-
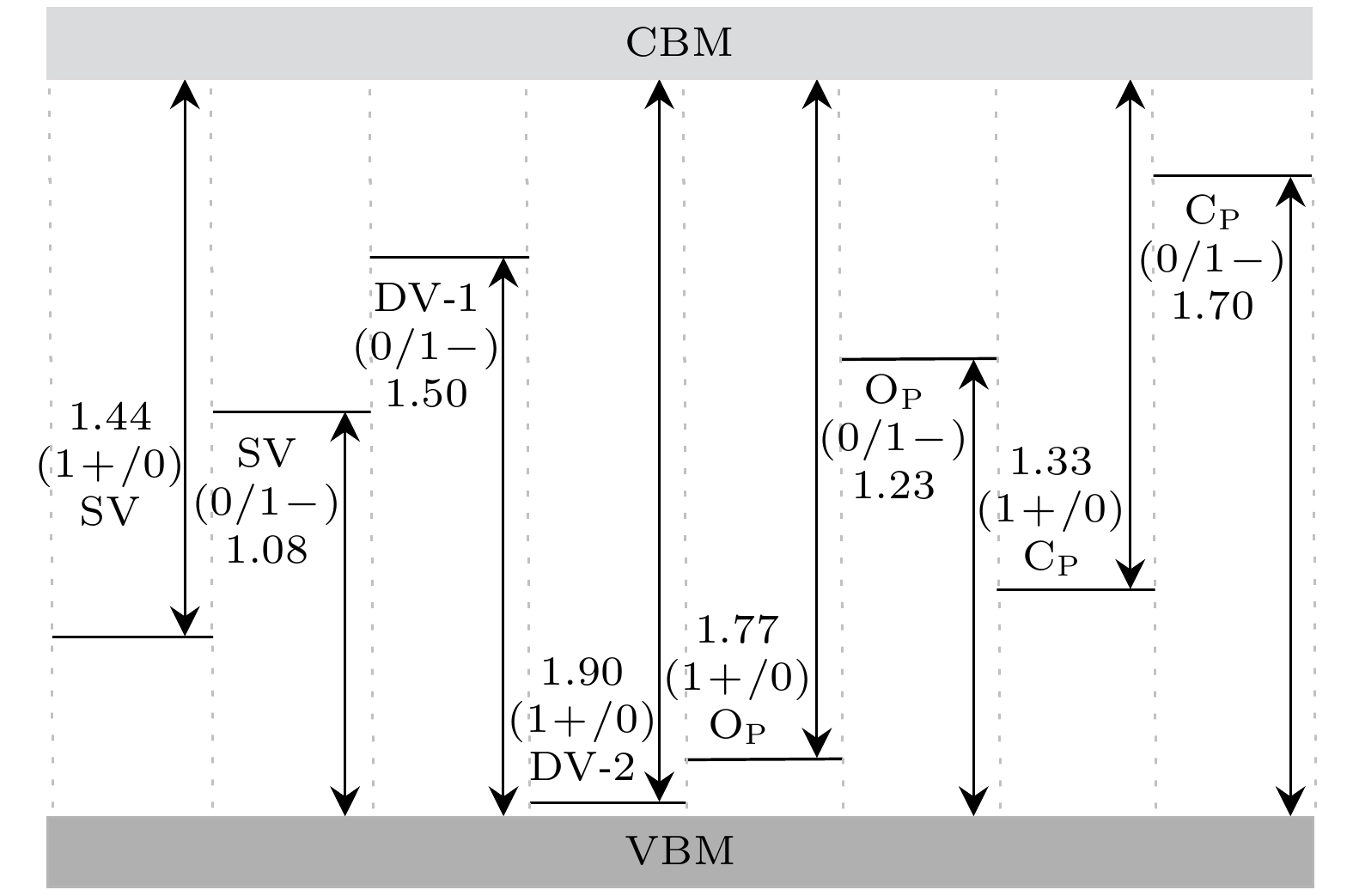
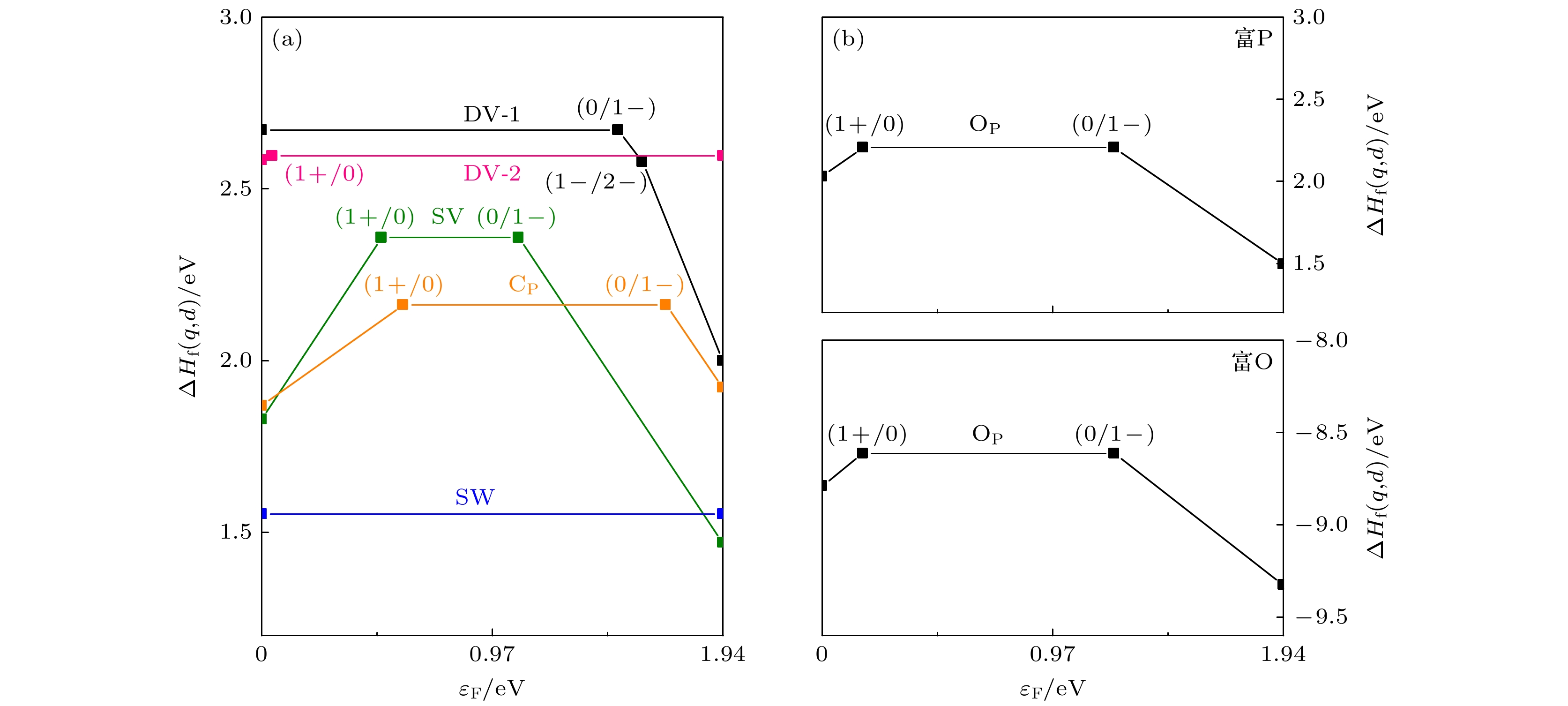
作为一种新型的二维材料, 蓝磷因其具有较高的载流子迁移率和较大的禁带宽度引起了研究者们极大的研究兴趣. 近年来已有研究讨论了蓝磷中的结构缺陷, 但是关于其缺陷带电性质的研究尚未见报道. 本文利用基于密度泛函理论的第一性原理计算, 讨论了蓝磷SW (Stone Wales)缺陷、单空位(SV)缺陷、两种双空位缺陷(DV-1和DV-2)以及两种替位缺陷(OP和CP)的带电性质. 利用带电缺陷体系能量依赖于晶胞尺寸的渐进表达式进行外推的方法, 修正了蓝磷带电缺陷体系的形成能. 研究结果表明, 在所讨论的缺陷中, 富O条件下OP具有最低的形成能, 最稳定; 而SV的离化能最小, 最容易电离. 引入的缺陷态会对蓝磷的禁带宽度产生影响. 缺陷带电过程中, 缺陷态位置变化导致绝大部分缺陷转变为深能级缺陷. 本研究结果对缺陷工程在二维材料上的应用提供了一定的理论指导.As a new two-dimensional material, blue phosphorus has attracted considerable research interest due to its high carrier mobility and large bandgap. Although the structural defects of blue phosphorus have been discussed recently, the charged properties of these defects have not been explored. In this paper, using first-principles calculations based on density functional theory, the six most stable point defects and their corresponding charged states in blue phosphorus are studied, including Stone Wales (SW), single vacancy (SV), two double-vacancy (DV-1 and DV-2) and two substitution defects (OP and CP). The converged ionization energy values of charged defects in blue phosphorus are obtained by extrapolating the asymptotic expression of the energy dependent on the cell size. Subsequently, the formation energy values for different charge states are modified to determine their structural stabilities. Finally, their electronic properties are analyzed through band structures. The results suggest that SV1– is easy to ionize, owing to its lowest ionization energy (1.08 eV). Furthermore, among the defects we are considering, OP1– is the most stable charged defect in blue phosphorus, with the lowest formation energy (–9.33 eV) under O-rich chemical potential condition. The negative formation energy indicates that O atoms can exist stably in blue phosphorus, implying that blue phosphorus is easily oxidized. The introduction of defect states will affect the bandgap of blue phosphorus, and the ionization of defects will cause the defect energy levels to shift, leading defects to transition between shallow and deep levels. This study provides theoretical guidance for the application of defect engineering in two-dimensional materials.
-
Keywords:
- first-principles calculations /
- blue phosphorus /
- charged defects /
- ionization energy
[1] Novoselov K S, Geim A K, Morozov S V, Jiang D, Zhang Y 2004 Science 306 666
 Google Scholar
Google Scholar
[2] Kourosh K, Ou J Z, Daeneke T, Strano M S, Martin P, Gras S L 2015 Adv. Funct. Mater. 25 5086
 Google Scholar
Google Scholar
[3] Xu M S, Liang T, Shi M M, Chen H Z 2013 Chem. Rev. 113 3766
 Google Scholar
Google Scholar
[4] Silvestrelli P L, Ambrosetti A 2018 Appl. Phys. Lett. 113 211603
 Google Scholar
Google Scholar
[5] Yoo H, Heo K, Ansari M H R, Cho S 2021 Nanomaterials 11 832
 Google Scholar
Google Scholar
[6] Zhang Z H, Zou X L, Crespi V H, Yakobson B I 2013 ACS Nano 7 10475
 Google Scholar
Google Scholar
[7] Wang R, Su Y, Yang G H, Zhang J F, Zhang S B 2020 Chem. Mater. 32 1545
 Google Scholar
Google Scholar
[8] Zhang J H, Guo Y, Li P G, Wang J, Zhou S, Zhao J J, Guo D H, Zhong D Y 2021 J. Phys. Chem. Lett. 12 2199
 Google Scholar
Google Scholar
[9] Pei W, Zhou S, Zhao J J, Du Y, Dou S X 2020 J. Mater. Chem. A 8 20570
 Google Scholar
Google Scholar
[10] Pisani L, Montanari B, Harrison N M 2008 New J. Phys. 10 033002
 Google Scholar
Google Scholar
[11] Han W, Kawakami R K, Gmitra M, Fabian J 2014 Nat. Nanotechnol. 9 794
 Google Scholar
Google Scholar
[12] Rasool H I, Ophus C, Zettl A 2015 Adv. Mater. 27 5771
 Google Scholar
Google Scholar
[13] Ma R R, Sun Y, Ge M, Ma C R, Zhang J F 2023 Phys. Chem. Chem. Phys. 25 8809
 Google Scholar
Google Scholar
[14] Qiu C, Cao R Y, Wang F, Deng H X 2021 Appl. Phys. Lett. 118 083102
 Google Scholar
Google Scholar
[15] Liu X F, Gao Z B, Wang V, Luo Z J, Lü B, Ding Z, Zhang Z F 2020 ACS Appl. Mater. Interfaces 12 17055
 Google Scholar
Google Scholar
[16] Wu Y N, Zhang X G, Pantelides S T 2017 Phys. Rev. Lett. 119 105501
 Google Scholar
Google Scholar
[17] Zhu G J, Yang J H, Gong X G 2020 Phys. Rev. B. 102 035202
 Google Scholar
Google Scholar
[18] Wang D, Han D, Li X B, Xie S Y, Chen N K, Tian W Q, West D, Sun H B, Zhang S B 2015 Phys. Rev. Lett. 114 196801
 Google Scholar
Google Scholar
[19] Wang D, Han D, Li X B, Chen N K, West D, Meunier V, Zhang S B, Sun H B 2017 Phys. Rev. B 96 155424
 Google Scholar
Google Scholar
[20] 王丹 2017 博士学位论文 (吉林: 吉林大学)
Wang D 2017 Ph. D. Dissertation (Jilin: Jilin University
[21] Xiao J, Long M Q, Deng C S, He J, Cui L L, Xu H 2016 J. Phys. Chem. C 120 4638
 Google Scholar
Google Scholar
[22] Xiao J, Long M Q, Zhang X J, Ouyang J, Xu H, Gao Y L 2015 Sci. Rep. 5 9961
 Google Scholar
Google Scholar
[23] Zhu Z, Tománek D 2014 Phys. Rev. Lett. 112 176802
 Google Scholar
Google Scholar
[24] Zhang J L, Zhao S T, Han C, Wang Z Z, Zhong S, Sun S, Guo R, Zhou X, Gu C D, Yuan K D, Li Z Y, Chen W 2016 Nano Lett. 16 4903
 Google Scholar
Google Scholar
[25] Zeng J, Cui P, Zhang Z Y 2017 Phys. Rev. Lett. 118 046101
 Google Scholar
Google Scholar
[26] Safari F, Fathipour M, Goharrizi A Y 2020 Physica E 118 113938
 Google Scholar
Google Scholar
[27] Wang C, You Y Z, Choi J H 2020 Mater. Res. Express 7 015005
 Google Scholar
Google Scholar
[28] Bai R M, Chen Z, Gou M M, Zhang Y X 2018 Solid State Commun. 270 76
 Google Scholar
Google Scholar
[29] Sun M L, Chou J P, Yu J, Tang W C 2017 Phys. Chem. Chem. Phys. 19 17324
 Google Scholar
Google Scholar
[30] Sun M L, Chou J P, Hu A, Schwingenschlögl U 2019 Chem. Mater. 31 8129
 Google Scholar
Google Scholar
[31] Sun S Y, Hussain T, Zhang W, Karton A 2019 Appl. Surf. Sci. 486 52
 Google Scholar
Google Scholar
[32] Zheng H L, Yang H, Wang H X, Du X B, Yan Y 2016 J. Magn. Magn. Mater. 408 121
 Google Scholar
Google Scholar
[33] Zhang W, Enriquez H, Tong Y, Bendounan A, Kara A, Seitsonen A P, Mayne A J, Dujardin G, Oughaddou H 2018 Small 14 1804066
 Google Scholar
Google Scholar
[34] Xie J F, Si M S, Yang D Z, Zhang Z Y, Xue D S 2014 J. Appl. Phys. 116 073704
 Google Scholar
Google Scholar
[35] Kresse G, Furthmüller J 1996 Phys. Rev. B 54 11169
 Google Scholar
Google Scholar
[36] Kresse G, Hafner J 1994 Phys. Rev. B 49 14251
 Google Scholar
Google Scholar
[37] Kresse G, Furthmüller J 1996 Comp. Mater. Sci. 6 15
 Google Scholar
Google Scholar
[38] Blöchl P E 1994 Phys. Rev. B 50 17953
 Google Scholar
Google Scholar
[39] Perdew J P, Burke K, Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[40] Nicholas L M, Jim G P, Rebecca J N, Salvy P R, Dougal G M 2017 Phys. Rev. B 96 144106
 Google Scholar
Google Scholar
[41] Wang D, Li X B, Sun H B 2021 Nano Lett. 21 6298
 Google Scholar
Google Scholar
-
图 1 蓝磷中6种完全弛豫的缺陷结构模型 (a) SV缺陷; (b) DV-1缺陷; (c) DV-2缺陷 ; (d) SW缺陷 ; (e) OP缺陷; (f) CP缺陷. 图(e), (f) 用箭头指出的分别为O原子(红色)和C原子(蓝色)
Fig. 1. Six fully relaxed atomic defect structures in monolayer blue P: (a) SV; (b) DV-1; (c) DV-2; (d) SW; (e) OP; (f) CP. The arrows indicate O atoms (red) and C atoms (blue) in panels (e), (f), respectively.
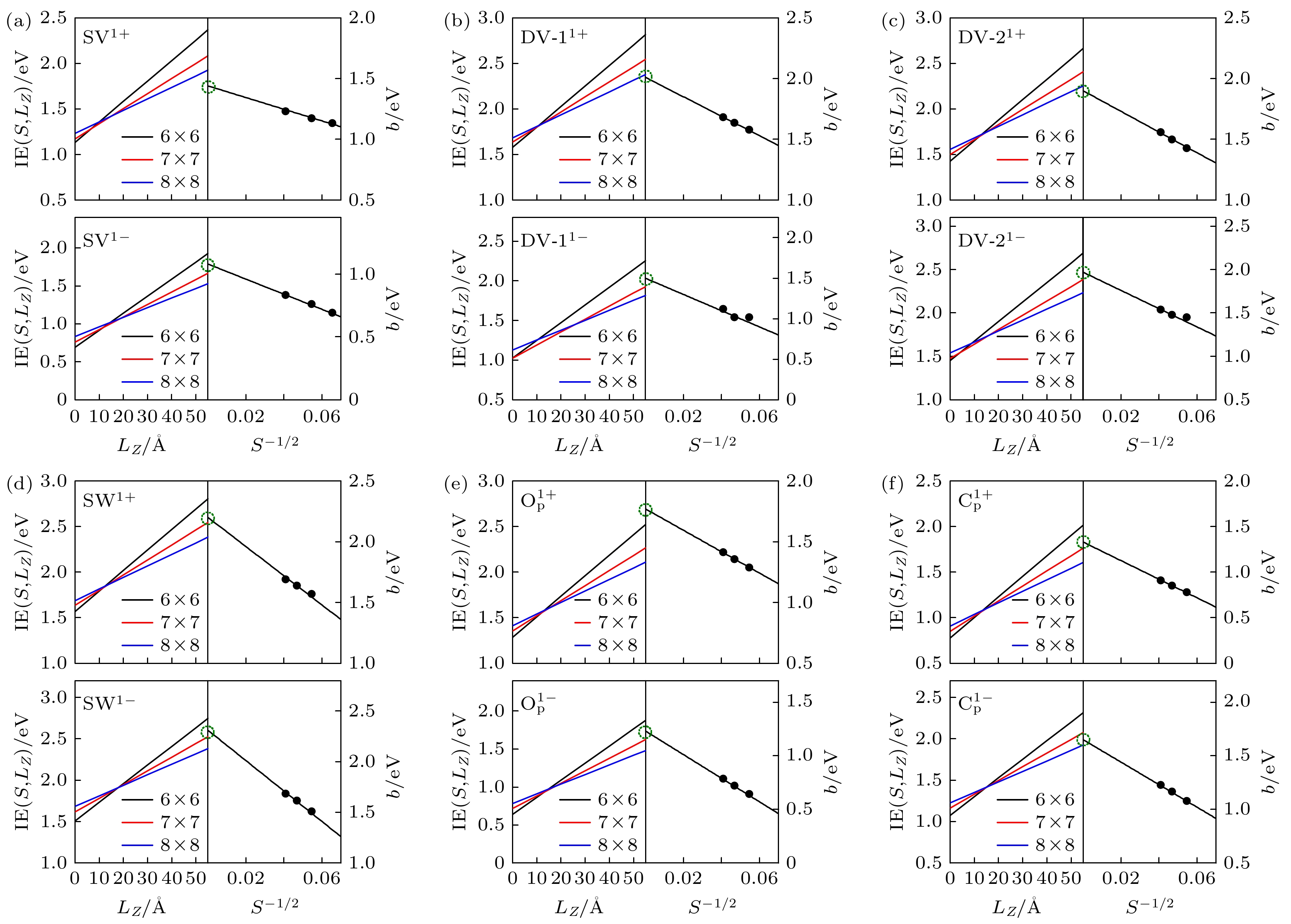
图 2 蓝磷的6种缺陷在3种不同晶胞尺寸下, IE与LZ, b与S –1/2的拟合关系图 (a) SV缺陷; (b) DV-1缺陷; (c) DV-2缺陷; (d) SW缺陷; (e) OP缺陷; (f) CP缺陷. 每幅图左半部分为IE与LZ的拟合关系图, 黑色、红色、蓝色图线分别表示6×6, 7×7, 8×8的晶胞; 每幅图右半部分为b与S –1/2的拟合关系图, 收敛的IE0的值用绿色虚线圆标出
Fig. 2. Calculated IE (left panels) and b (right panels) fitted for six defects in monolayer blue P at different lateral dimensions (S for 6×6, 7×7 and 8×8) as functions of LZ and S –1/2, respectively: (a) SV; (b) DV-1; (c) DV-2; (d) SW; (e) OP; (f) CP. The converged IE0 has been labeled by the green dash circle.
图 5 蓝磷完美和带电缺陷的能带图 (a) 完美蓝磷; (b) DV-1缺陷; (c) SV缺陷; (d) OP缺陷; (e) CP缺陷. 红色实线、黑色实线和蓝色实线分别代表1+, 0和1–价态的情况, 水平方向的虚线代表费米能级, 0价态缺陷的费米能级设置为0, 其他价态的VBM与0价的对齐
Fig. 5. Band structures diagrams of blue P perfection and defective: (a) Perfect blue P; (b) DV-1; (c) SV; (d) OP; (e) CP. Red, black and blue solid lines represent 1+, 0 and 1– states, respectively. The dashed lines in the horizontal direction represent the Fermi level. The Fermi level of the 0 valence defect is set to zero and the VBM of the other valence states is aligned with that of the 0 valence.
-
[1] Novoselov K S, Geim A K, Morozov S V, Jiang D, Zhang Y 2004 Science 306 666
 Google Scholar
Google Scholar
[2] Kourosh K, Ou J Z, Daeneke T, Strano M S, Martin P, Gras S L 2015 Adv. Funct. Mater. 25 5086
 Google Scholar
Google Scholar
[3] Xu M S, Liang T, Shi M M, Chen H Z 2013 Chem. Rev. 113 3766
 Google Scholar
Google Scholar
[4] Silvestrelli P L, Ambrosetti A 2018 Appl. Phys. Lett. 113 211603
 Google Scholar
Google Scholar
[5] Yoo H, Heo K, Ansari M H R, Cho S 2021 Nanomaterials 11 832
 Google Scholar
Google Scholar
[6] Zhang Z H, Zou X L, Crespi V H, Yakobson B I 2013 ACS Nano 7 10475
 Google Scholar
Google Scholar
[7] Wang R, Su Y, Yang G H, Zhang J F, Zhang S B 2020 Chem. Mater. 32 1545
 Google Scholar
Google Scholar
[8] Zhang J H, Guo Y, Li P G, Wang J, Zhou S, Zhao J J, Guo D H, Zhong D Y 2021 J. Phys. Chem. Lett. 12 2199
 Google Scholar
Google Scholar
[9] Pei W, Zhou S, Zhao J J, Du Y, Dou S X 2020 J. Mater. Chem. A 8 20570
 Google Scholar
Google Scholar
[10] Pisani L, Montanari B, Harrison N M 2008 New J. Phys. 10 033002
 Google Scholar
Google Scholar
[11] Han W, Kawakami R K, Gmitra M, Fabian J 2014 Nat. Nanotechnol. 9 794
 Google Scholar
Google Scholar
[12] Rasool H I, Ophus C, Zettl A 2015 Adv. Mater. 27 5771
 Google Scholar
Google Scholar
[13] Ma R R, Sun Y, Ge M, Ma C R, Zhang J F 2023 Phys. Chem. Chem. Phys. 25 8809
 Google Scholar
Google Scholar
[14] Qiu C, Cao R Y, Wang F, Deng H X 2021 Appl. Phys. Lett. 118 083102
 Google Scholar
Google Scholar
[15] Liu X F, Gao Z B, Wang V, Luo Z J, Lü B, Ding Z, Zhang Z F 2020 ACS Appl. Mater. Interfaces 12 17055
 Google Scholar
Google Scholar
[16] Wu Y N, Zhang X G, Pantelides S T 2017 Phys. Rev. Lett. 119 105501
 Google Scholar
Google Scholar
[17] Zhu G J, Yang J H, Gong X G 2020 Phys. Rev. B. 102 035202
 Google Scholar
Google Scholar
[18] Wang D, Han D, Li X B, Xie S Y, Chen N K, Tian W Q, West D, Sun H B, Zhang S B 2015 Phys. Rev. Lett. 114 196801
 Google Scholar
Google Scholar
[19] Wang D, Han D, Li X B, Chen N K, West D, Meunier V, Zhang S B, Sun H B 2017 Phys. Rev. B 96 155424
 Google Scholar
Google Scholar
[20] 王丹 2017 博士学位论文 (吉林: 吉林大学)
Wang D 2017 Ph. D. Dissertation (Jilin: Jilin University
[21] Xiao J, Long M Q, Deng C S, He J, Cui L L, Xu H 2016 J. Phys. Chem. C 120 4638
 Google Scholar
Google Scholar
[22] Xiao J, Long M Q, Zhang X J, Ouyang J, Xu H, Gao Y L 2015 Sci. Rep. 5 9961
 Google Scholar
Google Scholar
[23] Zhu Z, Tománek D 2014 Phys. Rev. Lett. 112 176802
 Google Scholar
Google Scholar
[24] Zhang J L, Zhao S T, Han C, Wang Z Z, Zhong S, Sun S, Guo R, Zhou X, Gu C D, Yuan K D, Li Z Y, Chen W 2016 Nano Lett. 16 4903
 Google Scholar
Google Scholar
[25] Zeng J, Cui P, Zhang Z Y 2017 Phys. Rev. Lett. 118 046101
 Google Scholar
Google Scholar
[26] Safari F, Fathipour M, Goharrizi A Y 2020 Physica E 118 113938
 Google Scholar
Google Scholar
[27] Wang C, You Y Z, Choi J H 2020 Mater. Res. Express 7 015005
 Google Scholar
Google Scholar
[28] Bai R M, Chen Z, Gou M M, Zhang Y X 2018 Solid State Commun. 270 76
 Google Scholar
Google Scholar
[29] Sun M L, Chou J P, Yu J, Tang W C 2017 Phys. Chem. Chem. Phys. 19 17324
 Google Scholar
Google Scholar
[30] Sun M L, Chou J P, Hu A, Schwingenschlögl U 2019 Chem. Mater. 31 8129
 Google Scholar
Google Scholar
[31] Sun S Y, Hussain T, Zhang W, Karton A 2019 Appl. Surf. Sci. 486 52
 Google Scholar
Google Scholar
[32] Zheng H L, Yang H, Wang H X, Du X B, Yan Y 2016 J. Magn. Magn. Mater. 408 121
 Google Scholar
Google Scholar
[33] Zhang W, Enriquez H, Tong Y, Bendounan A, Kara A, Seitsonen A P, Mayne A J, Dujardin G, Oughaddou H 2018 Small 14 1804066
 Google Scholar
Google Scholar
[34] Xie J F, Si M S, Yang D Z, Zhang Z Y, Xue D S 2014 J. Appl. Phys. 116 073704
 Google Scholar
Google Scholar
[35] Kresse G, Furthmüller J 1996 Phys. Rev. B 54 11169
 Google Scholar
Google Scholar
[36] Kresse G, Hafner J 1994 Phys. Rev. B 49 14251
 Google Scholar
Google Scholar
[37] Kresse G, Furthmüller J 1996 Comp. Mater. Sci. 6 15
 Google Scholar
Google Scholar
[38] Blöchl P E 1994 Phys. Rev. B 50 17953
 Google Scholar
Google Scholar
[39] Perdew J P, Burke K, Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[40] Nicholas L M, Jim G P, Rebecca J N, Salvy P R, Dougal G M 2017 Phys. Rev. B 96 144106
 Google Scholar
Google Scholar
[41] Wang D, Li X B, Sun H B 2021 Nano Lett. 21 6298
 Google Scholar
Google Scholar
计量
- 文章访问数: 4511
- PDF下载量: 168
- 被引次数: 0














 下载:
下载: