-
富锂层状氧化物因能量密度高和成本低, 有望成为下一代锂离子电池正极的重要候选材料. 然而, 富锂正极材料中阴离子氧化还原反应使晶格氧不稳定, 导致电压衰减和不可逆容量损失. 尽管铁代无钴富锂材料可以实现较少的电压衰减, 但存在严重的阳离子混排和较差的动力学. 采用一种简单易行的高价离子掺杂策略, 在Li1.2Ni0.13Fe0.13Mn0.54O2 (LNFMO)中掺入Mo元素, 拓宽了锂层间距, 为Li+的传输提供了更宽的通道, 改善了Li+的扩散动力学, 有效抑制了阳离子混排, 进一步稳定了层状结构. 得益于此, Mo掺杂后的富锂材料表现出显著增强的电化学性能, 在0.2 C电流密度下表现出209.48 mAh/g的初始放电比容量. 1 C下的初始放电比容量从137.02 mAh/g提高到165.15 mAh/g; 循环300次后, 仍有117.49 mAh/g的放电比容量, 电压衰减由2.09 mV/cycle降低为1.66 mV/cycle. 本文对Mo掺杂后的正极材料进行了系统表征并揭示了循环稳定的机理, 为高性能富锂正极材料的设计提供了重要参考.Li-ion batteries (LIBs) are widely used in mobile devices and electric vehicles, but the traditional layered transition metal cathode material, LiTMO2 (TM=Ni, Co, Mn, or Al), has a low energy density that cannot satisfy the demand of commercial applications. The Li-rich Mn-based layered oxides (LRLOs) are a strong competitor to the traditional layered cathode materials for their specific capacity of more than 200 mAh/g. Due to the high energy density and low cost, Li-rich Mn-based layered oxides (LRLO) have been a promising candidate cathode for next-generation Li-ion batteries. The anionic redox reaction (ARR) in LRLO destabilizes the lattice oxygen, leading to voltage degradation and capacity loss. Although iron-substituted cobalt-free Li-rich materials can achieve less voltage decay, they suffer severe cation disorder and poor kinetics. Here, we develop a simple and feasible high-valent ion doping strategy by doping Mo into Li1.2Ni0.13Fe0.13Mn0.54O2(LNFMO), which expands the Li layer spacing and provides a broader channel for Li+ transport, thereby improving the diffusion kinetics of Li+, effectively suppressing the cation disorder, and further stabilizing the layered structure. As a result, the Mo-doped LRLO exhibits significantly enhanced electrochemical performance, with an initial reversible capacity of 209.48 mAh/g at 0.2 C, and the initial specific capacity increasing from 137.02 mAh/g to 165.15 mAh/g at 1 C. After 300 cycles, specific capacity remains 117.49 mAh/g for the Mo-doped cathode, and the voltage decay decreases from 2.09 mV/cycle to 1.66 mV/cycle. The Mo-doped LRLO is systematically characterized, and the mechanism of cycle stabilization is revealed, which provides an important reference for designing high performance Li-rich cathode.
-
Keywords:
- Li-ion batteries /
- Li-rich layered oxides /
- cathode materials /
- cationic disorder
[1] Chen Q, Pei Y, Chen H, Song Y, Zhen L, Xu C Y, Xiao P, Henkelman G 2020 Nat. Commun. 11 3411
 Google Scholar
Google Scholar
[2] He W, Zhang C, Wang M, Wei B, Zhu Y, Wu J, Liang C, Chen L, Wang P, Wei W 2022 Adv. Funct. Mater. 32 2200322
 Google Scholar
Google Scholar
[3] Seo D-H, Lee J, Urban A, Malik R, Kang S, Ceder G 2016 Nat. Chem. 8 692
 Google Scholar
Google Scholar
[4] Li X, Li X, Monluc L, et al. 2022 Adv. Energy Mater. 12 2200427
 Google Scholar
Google Scholar
[5] Jiao J, Zhang Z, Kuroiwa Y, Zhao E, Yin W, Wang B, Wang F, Zhao J, Zhang X, Xiao X 2023 Chem. Eng. J. 454 140327
 Google Scholar
Google Scholar
[6] Zhang K, Li B, Zuo Y, Song J, Shang H, Ning F, Xia D 2019 Electrochem. Energy Rev. 2 606
 Google Scholar
Google Scholar
[7] Eum D, Kim B, Kim S J, et al. 2020 Nat. Mater. 19 419
 Google Scholar
Google Scholar
[8] Liu W, Li J, Li W, Xu H, Zhang C, Qiu X 2020 Nat. Commun. 11 3629
 Google Scholar
Google Scholar
[9] Asl H Y, Manthiram A 2020 Science 369 140
 Google Scholar
Google Scholar
[10] Manthiram A, Knight J C, Myung S T, Oh S-M, Sun Y K 2016 Energy Mater. 6 1501010
 Google Scholar
Google Scholar
[11] Wu K, Zhao E, Ran P, Yin W, Zhang Z, Wang B, Ikeda K, Otomo T, Xiao X, Wang F, Zhao J 2023 Small 19 2300419
 Google Scholar
Google Scholar
[12] Hu S, Pillai Anoop S, Liang G, Pang W K, Wang H, Li Q, Guo Z 2019 Electrochem. Energy Rev. 2 277
 Google Scholar
Google Scholar
[13] Zhao H, Lam W A, Sheng L, Wang L, Bai P, Yang Y, Ren D, Xu H, He X 2022 Adv. Energy Mater. 12 2103894
 Google Scholar
Google Scholar
[14] Zhao E, Zhang M, Wang X, et al. 2020 Energy Storage Mater. 24 384
 Google Scholar
Google Scholar
[15] Zhao H, Li W, Li J, Xu H, Zhang C, Li J, Han C, Li Z, Chu M, Qiu X 2022 Nano Energy 92 106760
 Google Scholar
Google Scholar
[16] Billaud J, Sheptyakov D, Sallard S, Leanza D, Talianker M, Grinblat J, Sclar H, Aurbach D, Novák P, Villevieille C 2019 J. Mater. Chem. A 7 15215
 Google Scholar
Google Scholar
[17] Zhao T, Ji R, Yang H, Zhang Y, Sun X, Li Y, Li L, Chen R 2019 J. Energy Chem. 33 37
 Google Scholar
Google Scholar
[18] Lee Y, Park H, Cho M, Ahn J, Ko W, Kang J, Choi Y J, Kim H, Park I, Ryu W, Hong J, Kim J 2022 Adv. Funct. Mater. 32 2204354
 Google Scholar
Google Scholar
[19] Nayak P K, Grinblat J, Levi M, Levi E, Kim S, Choi J W, Aurbach D 2016 Adv. Energy Mater. 6 1502398
 Google Scholar
Google Scholar
[20] Dahiya P P, Ghanty C, Sahoo K, Basu S, Majumder S B 2018 J. Electrochem. Soc. 165 A3114
 Google Scholar
Google Scholar
[21] Wang E, Xiao D, Wu T, Liu X, Zhou Y, Wang B, Lin T, Zhang X, Yu H 2022 Adv. Funct. Mater. 32 2201744
 Google Scholar
Google Scholar
[22] Kroger F A 1977 Annu. Rev. Mater. Sci. 7 449
 Google Scholar
Google Scholar
[23] Zu C X, Li H 2011 Energy Environ. Sci. 4 2614
 Google Scholar
Google Scholar
[24] Li X, Xin H, Liu Y, Li D, Yuan X, Qin X 2015 RSC Adv. 5 45351
 Google Scholar
Google Scholar
[25] Liu X, Yu B, Wang M, Jin Y, Fu Z, Chen J, Ma Z, Guo B, Huang Y, Li X 2022 Mater. Today Commun. 32 104170
 Google Scholar
Google Scholar
[26] Yang J, Chen Y, Li Y, Xi X, Zheng J, Zhu Y, Xiong Y, Liu S 2021 ACS Appl. Mater. Interfaces 13 25981
 Google Scholar
Google Scholar
[27] Meng J, Xu L, Ma Q, Yang M, Fang Y, Wan G, Li R, Yuan J, Zhang X, Yu H, Liu L, Liu T 2022 Adv. Funct. Mater. 32 2113013
 Google Scholar
Google Scholar
[28] Morales J, Pérez-Vicente C, Tirado J L 1990 Mater. Res. Bull. 25 623
 Google Scholar
Google Scholar
[29] Zhao J, Zhang W, Huq A, Misture S T, Zhang B, Guo S, Wu L, Zhu Y, Chen Z, Amine K, Pan F, Bai J, Wang F 2017 Adv. Energy Mater. 7 1601266
 Google Scholar
Google Scholar
[30] Li Q, Wang Y, Wang X, Sun X, Zhang J N, Yu X, Li H 2020 ACS Appl. Mater. Interfaces 12 2319
 Google Scholar
Google Scholar
-
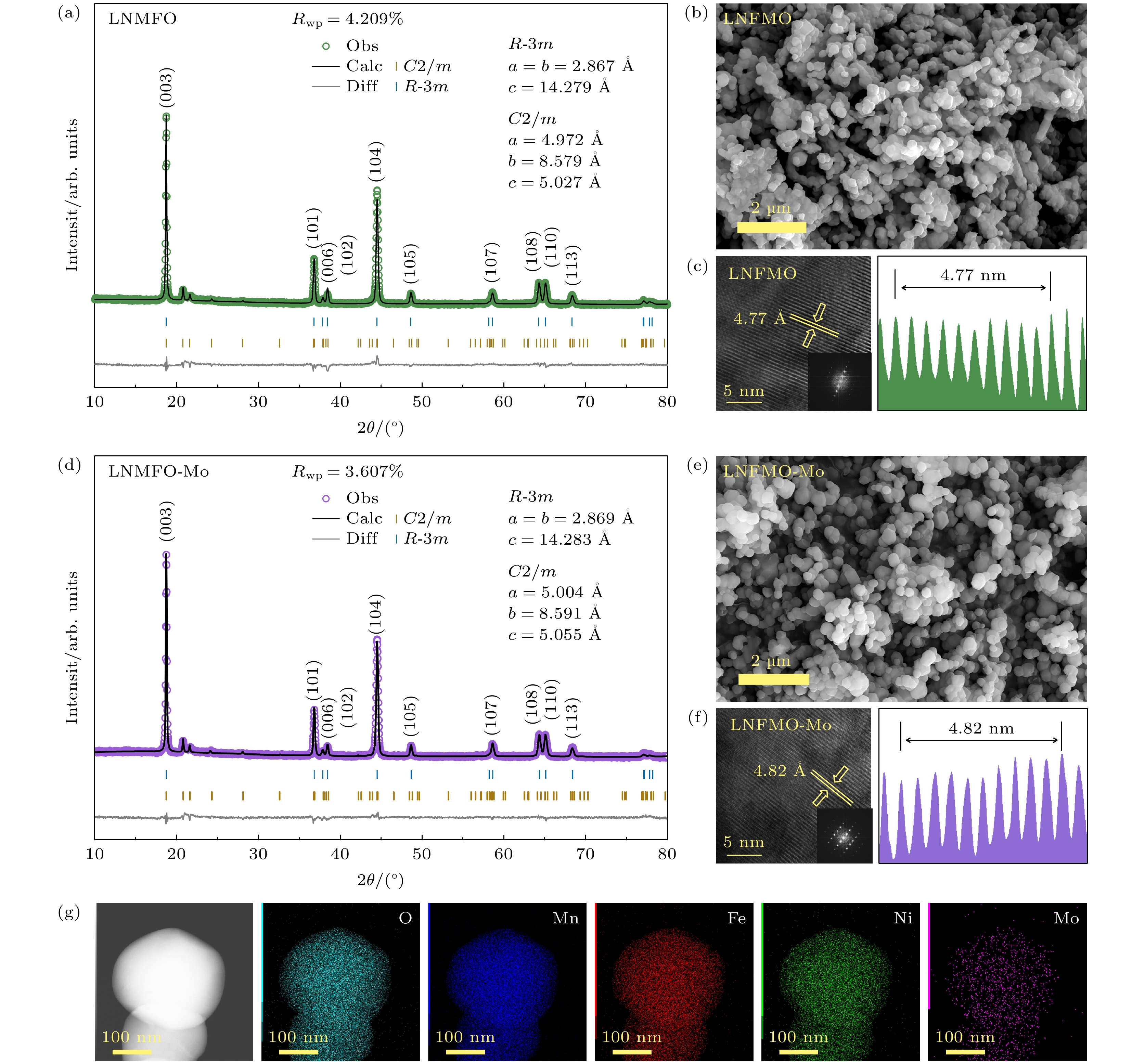
图 1 Rietveld精修的样品LNFMO (a)和LNFMO-Mo (d) XRD图谱; 样品LNFMO (b)和LNFMO-Mo (e)的SEM图; 样品LNFMO (c)和LNFMO-Mo (f)的TEM图及层间距; LNFMO-Mo (g)表面不同元素的EDS分布图
Fig. 1. Rietveld refined XRD patterns of samples LNFMO (a) and LNFMO-Mo (d); SEM images of samples LNFMO (b) and LNFMO-Mo (e); TEM images and layer spacing of samples LNFMO (c) and LNFMO-Mo (f); EDS images of distributions of different elements on the surface of LNFMO-Mo (g).
图 2 样品LNFMO (a)和LNFMO-Mo (b)前3次循环的CV曲线; 在不同电流密度下的倍率性能 (c); 0.2 C电流密度下的初始循环曲线 (d); LNFMO (e)和LNFMO-Mo (f)的GITT曲线; 两种样品0.2 C (g)、0.5 C (h)、1 C (i)电流密度下的长循环性能
Fig. 2. CV curves for the first 3 cycles of samples LNFMO (a) and LNFMO-Mo (b); rate performance at different current density (c); initial cycling curves at 0.2 C current density (d); GITT curves of samples LNFMO (e) and LNFMO-Mo (f); the long-cycle performance of the two samples at 0.2 C (g), 0.5 C (h), and 1 C (i) current density.
图 3 LNFMO (a)和LNFMO-Mo (b)在原始、第1次充电至4.5 V、 4.8 V, 第1次放电至2.0 V状态下的XRD谱图及I(003)/(104)比值的变化; LNFMO(c)和LNFMO-Mo(d)在原始、第1次充电至4.8 V, 第1次放电至2.0 V状态下的O 1s XPS光谱
Fig. 3. XRD spectra of LNFMO (a) and LNFMO-Mo (b) in the pristine, first charge to 4.5 V, 4.8 V, and first discharge to 2.0 V states and the variation of the I(003)/(104) ratio; O 1s XPS spectra of LNFMO (c) and LNFMO-Mo (d) in the pristine, first charge to 4.8 V, and first discharge to 2.0 V states.
-
[1] Chen Q, Pei Y, Chen H, Song Y, Zhen L, Xu C Y, Xiao P, Henkelman G 2020 Nat. Commun. 11 3411
 Google Scholar
Google Scholar
[2] He W, Zhang C, Wang M, Wei B, Zhu Y, Wu J, Liang C, Chen L, Wang P, Wei W 2022 Adv. Funct. Mater. 32 2200322
 Google Scholar
Google Scholar
[3] Seo D-H, Lee J, Urban A, Malik R, Kang S, Ceder G 2016 Nat. Chem. 8 692
 Google Scholar
Google Scholar
[4] Li X, Li X, Monluc L, et al. 2022 Adv. Energy Mater. 12 2200427
 Google Scholar
Google Scholar
[5] Jiao J, Zhang Z, Kuroiwa Y, Zhao E, Yin W, Wang B, Wang F, Zhao J, Zhang X, Xiao X 2023 Chem. Eng. J. 454 140327
 Google Scholar
Google Scholar
[6] Zhang K, Li B, Zuo Y, Song J, Shang H, Ning F, Xia D 2019 Electrochem. Energy Rev. 2 606
 Google Scholar
Google Scholar
[7] Eum D, Kim B, Kim S J, et al. 2020 Nat. Mater. 19 419
 Google Scholar
Google Scholar
[8] Liu W, Li J, Li W, Xu H, Zhang C, Qiu X 2020 Nat. Commun. 11 3629
 Google Scholar
Google Scholar
[9] Asl H Y, Manthiram A 2020 Science 369 140
 Google Scholar
Google Scholar
[10] Manthiram A, Knight J C, Myung S T, Oh S-M, Sun Y K 2016 Energy Mater. 6 1501010
 Google Scholar
Google Scholar
[11] Wu K, Zhao E, Ran P, Yin W, Zhang Z, Wang B, Ikeda K, Otomo T, Xiao X, Wang F, Zhao J 2023 Small 19 2300419
 Google Scholar
Google Scholar
[12] Hu S, Pillai Anoop S, Liang G, Pang W K, Wang H, Li Q, Guo Z 2019 Electrochem. Energy Rev. 2 277
 Google Scholar
Google Scholar
[13] Zhao H, Lam W A, Sheng L, Wang L, Bai P, Yang Y, Ren D, Xu H, He X 2022 Adv. Energy Mater. 12 2103894
 Google Scholar
Google Scholar
[14] Zhao E, Zhang M, Wang X, et al. 2020 Energy Storage Mater. 24 384
 Google Scholar
Google Scholar
[15] Zhao H, Li W, Li J, Xu H, Zhang C, Li J, Han C, Li Z, Chu M, Qiu X 2022 Nano Energy 92 106760
 Google Scholar
Google Scholar
[16] Billaud J, Sheptyakov D, Sallard S, Leanza D, Talianker M, Grinblat J, Sclar H, Aurbach D, Novák P, Villevieille C 2019 J. Mater. Chem. A 7 15215
 Google Scholar
Google Scholar
[17] Zhao T, Ji R, Yang H, Zhang Y, Sun X, Li Y, Li L, Chen R 2019 J. Energy Chem. 33 37
 Google Scholar
Google Scholar
[18] Lee Y, Park H, Cho M, Ahn J, Ko W, Kang J, Choi Y J, Kim H, Park I, Ryu W, Hong J, Kim J 2022 Adv. Funct. Mater. 32 2204354
 Google Scholar
Google Scholar
[19] Nayak P K, Grinblat J, Levi M, Levi E, Kim S, Choi J W, Aurbach D 2016 Adv. Energy Mater. 6 1502398
 Google Scholar
Google Scholar
[20] Dahiya P P, Ghanty C, Sahoo K, Basu S, Majumder S B 2018 J. Electrochem. Soc. 165 A3114
 Google Scholar
Google Scholar
[21] Wang E, Xiao D, Wu T, Liu X, Zhou Y, Wang B, Lin T, Zhang X, Yu H 2022 Adv. Funct. Mater. 32 2201744
 Google Scholar
Google Scholar
[22] Kroger F A 1977 Annu. Rev. Mater. Sci. 7 449
 Google Scholar
Google Scholar
[23] Zu C X, Li H 2011 Energy Environ. Sci. 4 2614
 Google Scholar
Google Scholar
[24] Li X, Xin H, Liu Y, Li D, Yuan X, Qin X 2015 RSC Adv. 5 45351
 Google Scholar
Google Scholar
[25] Liu X, Yu B, Wang M, Jin Y, Fu Z, Chen J, Ma Z, Guo B, Huang Y, Li X 2022 Mater. Today Commun. 32 104170
 Google Scholar
Google Scholar
[26] Yang J, Chen Y, Li Y, Xi X, Zheng J, Zhu Y, Xiong Y, Liu S 2021 ACS Appl. Mater. Interfaces 13 25981
 Google Scholar
Google Scholar
[27] Meng J, Xu L, Ma Q, Yang M, Fang Y, Wan G, Li R, Yuan J, Zhang X, Yu H, Liu L, Liu T 2022 Adv. Funct. Mater. 32 2113013
 Google Scholar
Google Scholar
[28] Morales J, Pérez-Vicente C, Tirado J L 1990 Mater. Res. Bull. 25 623
 Google Scholar
Google Scholar
[29] Zhao J, Zhang W, Huq A, Misture S T, Zhang B, Guo S, Wu L, Zhu Y, Chen Z, Amine K, Pan F, Bai J, Wang F 2017 Adv. Energy Mater. 7 1601266
 Google Scholar
Google Scholar
[30] Li Q, Wang Y, Wang X, Sun X, Zhang J N, Yu X, Li H 2020 ACS Appl. Mater. Interfaces 12 2319
 Google Scholar
Google Scholar
计量
- 文章访问数: 5704
- PDF下载量: 129
- 被引次数: 0













 下载:
下载:


