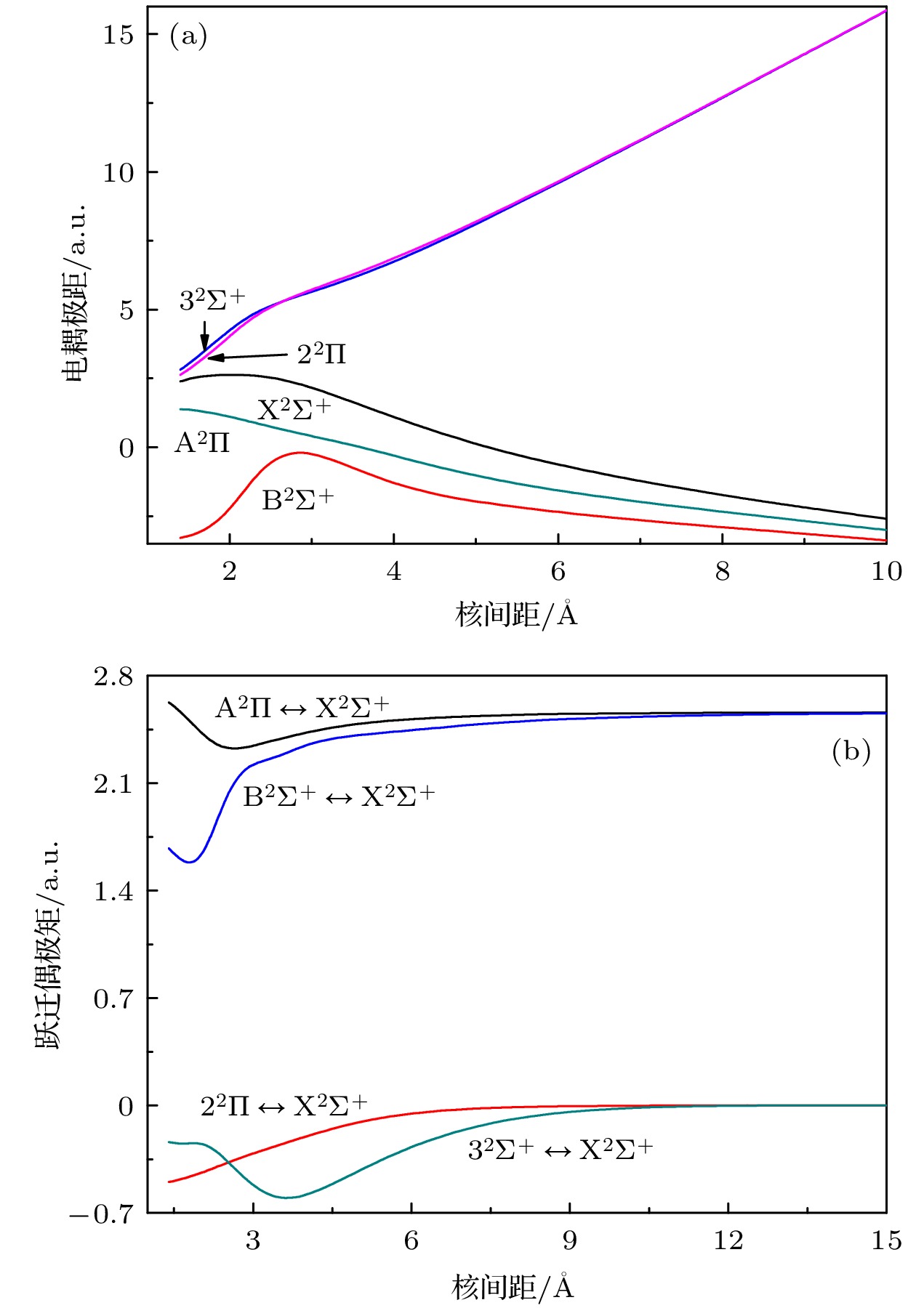
-
采用多参考组态相互作用方法结合全电子基组计算了LiCl- 阴离子5个电子态 (X2Σ+, A2∏, B2Σ+, 32Σ+, 22∏) 的电子结构. 为了得到精确的光谱常数, 计算中考虑了Davidson 修正、芯-价电子关联效应和自旋-轨道耦合效应. 拟合得到各电子态的光谱常数、分子常数、自发辐射速率和自发辐射寿命. 基态的光谱常数与实验值和其他理论值符合较好, 同时报道了LiCl– 阴离子激发态的光谱常数以及其到基态的跃迁性质. 计算结果表明A2∏
$\leftrightarrow $ X2Σ+跃迁具有高对角分布的弗兰克-康登因子f00, 第一激发态A2∏有较短的自发辐射寿命. 构造A2∏ (ν′)$\leftrightarrow $ X2Σ+ ($\nu''$ )准循环跃迁进行激光冷却LiCl– 阴离子需要一束主激光和两束抽运激光. 以上结果预测了激光冷却LiCl–阴离子是可行的.The electronic structure of the X2Σ+, A2Π, B2Σ+, 32Σ+, and 22Π state of LiCl– anion are performed at an MRCI+Q level. Davison correction, core-valence correction and spin-orbit coupling effect are also considered. The ground state X2Σ+ of LiCl– anion correlates with the lowest dissociation channel Li(2Sg) + Cl–(1Sg); the A2∏ state and B2Σ+ state correlate with the second dissociation channel Li(2Pu) + Cl–(1Sg); the 32Σ+ state and 22Π state correlate with the third dissociation channel Li–(1Sg) + Cl–(2Pu). Spectroscopic parameters are calculated by solving the radial Schröedinger equation. The equilibrium internuclear distance Re of the ground state X2Σ+ is 2.1352 Å, which is a little bigger than the experimental datum, with an error being 0.5%. It is a deep potential well, and the dissociation energy De is 1.886 eV. These values are in good agreement with experimental data. The A2∏ state is at 13431.93 cm–1 above the X2Σ+ state. The Re is 2.1198 Å, which is only 0.0154 Å smaller than that of the X2Σ+ state. The values of energy level Gν and rotational constant Bν of five Λ-S states are also calculated. The values are in good agreement with available theoretical ones. The electronic structures of the excited states are also reported. The SOC effect weakly influences the spectroscopic parameters for the $ {\text{X}}{}^2\Sigma _{1/2}^ + $ ,$ {\text{A}}{}^2{\Pi _{1/2}} $ ,$ {\text{A}}{}^2{\Pi _{3/2}} $ , and$ {\text{B}}{}^2\Sigma _{1/2}^ + $ state. From the analysis of the SO matrix, it can be seen that the SOC effect plays a little role in realizing the A2Π$\leftrightarrow $ X2Σ+ transition, so, it can be ignored.The scheme of laser cooling of LiCl– anion has constructed at a spin – free level. The A2∏(ν′) $\leftrightarrow $ X2Σ+($v'' $ ) transition has a highly diagonally distributed Franck-Condon factor f00 = 0.9898, the calculated branching ratio of the diagonal term R00 is 0.9893, and spontaneous radiative lifetime of A2∏ is 35.45 ns. A main pump laser and two repumping lasers for driving the A2∏(ν′)$\leftrightarrow $ X2Σ+($v'' $ ) transitions are required. The laser wavelengths are 744.10, 774.30 and 772.42 nm, respectively. Owing to the summation of R00, R01, and R02 being closer to 1, the A2∏(ν′)$\leftrightarrow $ X2Σ+($v'' $ ) transition is a quasicycling transition. These results imply that the LiCl– anion is a candidate for laser cooling.-
Keywords:
- spin-orbit coupling effects /
- spectroscopic parameters /
- molecular parameters /
- laser cooling
[1] Micheli A, Brennen G, Zoller P 2006 Nat. Phys. 2 341
 Google Scholar
Google Scholar
[2] Baron J et al., (The ACME Collaboration). 2014 Science 343 269
 Google Scholar
Google Scholar
[3] Krems R V 2008 Phys. Chem. Chem. Phys. 10 4079
 Google Scholar
Google Scholar
[4] Shuman E S, Barry J F, DeMille D 2010 Nature 467 820
 Google Scholar
Google Scholar
[5] Hummon M T, Yeo M, Stuhl B K, Collopy A L, Xia Y, Ye J 2013 Phys. Rev. Lett. 110 143001
 Google Scholar
Google Scholar
[6] You Y, Yang C L, Wang M S, Ma X G, Liu W W 2015 Phys. Rev. A 92 032502
 Google Scholar
Google Scholar
[7] Wan M J, Shao J X, Huang D H, Jin C G, Yu Y, Wang F H 2015 Phys. Chem. Chem. Phys. 17 26731
 Google Scholar
Google Scholar
[8] Wan M J, Shao J X, Gao Y F, Huang D H, Yang J S, Cao Q L, Jin C G, Wang F H 2015 J Chem. Phys. 143 024302
 Google Scholar
Google Scholar
[9] Yang Q S, Li S C, Yu Y, Gao T 2018 J. Phys. Chem. A 122 3021
 Google Scholar
Google Scholar
[10] Fu M K, Ma H T, Cao J W, Bian W S 2016 J. Chem. Phys. 144 184302
 Google Scholar
Google Scholar
[11] Wan M J, Yuan D, Jin C G, Wang F H, Yang Y J, Yu Y, Shao J X 2016 J. Chem. Phys. 145 024309
 Google Scholar
Google Scholar
[12] Yuan X, Yin S, Shen Y, Liu Y, Lian Y, Xu H F, Yan B 2018 J. Chem. Phys. 149 094306
 Google Scholar
Google Scholar
[13] Yzombard P, Hamamda M, Gerber S, Doser M, Comparat D 2015 Phys. Rev. Lett. 114 213001
 Google Scholar
Google Scholar
[14] Zhang Q Q, Yang C L, Wang M S, Ma X G, Liu W W 2017 Spectrochim. Acta, Part A. 182 130
 Google Scholar
Google Scholar
[15] Zhang Q Q, Yang C L, Wang M S, Ma X G, Liu W W 2017 Spectrochim. Acta, Part A. 185 365
 Google Scholar
Google Scholar
[16] Wan M J, Huang D H, Yu Y, Zhang Y G 2017 Phys. Chem. Chem. Phys. 17 27360
[17] 万明杰, 李松, 金成国, 罗华锋 2019 68 063103
 Google Scholar
Google Scholar
Wan M J, Li S, Jin C G, Luo H F 2019 Acta Phys. Sin. 68 063103
 Google Scholar
Google Scholar
[18] 万明杰, 罗华锋, 袁娣, 李松 2019 68 173102
 Google Scholar
Google Scholar
Wan M J, Luo H F, Yuan D, Li S 2019 Acta Phys. Sin. 68 173102
 Google Scholar
Google Scholar
[19] 万明杰, 柳福提, 黄多辉 2021 70 033101
 Google Scholar
Google Scholar
Wan M J, Liu F T, Huang D H, 2021 Acta Phys. Sin. 70 033101
 Google Scholar
Google Scholar
[20] Carlsten J L, Peterson J R. Lineberger W C 1976 Chem. Phys. Lett. 37 5
 Google Scholar
Google Scholar
[21] Miller T M, Leopold D G, Murray K K, Lineberger W C 1986 J. Chem. Phys. 85 2368
 Google Scholar
Google Scholar
[22] Jordan K D, Luken W 1976 J Chem. Phys. 64 2760
 Google Scholar
Google Scholar
[23] Li S, Zheng R, Chen S J, Fan Q C 2015 Mol. Phys. 113 1433
 Google Scholar
Google Scholar
[24] Weck P F, Kirby K, Stancil P C 2004 J. Chem. Phys. 120 4216
 Google Scholar
Google Scholar
[25] Kurosaki Y, Yokoyama K 2012 J. Chem. Phys. 137 064305
 Google Scholar
Google Scholar
[26] Werner H J, Knowles P J, Knizia G, et al. 2010 MOLPRO, a Package of ab initio Programs (Version 2010.1)
[27] Knowles P J, Werner H J 1985 J. Chem. Phys. 82 5053
 Google Scholar
Google Scholar
[28] Knowles P J, Werner H J 1985 Chem. Phys. Lett. 115 259
 Google Scholar
Google Scholar
[29] Werner H J, Knowles P J 1988 J. Chem. Phys. 89 5803
 Google Scholar
Google Scholar
[30] Langhoff S R, Davidson E R 1974 Int. J. Quantum Chem. 8 61
 Google Scholar
Google Scholar
[31] Berning A, Schweizer M, Werner H J, Knowles P J, Palmieri P 2000 Mol. Phys. 98 1283
[32] Xiao K L, Yang C L, Wang M S, Ma X G, Liu W W 2013 J. Chem. Phys. 139 074305
 Google Scholar
Google Scholar
[33] Weigend F 2008 J. Comput. Chem. 29 167
 Google Scholar
Google Scholar
[34] Peterson K A, Dunning T H 2002 J. Chem. Phys. 117 10548
 Google Scholar
Google Scholar
[35] Le Roy R J 2007 LEVEL 8.0: a Computer Program for Solving the Radial Schröinger Equation for Bound and Quasibound Levels (Waterloo: University of Waterloo) Chemical Physics Research Report CP-663)
[36] Haeffler G, Hanstrorp D, Kiyan I, Klinkmueller A E, Ljungblad U, Pegg D 1996 Phys. Rev. A 53 4127
 Google Scholar
Google Scholar
[37] Berzinsh U, Gustafsson M, Hanstorp D, Klinkmueller A E, Ljungblad U, Maartensson-Pendrill A M 1995 Phys. Rev. A 51 231
 Google Scholar
Google Scholar
[38] Moore C E 1971 Atomic Energy Levels (Vol. 1) (Washington, DC: US Govt Printing Office) pp9, 195
[39] 尹俊豪, 杨涛, 印建平 2021 70 163302
 Google Scholar
Google Scholar
Yin J H, Yang T, Yin J P 2021 Acta Phys. Sin. 70 163302
 Google Scholar
Google Scholar
-
表 1 LiCl–阴离子Λ-S态的离解关系
Table 1. Calculated dissociation relationships of the Λ-S states of LiCl– anion.
表 2 LiCl–阴离子Λ-S态的光谱常数
Table 2. Spectroscopic parameters of the Λ-S states of LiCl– anion.
电子态 Re/Å ωe/cm–1 ωeχe/cm–1 Be/cm–1 De/eV D0/eV Te/cm–1 X2Σ+ 2.1352 535.33 5.8173 0.7205 1.8886 1.856 0 实验值[20] 2.18(4) 0 实验值[21] 2.123(15) 1.75(2) 0 理论值[22] 2.12a 0 理论值[23] 2.1354 537.7b 6.34b 0.7203b 1.81 0 A2∏ 2.1198 554.65 5.7157 0.7310 1.9902 1.9559 13431.93 B2Σ+ 2.0282 652.79 6.1252 0.7985 1.6653 1.6250 17491.75 32Σ+ 5.8594 30.19 0.8483 0.0963 0.0362 0.0353 38607.64 22∏ 7.1411 39.08 0.5616 0.0638 0.0136 0.0112 38855.32 a采用HF方法计算得到基态的核间距. 表 3 X2Σ+, A2∏, B2Σ+, 32Σ+和22∏态的振动能级和转动常数
Table 3. Vibrational energy levels, rotational constants of the X2Σ+, A2∏, B2Σ+, 32Σ+ and 22∏ states.
ν X2Σ+ A2∏ B2Σ+ 32Σ+ 22∏ Gν Bν Gν Bν Gν Bν Gν Bν Gν Bν 本文工作 文献[23] 本文工作 文献[23] 本文工作 本文工作 本文工作 本文工作 本文工作 本文工作 本文工作 本文工作 0 266.72 264.07 0.7146 0.7143 276.48 0.7252 325.33 0.7927 14.96 0.0940 6.12 0.0631 1 790.93 791.77 0.7029 0.7023 820.21 0.7136 966.07 0.7811 43.02 0.0893 18.15 0.0618 2 1303.27 1307.01 0.6911 0.6903 1352.13 0.7021 1594.23 0.7697 68.84 0.0857 28.92 0.0572 3 1803.71 1809.92 0.6794 0.6784 1872.34 0.6907 2210.02 0.7584 93.22 0.0826 29.30 0.0095 4 2292.31 2300.65 0.6677 0.6666 2380.97 0.6793 2813.56 0.7471 116.24 0.0796 31.32 0.0112 5 2769.15 2779.34 0.6560 0.6548 2878.13 0.6680 3404.89 0.7359 138.17 0.0765 33.19 0.0125 6 3234.29 3246.11 0.6444 0.6430 3363.87 0.6567 3984.09 0.7247 158.98 0.0725 35.07 0.0140 7 3687.83 3701.10 0.6328 0.6313 3838.29 0.6455 4551.20 0.7135 178.26 0.0678 36.53 0.0310 8 4129.88 4144.45 0.6212 0.6197 4301.42 0.6343 5106.17 0.7023 195.60 0.0626 37.33 0.0257 9 4560.61 4576.29 0.6097 0.6081 4753.37 0.6231 5648.95 0.6911 210.77 0.0571 39.11 0.0188 表 4 LiCl–阴离子Ω态的离解关系
Table 4. Calculated dissociation relationships of the Ω states of LiCl– anion.
表 5 LiCl–阴离子Ω态的光谱常数
Table 5. Spectroscopic parameters of the Ω states of LiCl– anion at icMRCI+Q level.
Ω态 Re/Å ωe/cm–1 ωeχe /cm–1 Be/cm–1 De/eV Te/cm–1 $ {\text{X}}{}^2\Sigma _{1/2}^ + $ 2.1352 535.32 5.8172 0.7205 1.8886 0 $ {\text{A}}{}^2{\Pi _{1/2}} $ 2.1196 554.87 5.7153 0.7311 2.0094 13419.77 $ {\text{A}}{}^2{\Pi _{3/2}} $ 2.1200 554.41 5.7160 0.7308 2.0059 13444.07 $ {\text{B}}{}^2\Sigma _{1/2}^ + $ 2.0282 652.79 6.1253 0.7985 1.6679 17491.75 表 6 LiCl–阴离子的自旋-轨道矩阵元
Table 6. Notation of spin-orbit matrix element.
SO矩阵元 $ {\text{S}}{{\text{O}}_1} = - {\text{i}}\left\langle {{{\text{X}}^2}{\Sigma ^ + }} \right|\hat H_{{\text{SO}}}^{{\text{BP}}}\left| {{{\text{A}}^2}{\Pi _y}} \right\rangle $ $ {\text{S}}{{\text{O}}_2} = \left\langle {{{\text{X}}^2}{\Sigma ^ + }} \right|\hat H_{{\text{SO}}}^{{\text{BP}}}\left| {{{\text{A}}^2}{\Pi _x}} \right\rangle $ $ {\text{S}}{{\text{O}}_3} = - {\text{i}}\left\langle {{{\text{B}}^2}{\Sigma ^ + }} \right|\hat H_{{\text{SO}}}^{{\text{BP}}}\left| {{{\text{A}}^2}{\Pi _y}} \right\rangle $ $ {\text{S}}{{\text{O}}_4} = \left\langle {{{\text{B}}^2}{\Sigma ^ + }} \right|\hat H_{{\text{SO}}}^{{\text{BP}}}\left| {{{\text{A}}^2}{\Pi _x}} \right\rangle $ $ {\text{S}}{{\text{O}}_5} = - {\text{i}}\left\langle {{3^2}{\Sigma ^ + }} \right|\hat H_{{\text{SO}}}^{{\text{BP}}}\left| {{{\text{A}}^2}{\Pi _y}} \right\rangle $ $ {\text{S}}{{\text{O}}_6} = \left\langle {{3^2}{\Sigma ^ + }} \right|\hat H_{{\text{SO}}}^{{\text{BP}}}\left| {{{\text{A}}^2}{\Pi _x}} \right\rangle $ $ {\text{S}}{{\text{O}}_7} = - {\text{i}}\left\langle {{{\text{X}}^2}{\Sigma ^ + }} \right|\hat H_{{\text{SO}}}^{{\text{BP}}}\left| {{2^2}{\Pi _y}} \right\rangle $ $ {\text{S}}{{\text{O}}_8} = \left\langle {{{\text{X}}^2}{\Sigma ^ + }} \right|\hat H_{{\text{SO}}}^{{\text{BP}}}\left| {{2^2}{\Pi _x}} \right\rangle $ $ {\text{S}}{{\text{O}}_9} = - {\text{i}}\left\langle {{{\text{B}}^2}{\Sigma ^ + }} \right|\hat H_{{\text{SO}}}^{{\text{BP}}}\left| {{2^2}{\Pi _y}} \right\rangle $ $ {\text{S}}{{\text{O}}_{10}} = \left\langle {{{\text{B}}^2}{\Sigma ^ + }} \right|\hat H_{{\text{SO}}}^{{\text{BP}}}\left| {{2^2}{\Pi _x}} \right\rangle $ $ {\text{S}}{{\text{O}}_{11}} = - {\text{i}}\left\langle {{3^2}{\Sigma ^ + }} \right|\hat H_{{\text{SO}}}^{{\text{BP}}}\left| {{2^2}{\Pi _y}} \right\rangle $ $ {\text{S}}{{\text{O}}_{12}} = \left\langle {{3^2}{\Sigma ^ + }} \right|\hat H_{{\text{SO}}}^{{\text{BP}}}\left| {{2^2}{\Pi _x}} \right\rangle $ $ {\text{S}}{{\text{O}}_{13}} = {\text{i}}\left\langle {{{\text{A}}^2}{\Pi _x}} \right|\hat H_{{\text{SO}}}^{{\text{BP}}}\left| {{{\text{A}}^2}{\Pi _y}} \right\rangle $ $ {\text{S}}{{\text{O}}_{14}} = {\text{i}}\left\langle {{2^2}{\Pi _x}} \right|\hat H_{{\text{SO}}}^{{\text{BP}}}\left| {{{\text{A}}^2}{\Pi _y}} \right\rangle $ $ {\text{S}}{{\text{O}}_{15}} = {\text{i}}\left\langle {{2^2}{\Pi _y}} \right|\hat H_{{\text{SO}}}^{{\text{BP}}}\left| {{{\text{A}}^2}{\Pi _x}} \right\rangle $ $ {\text{S}}{{\text{O}}_{16}} = {\text{i}}\left\langle {{2^2}{\Pi _x}} \right|\hat H_{{\text{SO}}}^{{\text{BP}}}\left| {{2^2}{\Pi _y}} \right\rangle $ 表 7 A2∏
$\leftrightarrow $ X2Σ+和B2Σ+$\leftrightarrow $ X2Σ+跃迁的弗兰克-康登因子fν'ν'', 爱因斯坦系数Aν'ν''和自发辐射寿命 (单位: ns)Table 7. Franck-Condon Factors fν'ν'', Einstein coefficients Aν'ν'', and radiative lifetimes τ of the A2∏
$\leftrightarrow $ X2Σ+ and B2Σ+$\leftrightarrow $ X2Σ+ transitions of LiCl– anion(in ns).跃迁 f00 f01 f02 f03 A00 A01 A02 A03 τ = 1/ΣA f10 f11 f12 f13 A10 A11 A12 A13 A2∏ $\leftrightarrow $ X2Σ+ 0.9898 0.0101 0.0001 8.70(–7) 27904200 298313 3848.85 44.60 35.45 0.0102 0.9686 0.0209 0.0004 269660 27336600 607335 12332 35.43 B2Σ+ $\leftrightarrow $ X2Σ+ 0.5908 0.2909 0.0894 0.0225 18316800 7658290 2038730 452210 34.99 0.3266 0.1286 0.2888 0.1671 12279600 4261780 7988480 3979390 33.00 -
[1] Micheli A, Brennen G, Zoller P 2006 Nat. Phys. 2 341
 Google Scholar
Google Scholar
[2] Baron J et al., (The ACME Collaboration). 2014 Science 343 269
 Google Scholar
Google Scholar
[3] Krems R V 2008 Phys. Chem. Chem. Phys. 10 4079
 Google Scholar
Google Scholar
[4] Shuman E S, Barry J F, DeMille D 2010 Nature 467 820
 Google Scholar
Google Scholar
[5] Hummon M T, Yeo M, Stuhl B K, Collopy A L, Xia Y, Ye J 2013 Phys. Rev. Lett. 110 143001
 Google Scholar
Google Scholar
[6] You Y, Yang C L, Wang M S, Ma X G, Liu W W 2015 Phys. Rev. A 92 032502
 Google Scholar
Google Scholar
[7] Wan M J, Shao J X, Huang D H, Jin C G, Yu Y, Wang F H 2015 Phys. Chem. Chem. Phys. 17 26731
 Google Scholar
Google Scholar
[8] Wan M J, Shao J X, Gao Y F, Huang D H, Yang J S, Cao Q L, Jin C G, Wang F H 2015 J Chem. Phys. 143 024302
 Google Scholar
Google Scholar
[9] Yang Q S, Li S C, Yu Y, Gao T 2018 J. Phys. Chem. A 122 3021
 Google Scholar
Google Scholar
[10] Fu M K, Ma H T, Cao J W, Bian W S 2016 J. Chem. Phys. 144 184302
 Google Scholar
Google Scholar
[11] Wan M J, Yuan D, Jin C G, Wang F H, Yang Y J, Yu Y, Shao J X 2016 J. Chem. Phys. 145 024309
 Google Scholar
Google Scholar
[12] Yuan X, Yin S, Shen Y, Liu Y, Lian Y, Xu H F, Yan B 2018 J. Chem. Phys. 149 094306
 Google Scholar
Google Scholar
[13] Yzombard P, Hamamda M, Gerber S, Doser M, Comparat D 2015 Phys. Rev. Lett. 114 213001
 Google Scholar
Google Scholar
[14] Zhang Q Q, Yang C L, Wang M S, Ma X G, Liu W W 2017 Spectrochim. Acta, Part A. 182 130
 Google Scholar
Google Scholar
[15] Zhang Q Q, Yang C L, Wang M S, Ma X G, Liu W W 2017 Spectrochim. Acta, Part A. 185 365
 Google Scholar
Google Scholar
[16] Wan M J, Huang D H, Yu Y, Zhang Y G 2017 Phys. Chem. Chem. Phys. 17 27360
[17] 万明杰, 李松, 金成国, 罗华锋 2019 68 063103
 Google Scholar
Google Scholar
Wan M J, Li S, Jin C G, Luo H F 2019 Acta Phys. Sin. 68 063103
 Google Scholar
Google Scholar
[18] 万明杰, 罗华锋, 袁娣, 李松 2019 68 173102
 Google Scholar
Google Scholar
Wan M J, Luo H F, Yuan D, Li S 2019 Acta Phys. Sin. 68 173102
 Google Scholar
Google Scholar
[19] 万明杰, 柳福提, 黄多辉 2021 70 033101
 Google Scholar
Google Scholar
Wan M J, Liu F T, Huang D H, 2021 Acta Phys. Sin. 70 033101
 Google Scholar
Google Scholar
[20] Carlsten J L, Peterson J R. Lineberger W C 1976 Chem. Phys. Lett. 37 5
 Google Scholar
Google Scholar
[21] Miller T M, Leopold D G, Murray K K, Lineberger W C 1986 J. Chem. Phys. 85 2368
 Google Scholar
Google Scholar
[22] Jordan K D, Luken W 1976 J Chem. Phys. 64 2760
 Google Scholar
Google Scholar
[23] Li S, Zheng R, Chen S J, Fan Q C 2015 Mol. Phys. 113 1433
 Google Scholar
Google Scholar
[24] Weck P F, Kirby K, Stancil P C 2004 J. Chem. Phys. 120 4216
 Google Scholar
Google Scholar
[25] Kurosaki Y, Yokoyama K 2012 J. Chem. Phys. 137 064305
 Google Scholar
Google Scholar
[26] Werner H J, Knowles P J, Knizia G, et al. 2010 MOLPRO, a Package of ab initio Programs (Version 2010.1)
[27] Knowles P J, Werner H J 1985 J. Chem. Phys. 82 5053
 Google Scholar
Google Scholar
[28] Knowles P J, Werner H J 1985 Chem. Phys. Lett. 115 259
 Google Scholar
Google Scholar
[29] Werner H J, Knowles P J 1988 J. Chem. Phys. 89 5803
 Google Scholar
Google Scholar
[30] Langhoff S R, Davidson E R 1974 Int. J. Quantum Chem. 8 61
 Google Scholar
Google Scholar
[31] Berning A, Schweizer M, Werner H J, Knowles P J, Palmieri P 2000 Mol. Phys. 98 1283
[32] Xiao K L, Yang C L, Wang M S, Ma X G, Liu W W 2013 J. Chem. Phys. 139 074305
 Google Scholar
Google Scholar
[33] Weigend F 2008 J. Comput. Chem. 29 167
 Google Scholar
Google Scholar
[34] Peterson K A, Dunning T H 2002 J. Chem. Phys. 117 10548
 Google Scholar
Google Scholar
[35] Le Roy R J 2007 LEVEL 8.0: a Computer Program for Solving the Radial Schröinger Equation for Bound and Quasibound Levels (Waterloo: University of Waterloo) Chemical Physics Research Report CP-663)
[36] Haeffler G, Hanstrorp D, Kiyan I, Klinkmueller A E, Ljungblad U, Pegg D 1996 Phys. Rev. A 53 4127
 Google Scholar
Google Scholar
[37] Berzinsh U, Gustafsson M, Hanstorp D, Klinkmueller A E, Ljungblad U, Maartensson-Pendrill A M 1995 Phys. Rev. A 51 231
 Google Scholar
Google Scholar
[38] Moore C E 1971 Atomic Energy Levels (Vol. 1) (Washington, DC: US Govt Printing Office) pp9, 195
[39] 尹俊豪, 杨涛, 印建平 2021 70 163302
 Google Scholar
Google Scholar
Yin J H, Yang T, Yin J P 2021 Acta Phys. Sin. 70 163302
 Google Scholar
Google Scholar
计量
- 文章访问数: 6232
- PDF下载量: 92
- 被引次数: 0






















 下载:
下载: