-
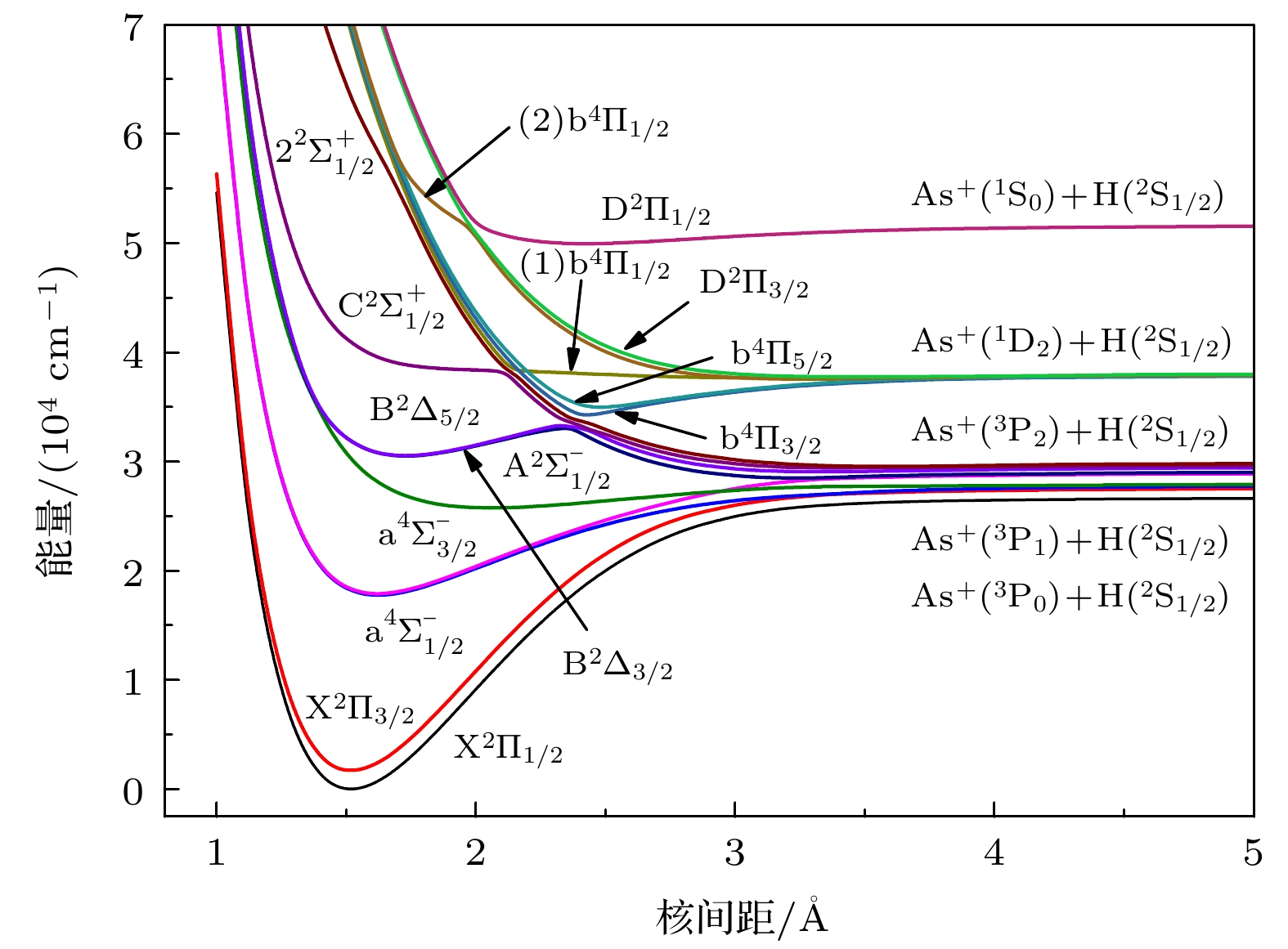
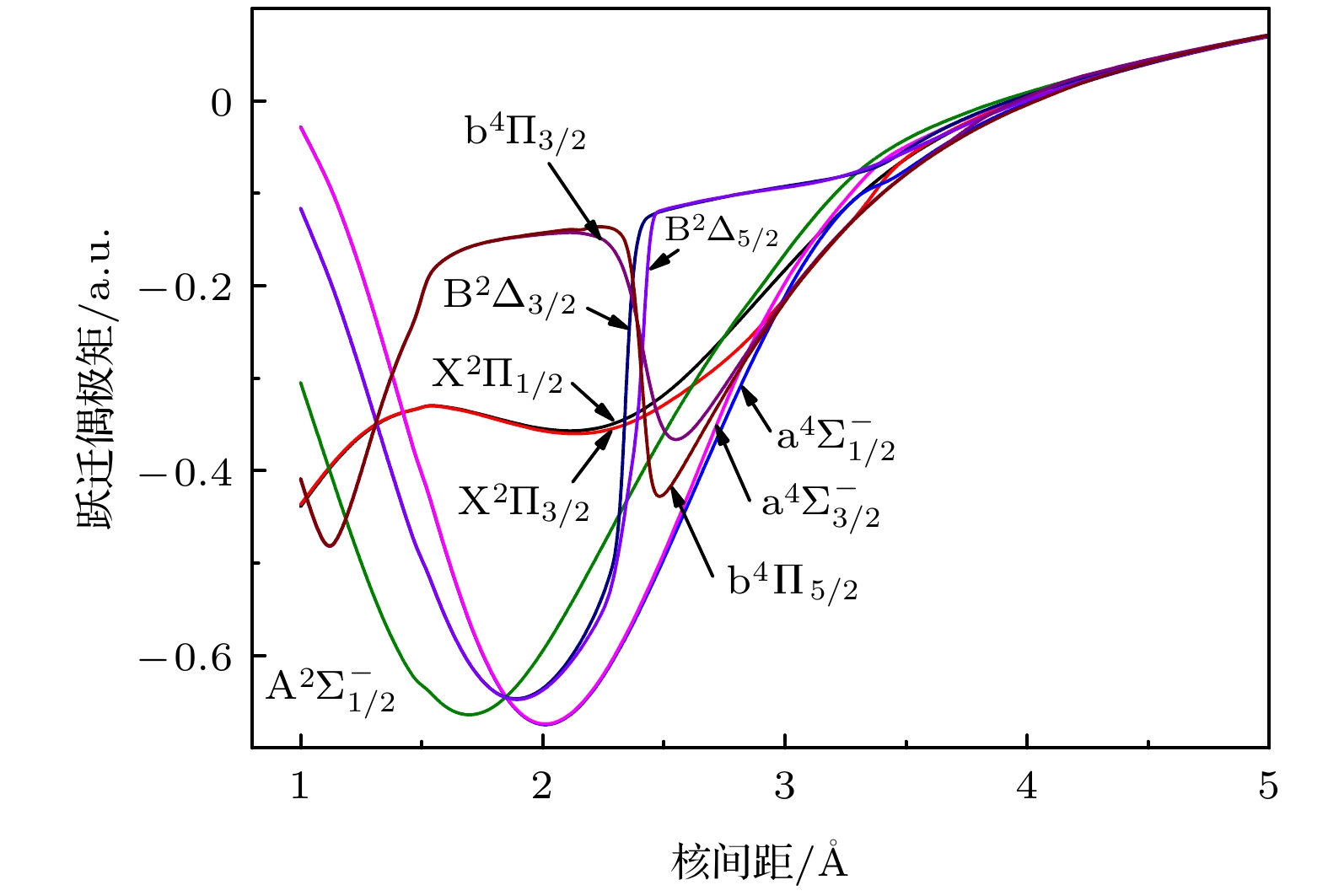
采用多参考组态相互作用方法计算了AsH+离子前3个离解极限所对应的8个电子态 (X2Π, a4Σ–, A2Σ–, b4Π, B2Δ, C2Σ+, D2Π, 22Σ+) 的电子结构. As原子选择了aug-cc-pwCV5Z-PP相对论赝势基组. 在计算中考虑了Davidson修正, 芯-价电子关联和自旋-轨道耦合效应. 拟合得到了所有态的光谱常数, 离解能越大的电子态, 其谐振频率越大, 平衡核间距越小. 考虑自旋-轨道耦合效应后, 由于避免交叉, B2Δ3/2和B2Δ5/2变为双势阱结构. 最后预测了
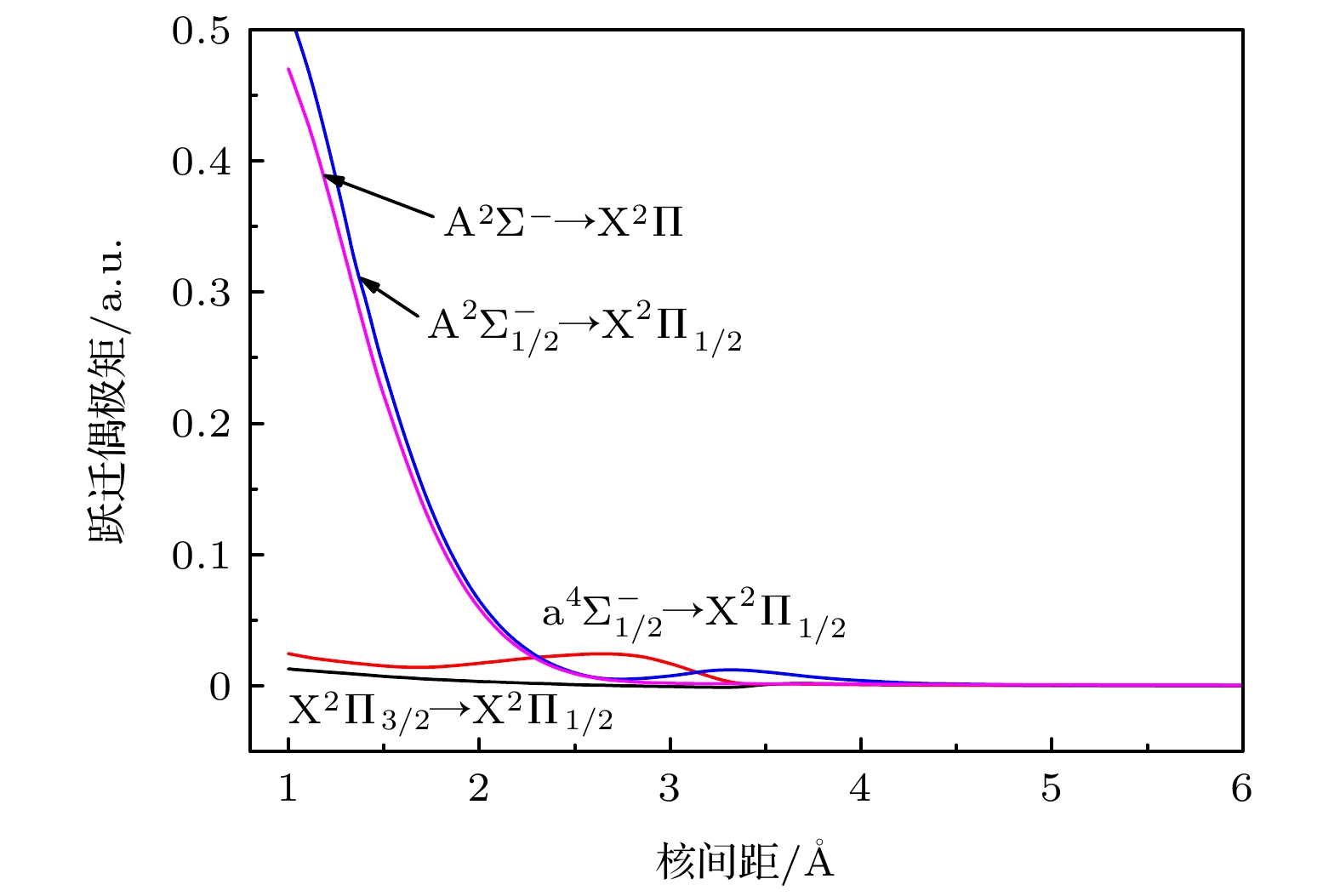
$ {{{\rm{A}}^2}}{\Sigma ^ - } \to {{{\rm{X}}^2}}\Pi $ ,$ {{{\rm{a}}^4}}\Sigma _{1/2}^ - \to {{{\rm{X}}^2}}{\Pi _{1/2}} $ 和$ {{{\rm{A}}^2}}\Sigma _{1/2}^ - \to {{{\rm{X}}^2}}{\Pi _{1/2}} $ 跃迁的弗兰克-康登因子、自发辐射速率和自发辐射寿命, 计算结果表明$ {{{\rm{a}}^4}}\Sigma _{1/2}^ - \to {{{\rm{X}}^2}}{\Pi _{1/2}} $ 阻禁跃迁的强度很小. 本文的计算结果为以后AsH+离子的光谱实验研究提供理论基础.Potential energy curves (PECs), dipole moments (DMs) and transition dipole moments (TDMs) of the X2Π, a4Σ–, A2Σ–, b4Π, B2Δ, C2Σ+, D2Π, 22Σ+ states correlating with the three lowest dissociation channels of AsH+ cation are calculated by using the multireference configuration interaction (MRCI) method. The Davidson correction, core-valence (CV) correlation, and spin-orbit coupling (SOC) effect are all considered. The aug-cc-pV5Z all-electron basis set of H atom and the aug-cc-pwCV5Z-PP pseudopotential basis set of As atom are both selected in the calculation. In the complete active space self-consistent field (CASSCF) calculation, H (1s) and As (4s4p) shell are selected as active orbitals, As (3p3d) shells are selected as closed orbitals, which keeps doubly occupation, the remaining electrons are in the frozen orbitals. In the MRCI calculation, As (3p3d) shells are used for CV correlation, and the calculation accuracy can be improved. The SOC effects are considered with Breit-Pauli operators. All calculated states are bound states. The X2Π is the ground state, which is a deep potential well, the dissociation energy is 3.100 eV. The b4Π, C2Σ+ and D2Π are weakly bound states. The spectroscopic parameters are obtained by solving radial Schrodinger equation. To the best of our knowledge, there has been no study of the spectroscopy of AsH+ cation so far. Comparing with Ⅴ-hydride cations MH+ (M = N, P, As), the orders of the energy levels of the low-lying states for three ions are identical. The dissociation energy and harmonic frequency both decrease with the increase of the atomic weight of M. At spin-free level, the PEC of b4Π state and the PEC of B2Δ state cross at about 1.70 Å. When SOC effects are taken into account, according to the rule of avoid-crossing, the $ {{{\rm{B}}^2}}{\Delta _{3/2}} $ state and$ {{{\rm{B}}^2}}{\Delta _{5/2}} $ state change to the double potential wells, and the avoided crossing between the$ {{{\rm{B}}^2}}{\Delta _{3/2}} $ ($ {{{\rm{B}}^2}}{\Delta _{3/2}} $ ) state and${{\rm{b}}^4}{\Pi _{3/2}}$ (${{\rm{b}}^4}{\Pi _{5/2}}$ ) state is observed. The transition dipole moment (TDM) of the$ {{{\rm{A}}^2}}{\Sigma ^ - } \to {{{\rm{X}}^2}}\Pi $ ,$ {{{\rm{a}}^4}}\Sigma _{1/2}^ - \to {{{\rm{X}}^2}}{\Pi _{1/2}} $ and$ {{{\rm{A}}^2}}\Sigma _{1/2}^ - \to {{{\rm{X}}^2}}{\Pi _{1/2}} $ transition are also calculated. The TDM at the equilibrium distance of the$ {{{\rm{a}}^4}}\Sigma _{1/2}^ - \to {{{\rm{X}}^2}}{\Pi _{1/2}} $ spin-forbidden reaches 0.036 Debye, therefore, the SOC effect plays an important role. Based on the accurate PECs and PDMs, the Franck-Condon factors, spontaneous radiative coefficients, and spontaneous radiative lifetimes of the$ {{{\rm{A}}^2}}{\Sigma ^ - } \to {{{\rm{X}}^2}}\Pi $ ,$ {{{\rm{a}}^4}}\Sigma _{1/2}^ - \to {{{\rm{X}}^2}}{\Pi _{1/2}} $ , and$ {{{\rm{A}}^2}}\Sigma _{1/2}^ - \to {{{\rm{X}}^2}}{\Pi _{1/2}} $ transition are also calculated.-
Keywords:
- spin-orbit coupling effects /
- spectroscopic parameters /
- Franck-Condon factors /
- spontaneous radiative lifetimes
[1] Dixon R N, Duxbury G, Lamberton H M 1968 Proc. R. Soc. London, Ser. A. 305 271
 Google Scholar
Google Scholar
[2] Arens M, Richter W 1990 J. Chem. Phys. 93 7094
 Google Scholar
Google Scholar
[3] Beutel M, Setzer K D, Shestakov O, Fink E H 1996 J. Mol. Spectrosc. 178 165
 Google Scholar
Google Scholar
[4] Pettersson L G, Langhoff S R 1986 J. Chem. Phys. 85 3130
 Google Scholar
Google Scholar
[5] Matsushita T, Marian C M, Klotz R, Peyerimho S D 1987 Can. J. Phys. 65 155
 Google Scholar
Google Scholar
[6] Balasubramanian K, Nannegari V 1989 J. Mol. Spectrosc. 138 482
 Google Scholar
Google Scholar
[7] Shi D H, Liu H, Sun J F, Zhang J P, Liu Y F, Zhu Z L 2009 J. Mol. Struct. 911 8
 Google Scholar
Google Scholar
[8] Bian W S, Li D H, Cao J W, Ma H T 2022 Phys. Chem. Chem. Phys. 24 10114
 Google Scholar
Google Scholar
[9] 赵东锋, 秦成兵, 张群, 陈旸 2009 科学通报 54 3190
 Google Scholar
Google Scholar
Zhao D F, Qin C B, Zhang Q, Chen Y 2009 Chin. Sci. Bull. 54 3190
 Google Scholar
Google Scholar
[10] Wan M J, Zhang Y G, Song C Q, Gao Tao 2008 J. Phys. B:At. Mol. Opt. Phys. 41 215102
 Google Scholar
Google Scholar
[11] Yang C L, You Y, Wang M S, Ma X G, Liu W W 2015 Phys. Rev. A 92 032502
 Google Scholar
Google Scholar
[12] Bruna P J, Hirsch G, Peyerimhoff S D, Buenker R J 1981 Mol. Phys. 42 875
 Google Scholar
Google Scholar
[13] Li G X, Gao T, Zhang Y G 2008 Chin. Phys. B 17 2040
 Google Scholar
Google Scholar
[14] Yan B, Zhang X, Li X 2015 Spectrochim. Acta, Part A 142 1
 Google Scholar
Google Scholar
[15] 邢伟, 孙金锋, 施德恒, 朱遵略 2018 67 193101
 Google Scholar
Google Scholar
Xing W, Sun J F, Shi D H, Zhu Z L 2018 Acta Phys. Sin. 67 193101
 Google Scholar
Google Scholar
[16] 滑亚文, 刘以良, 万明杰 2020 69 153101
 Google Scholar
Google Scholar
Hua Y W, Liu Y L, Wan M J 2020 Acta Phys. Sin. 69 153101
 Google Scholar
Google Scholar
[17] 高峰, 张红, 张常哲, 赵文丽, 孟庆田 2021 70 153301
 Google Scholar
Google Scholar
Gao F, Zhang H, Zhang C Z, Zhao W L, Meng Q T 2021 Acta Phys. Sin. 70 153301
 Google Scholar
Google Scholar
[18] Werner H J, Knowles P J, Knizia G, et al. 2010 MOLPRO, a Package of ab initio Programs (version 2010.1)
[19] Dunning Jr. T H 1989 J. Chem. Phys. 90 1007
 Google Scholar
Google Scholar
[20] Peterson K A, Yousaf K E 2010 J. Chem. Phys. 133 174116
 Google Scholar
Google Scholar
[21] Knowles P J, Werner H J 1985 J. Chem. Phys. 82 5053
 Google Scholar
Google Scholar
[22] Knowles P J, Werner H J 1985 Chem. Phys. Lett. 115 259
 Google Scholar
Google Scholar
[23] Werner H J, Knowles P J 1988 J. Chem. Phys. 89 5803
 Google Scholar
Google Scholar
[24] Knowles P J, Werner H J 1988 Chem. Phys. Lett. 145 514
 Google Scholar
Google Scholar
[25] Langhoff S R, Davidson E R 1974 Int. J. Quantum Chem. 8 61
 Google Scholar
Google Scholar
[26] Berning A, Schweizer M, Werner H J, Knowles P J, Palmieri P 2000 Mol. Phys. 98 1823
 Google Scholar
Google Scholar
[27] Le Roy R J 2007 LEVEL 8.0: a Computer Program for Solving the Radial Schröinger Equation for Bound and Quasibound Levels (Waterloo: University of Waterloo) Chemical Physics Research Report CP-663
[28] Moore C E 1971 Atomic Energy Levels vol. Ⅱ (Washington, DC: US Govt Printing Office) p144
[29] Huber K, Herzberg G 1979 Molecular Spectra and Molecular Structure Vol. 4. Constants of Diatomic Molecules (New York: Van Nostrand Reinhold) p460
[30] Tarroni R, Palmieri P, Mitrushenkov A, Tosi P, Bassi D 1997 J. Chem. Phys. 106 10265
 Google Scholar
Google Scholar
[31] Colin R 1989 J. Mol. Spectrosc. 136 387
 Google Scholar
Google Scholar
[32] Li R, Zhai Z, Zhang X M, Jin M X, Xu H F, Yan B 2015 J. Quant. Spectrosc. Radiat. Transfer 157 42
 Google Scholar
Google Scholar
[33] Xiao L D, Liu Y, Li R, Xiao Z Y, Yan B 2021 J. Quant. Spectrosc. Radiat. Transfer 266 107593
 Google Scholar
Google Scholar
-
表 1 AsH+离子Λ-S态的离解关系
Table 1. Dissociation relationships of Λ-S states of AsH+
原子态 Λ-S态 ΔE/cm–1 本文工作 实验值[28] As+(3Pg)+H(2Sg) X2Π, a4Σ–,
A2Σ–, b4Π0 0 As+(1Dg)+H(2Sg) B2Δ, C2Σ+, D2Π 8222.18 8752 As+(1Sg)+H(2Sg) 22Σ+ 20400.69 21252 表 2 Λ-S的光谱常数
Table 2. Spectroscopic parameters of the Λ-S states.
Λ-S states Re/Å ωe/cm–1 ωeχe/cm–1 Be/cm–1 De/eV Te/cm–1 X2∏ 1.5131 2222.58 42.08 7.3632 3.100 0 a4Σ– 1.6211 1433.42 52.39 6.4528 1.208 15260.18 A2Σ– 2.0523 598.03 35.70 4.0257 0.313 22481.02 b4∏ 3.9651 111.89 27.81 1.1024 0.015 24885.54 B2Δ 1.7260 1139.52 49.11 5.6847 0.821 26654.73 C2Σ+ 3.2885 140.54 22.86 1.6101 0.028 32911.82 D2∏ 3.3767 172.32 29.15 1.5005 0.035 32993.70 22Σ+ 2.4140 532.54 47.36 2.9263 0.188 43887.70 表 3 第五主族氢化物离子的光谱常数对比
Table 3. Comparison of the spectroscopy parameters of the Ⅴ-group hydride cations.
分子离子 Λ-S态 Re/Å ωe/cm–1 De/eV Te/cm–1 NH+ X2∏ 1.080a 2810.6a 4.40a 0 a4Σ– ~1.105b ~2520b 3.66c 509d A2Σ– 1.206a 1578.2a 1.76a 22161.27a B2Δ 1.161a 2011.2a 3.25a 23331a PH+ X2∏ 1.4226e 2412.79e 3.525e 0 a4Σ– 1.4816e 1832.51e 1.790e 13998e A2Σ– 1.7914e 823.68e 0.490e 24476e B2Δ 1.5454e 1512.20e 1.277e 26322e AsH+ X2∏ 1.5131f 2222.58f 3.100f 0 a4Σ– 1.6211f 1433.42f 1.208f 15260.18f A2Σ– 2.0523f 598.03f 0.313f 22481.02f B2Δ 1.7260f 1139.52f 0.188f 43887.70f 注: a文献[10] , b文献[29] , c文献[30] , d文献[31] , e文献[14], e本文计算值. 表 4 AsH+离子Ω态的离解关系
Table 4. Dissociation relationships of Ω states of AsH+.
原子态 Ω态 ΔE/cm–1 本文工作 实验值[28] As+(3P0)+H(2S1/2) 1/2 0 0 As+(3P1)+H(2S1/2) 3/2, 1/2, 1/2 1090.35 1061 As+(3P2)+H(2S1/2) 5/2, 3/2, 3/2, 1/2, 1/2 2721.66 2540 As+(1D2)+H(2S1/2) 5/2, 3/2, 3/2, 1/2, 1/2 11252.94 10093 As+(1S0)+H(2S1/2) 1/2 24909.88 22593 表 5 Ω的光谱常数
Table 5. Spectroscopic parameters of the Ω states.
Ω states Re /Å ωe /cm–1 ωeχe /cm–1 Be /cm–1 De /eV Te /cm–1 $ {{{\rm{X}}^2}}{\Pi _{1/2}} $ 1.5146 2339.14 41.99 7.3592 3.314 0 $ {{{\rm{X}}^2}}{\Pi _{3/2}} $ 1.5121 2344.11 42.99 7.3647 3.216 1696.92 $ {{{\rm{a}}^4}}\Sigma _{1/2}^ - $ 1.6222 1535.88 58.51 6.4447 1.248 17777.19 $ {{{\rm{a}}^4}}\Sigma _{3/2}^ - $ 1.6210 1688.21 69.69 6.4554 1.249 17910.08 $ {{{\rm{A}}^2}}\Sigma _{1/2}^ - $ 2.0581 685.64 35.99 3.9976 0.400 25773.03 $ {{{\rm{B}}^2}}{\Delta _{3/2}} $ 第一势阱 1.7285 1175.34 58.14 5.6857 0.321 30513.22 第二势阱 3.2405 333.26 49.99 1.7215 0.076 28497.77 $ {{{\rm{B}}^2}}{\Delta _{5/2}} $ 第一势阱 1.7259 1186.52 51.97 5.6923 0.356 30548.89 第二势阱 3.3318 283.60 49.29 1.5654 0.055 29071.95 表 6 AsH+离子的弗兰克-康登因子(单位: s-1)、总自发辐射速率和自发辐射寿命(单位: μs)
Table 6. Franck-Condon Factors, spontaneous emission rates (unit of s-1) and spontaneous radiative lifetimes τ (unit of μs) of the AsH+ cation.
跃迁 ν′ ν″ = 0 ν″ = 1 ν″ = 2 ν″ = 3 ν″ = 4 ν″ = 5 ΣA τ = 1/ΣA A2Σ– ↔ X2Π 0 0.0056 0.0280 0.0722 0.1275 0.1715 0.1843 2295.52 5374.75 6276.27 4791.31 2627.48 1065.87 22838.76 43.75 $ {{{\rm{a}}^4}}\Sigma _{1/2}^ - \to {{{\rm{X}}^2}}{\Pi _{1/2}} $ 0 0.6817 0.2447 0.0613 0.0107 0.0014 0.0001 1545.42 319.72 60.84 8.08 0.74 0.04 1934.85 517 $ {{{\rm{A}}^2}}\Sigma _{1/2}^ - \to {{{\rm{X}}^2}}{\Pi _{1/2}} $ 0 0.0041 0.0216 0.0588 0.1098 0.1565 0.1789 3036.67 7910.09 10373.6 8987.8 5672.57 2703.67 40012.76 24.99 -
[1] Dixon R N, Duxbury G, Lamberton H M 1968 Proc. R. Soc. London, Ser. A. 305 271
 Google Scholar
Google Scholar
[2] Arens M, Richter W 1990 J. Chem. Phys. 93 7094
 Google Scholar
Google Scholar
[3] Beutel M, Setzer K D, Shestakov O, Fink E H 1996 J. Mol. Spectrosc. 178 165
 Google Scholar
Google Scholar
[4] Pettersson L G, Langhoff S R 1986 J. Chem. Phys. 85 3130
 Google Scholar
Google Scholar
[5] Matsushita T, Marian C M, Klotz R, Peyerimho S D 1987 Can. J. Phys. 65 155
 Google Scholar
Google Scholar
[6] Balasubramanian K, Nannegari V 1989 J. Mol. Spectrosc. 138 482
 Google Scholar
Google Scholar
[7] Shi D H, Liu H, Sun J F, Zhang J P, Liu Y F, Zhu Z L 2009 J. Mol. Struct. 911 8
 Google Scholar
Google Scholar
[8] Bian W S, Li D H, Cao J W, Ma H T 2022 Phys. Chem. Chem. Phys. 24 10114
 Google Scholar
Google Scholar
[9] 赵东锋, 秦成兵, 张群, 陈旸 2009 科学通报 54 3190
 Google Scholar
Google Scholar
Zhao D F, Qin C B, Zhang Q, Chen Y 2009 Chin. Sci. Bull. 54 3190
 Google Scholar
Google Scholar
[10] Wan M J, Zhang Y G, Song C Q, Gao Tao 2008 J. Phys. B:At. Mol. Opt. Phys. 41 215102
 Google Scholar
Google Scholar
[11] Yang C L, You Y, Wang M S, Ma X G, Liu W W 2015 Phys. Rev. A 92 032502
 Google Scholar
Google Scholar
[12] Bruna P J, Hirsch G, Peyerimhoff S D, Buenker R J 1981 Mol. Phys. 42 875
 Google Scholar
Google Scholar
[13] Li G X, Gao T, Zhang Y G 2008 Chin. Phys. B 17 2040
 Google Scholar
Google Scholar
[14] Yan B, Zhang X, Li X 2015 Spectrochim. Acta, Part A 142 1
 Google Scholar
Google Scholar
[15] 邢伟, 孙金锋, 施德恒, 朱遵略 2018 67 193101
 Google Scholar
Google Scholar
Xing W, Sun J F, Shi D H, Zhu Z L 2018 Acta Phys. Sin. 67 193101
 Google Scholar
Google Scholar
[16] 滑亚文, 刘以良, 万明杰 2020 69 153101
 Google Scholar
Google Scholar
Hua Y W, Liu Y L, Wan M J 2020 Acta Phys. Sin. 69 153101
 Google Scholar
Google Scholar
[17] 高峰, 张红, 张常哲, 赵文丽, 孟庆田 2021 70 153301
 Google Scholar
Google Scholar
Gao F, Zhang H, Zhang C Z, Zhao W L, Meng Q T 2021 Acta Phys. Sin. 70 153301
 Google Scholar
Google Scholar
[18] Werner H J, Knowles P J, Knizia G, et al. 2010 MOLPRO, a Package of ab initio Programs (version 2010.1)
[19] Dunning Jr. T H 1989 J. Chem. Phys. 90 1007
 Google Scholar
Google Scholar
[20] Peterson K A, Yousaf K E 2010 J. Chem. Phys. 133 174116
 Google Scholar
Google Scholar
[21] Knowles P J, Werner H J 1985 J. Chem. Phys. 82 5053
 Google Scholar
Google Scholar
[22] Knowles P J, Werner H J 1985 Chem. Phys. Lett. 115 259
 Google Scholar
Google Scholar
[23] Werner H J, Knowles P J 1988 J. Chem. Phys. 89 5803
 Google Scholar
Google Scholar
[24] Knowles P J, Werner H J 1988 Chem. Phys. Lett. 145 514
 Google Scholar
Google Scholar
[25] Langhoff S R, Davidson E R 1974 Int. J. Quantum Chem. 8 61
 Google Scholar
Google Scholar
[26] Berning A, Schweizer M, Werner H J, Knowles P J, Palmieri P 2000 Mol. Phys. 98 1823
 Google Scholar
Google Scholar
[27] Le Roy R J 2007 LEVEL 8.0: a Computer Program for Solving the Radial Schröinger Equation for Bound and Quasibound Levels (Waterloo: University of Waterloo) Chemical Physics Research Report CP-663
[28] Moore C E 1971 Atomic Energy Levels vol. Ⅱ (Washington, DC: US Govt Printing Office) p144
[29] Huber K, Herzberg G 1979 Molecular Spectra and Molecular Structure Vol. 4. Constants of Diatomic Molecules (New York: Van Nostrand Reinhold) p460
[30] Tarroni R, Palmieri P, Mitrushenkov A, Tosi P, Bassi D 1997 J. Chem. Phys. 106 10265
 Google Scholar
Google Scholar
[31] Colin R 1989 J. Mol. Spectrosc. 136 387
 Google Scholar
Google Scholar
[32] Li R, Zhai Z, Zhang X M, Jin M X, Xu H F, Yan B 2015 J. Quant. Spectrosc. Radiat. Transfer 157 42
 Google Scholar
Google Scholar
[33] Xiao L D, Liu Y, Li R, Xiao Z Y, Yan B 2021 J. Quant. Spectrosc. Radiat. Transfer 266 107593
 Google Scholar
Google Scholar
计量
- 文章访问数: 7663
- PDF下载量: 74
- 被引次数: 0
























 下载:
下载: