 253 30
 Google Scholar
Google Scholar
[4] Chen M D, Li X B, Yang J, Zhang Q E 2006 J. Phys. Chem. A 110 4502
[5] Zhang C J 2006 J. Mol. Struc.: Theochem. 759 201
 Google Scholar
Google Scholar
[6] Barker B J, Antonov I O, Merritt J M, Bondybey V E, Heaven M C, Dawes R 2012 J. Chem. Phys. 137 214313
 Google Scholar
Google Scholar
[7] Wright J S, Kolbuszewski M 1993 J. Chem. Phys. 98 9725
 Google Scholar
Google Scholar
[8] Borin A C, Ornellas F R 1993 J. Chem. Phys. 98 8761
 Google Scholar
Google Scholar
[9] da Silva C O, Teixeira F E C, Azevedo, J A T, Da Silva E C, Nascimento M A C 1996 Int. J. Quant. Chem. 60 433
 Google Scholar
Google Scholar
[10] Pelegrini M, Roberto-Neto O, Ornellas F R, Machado F B C 2004 Chem. Phys. Lett. 383 143
 Google Scholar
Google Scholar
[11] Wells N, Lane I C 2011 Phys. Chem. Chem. Phys. 13 19036
 Google Scholar
Google Scholar
[12] Koch W, Frenking G, Gauss J, Cremer D, Sawaryn A, Schleyer P V R 1986 J. Am. Chem. Soc. 108 5732
 Google Scholar
Google Scholar
[13] Fioressi S E, Binning Jr R C, Bacelo D E 2014 Chem. Phys. 443 76
 Google Scholar
Google Scholar
[14] Patrick A D, Williams P, Blaisten-Barojas E 2007 J. Mol. Struc.: Theochem. 824 39
 Google Scholar
Google Scholar
[15] Ghouri M M, Yareeda L, Mainardi D S 2007 J. Phys. Chem. A 111 13133
 Google Scholar
Google Scholar
[16] Midda S, Das A K 2004 J. Mol. Spectrosc. 224 1
 Google Scholar
Google Scholar
[17] Borin A C, Ornellas F R 1995 Chem. Phys. 190 43
 Google Scholar
Google Scholar
[18] Borin A C, Ornellas F R A 1995 J. Mol. Struc.: Theochem 335 107
 Google Scholar
Google Scholar
[19] Teberekidis V I, Kerkines I S K, Carsky P, Tsipis C A, Mavridis A 2005 Int. J. Quant. Chem. 102 762
 Google Scholar
Google Scholar
[20] Deskevich M P, Nesbitt D J, Werner H J 2004 J. Chem. Phys. 120 7281
 Google Scholar
Google Scholar
[21] Dawes R, Jasper A W, Tao C, Richmond C, Mukarakate C, Kable S H, Reid S A 2010 J. Phys. Chem. Lett. 1 641
 Google Scholar
Google Scholar
[22] Werner H J, Knowles P J 1988 J. Chem. Phys. 89 5803
 Google Scholar
Google Scholar
[23] Knowles P J, Werner H J 1988 Chem. Phys. Lett. 145 514
 Google Scholar
Google Scholar
[24] Werner H J, Knowles P, Knizia G, Manby F R, Schütz M, Celani P, Korona T, Lindh R, Mitrushenkov A, Rauhut G 2010 Molpro Version 2010.1: A Package of ab initio Programs
[25] Peterson K A, Wilson A K, Woon D E, Dunning Jr T H 1997 Theor. Chem. Acc. 97 251
 Google Scholar
Google Scholar
[26] Woon D E, Dunning Jr T H 1995 J. Chem. Phys. 103 4572
 Google Scholar
Google Scholar
[27] Prascher B P, Woon D E, Peterson K A, Dunning T H, Wilson A K 2011 Theor. Chem. Acc. 128 69
 Google Scholar
Google Scholar
[28] Karton A, Martin J M L. 2006 Theor. Chem. Acc. 115 330
 Google Scholar
Google Scholar
[29] Halkier A, Helgaker T, Jørgesen P, Klopper W, Koch H, Olsen J, Wilson A K 1998 Chem. Phys. Lett. 286 243
 Google Scholar
Google Scholar
[30] Nakajima T, Hirao K 2000 J. Chem. Phys. 113 7786
 Google Scholar
Google Scholar
[31] Fedorov D G, Nakajima T, Hirao K 2003 J. Chem. Phys. 118 4970
 Google Scholar
Google Scholar
[32] Paulovic J, Nakajima T, Hirao K, Lindh R, Malmqvist P Å 2003 J. Chem. Phys. 119 798
 Google Scholar
Google Scholar
[33] NIST, Atomic Spectra Database, see http://www.nist.gov/pml/data/asd.cfm (2012)
[34] Haris K, Kramida A, 2017 Astrophys. J. Suppl. S. 233 16
 Google Scholar
Google Scholar
[35] LeRoy R J 2010 LEVEL 8.0: A Computer Program for Solving the Radial Schrödinger Equation for Bound and Quasibound Levels, Chemical Physics: Research Report CP-663 (Ontario: University of Waterloo)
[36] Herzberg G. 1950 Molecular Spectra and Molecular Structure I. Spectra of Diatomic Molecules(New York: Van Nostrand Reinhold) p658
[37] Murrel J N, Carter S, Farantos S C, Huxley P, Varandas A J C 1984 Molecular Potential Energy Functions (Wiley: Chichester, U. K.) p9
[38] 7D-Soft High Technology, Inc. 1st Opt User Manual (V.6.0) Beijing, People’s Republic of China, 2014
计量
- 文章访问数: 11109
- PDF下载量: 77
- 被引次数: 0































 下载:
下载: