-
钠离子电池近年来在大规模储能领域展现出优异的发展和应用前景. 由于钠离子层状过渡金属氧化物正极材料(NaxTMO2)具有比容量高、容易制备、电压可调和成本低的优势, 在学术界和产业界得到了广泛的关注与研究. 但较大的Na+半径和较强的Na+-Na+静电排斥作用, 导致NaxTMO2具有多种结构类型和复杂的结构转变, 以及由此形成了多重结构-性能关系. 本文详细介绍了NaxTMO2的结构类型, 综述了在Na+脱出/嵌入过程中引发的结构演变, 旨在揭示钠离子层状过渡金属氧化物正极材料结构转变机理及其对电化学性能的影响, 最后讨论了现存的挑战并提出了改进策略.Na-ion batteries possess great potential applications in the large-scale energy storage. The Na-ion layered oxide cathode (NaxTMO2) has received increasing attention in scientific and industrial research due to its high capacity, easy manufacture, adjustable voltage, and low cost. However, the larger the Na+ radius and the stronger the Na+-Na+ electrostatic repulsion is, which will lead to various structural configurations and complex structural transitions, resulting in multiple structure-property connections. In this paper, the structural types of Na-ion layered transition metal oxide cathode materials are introduced, and their structural evolutions during Na+ de/intercalation are summarized for revealing the mechanism for structural transformation of Na-ion layered transition-metal oxide cathode material and its effect on electrochemical performance; the existing challenges are discussed; the improvement strategies are proposed finally.
-
Keywords:
- Na-ion battery /
- layered oxide /
- cathode material /
- phase transformation mechanism
[1] 陆雅翔, 赵成龙, 容晓晖, 陈立泉, 胡勇胜 2018 67 120601
 Google Scholar
Google Scholar
Lu Y X, Zhao C L, Chen L Q, Hu Y S 2018 Acta Phys. Sin. 67 120601
 Google Scholar
Google Scholar
[2] Sun Y, Guo S, Zhou H 2019 Energy Environ Sci. 12 825
 Google Scholar
Google Scholar
[3] Kubota K, Kumakura S, Yoda Y, Kuroki K, Komaba S 2018 Advan. Energy Mater. 8 1703415
 Google Scholar
Google Scholar
[4] Liu Q, Hu Z, Chen M, Zou C, Jin H, Wang S, Chou S L, Dou S X 2019 Small 0 1805381
[5] Kim S W, Seo D H, Ma X, Ceder G, Kang K 2012 Advan. Energy Mater. 2 710
 Google Scholar
Google Scholar
[6] Kim H, Park I, Lee S, Kim H, Park K Y, Park Y U, Kim H, Kim J, Lim H D, Yoon W S, Kang K 2013 Chem. Mater. 25 3614
 Google Scholar
Google Scholar
[7] Bauer A, Song J, Vail S, Pan W, Barker J, Lu Y 2018 Advan. Energy Mater. 8 1702869
 Google Scholar
Google Scholar
[8] Wang L, Lu Y, Liu J, Xu M, Cheng J, Zhang D, Goodenough J B 2013 Angew. Chem. Int. Ed. 52 1964
 Google Scholar
Google Scholar
[9] Wang S, Wang L, Zhu Z, Hu Z, Zhao Q, Chen J 2014 Angew. Chem. Int. Ed. 53 5892
 Google Scholar
Google Scholar
[10] Wang Q, Zhao C, Lu Y, Li Y, Zheng Y, Qi Y, Rong X, Jiang L, Qi X, Shao Y, Pan D, Li B, Hu Y S, Chen L 2017 Small 13 1701835
 Google Scholar
Google Scholar
[11] Wu F, Zhao C, Chen S, Lu Y, Hou Y, Hu Y S, Maier J, Yu Y 2018 Mater. Today 21 960
 Google Scholar
Google Scholar
[12] Delmas C, Fouassier C, Hagenmuller P 1980 Physica B+C 99 81
 Google Scholar
Google Scholar
[13] 胡勇胜, 陆雅翔, 陈立泉 2020 钠离子电池科学与技术 (北京: 科学出版社) 第20页
Hu Y S, Lu Y X, Chen L Q 2020 Na-ion batteries:science and technology (Beijing: Science Press) p20 (in Chinese)
[14] Mortemard de Boisse B, Cheng J H, Carlier D, Guignard M, Pan C J, Bordère S, Filimonov D, Drathen C, Suard E, Hwang B-J, Wattiaux A, Delmas C 2015 J. Mater. Chem. A 3 10976
 Google Scholar
Google Scholar
[15] Mortemard de Boisse B, Liu G, Ma J, Nishimura S I, Chung S C, Kiuchi H, Harada Y, Kikkawa J, Kobayashi Y, Okubo M, Yamada A 2016 Nat. Commun. 7 11397
 Google Scholar
Google Scholar
[16] Nanba Y, Iwao T, Boisse B M d, Zhao W, Hosono E, Asakura D, Niwa H, Kiuchi H, Miyawaki J, Harada Y, Okubo M, Yamada A 2016 Chem. Mater. 28 1058
 Google Scholar
Google Scholar
[17] Perez A J, Batuk D, Saubanère M, Rousse G, Foix D, McCalla E, Berg E J, Dugas R, H. W. van den Bos K, Doublet M L, Gonbeau D, Abakumov A, Tendeloo G, Tarascon J-M 2016 Chem. Mater. 28 8278
 Google Scholar
Google Scholar
[18] Zhao C, Wang Q, Yao Z, Wang J, Sanchez-Lengeling B, Ding F, Qi X, Lu Y, Bai X, Li B, Li H, Aspuru-Guzik A, Huang X, Delmas C, Wagemaker M, Chen L, Hu Y S 2020 Science 370 708
[19] Liu J, Kan W H, Ling C D 2021 J. Power Sources 481 229139
 Google Scholar
Google Scholar
[20] Komaba S, Yabuuchi N, Nakayama T, Ogata A, Ishikawa T, Nakai I 2012 Inorg. Chem. 51 6211
 Google Scholar
Google Scholar
[21] Sathiya M, Jacquet Q, Doublet M-L, Karakulina O M, Hadermann J, Tarascon J M 2018 Advan. Energy Mater. 8 1702599
 Google Scholar
Google Scholar
[22] Croguennec L, Pouillerie C, Mansour A N, Delmas C 2001 J. Mater. Chem. 11 131
 Google Scholar
Google Scholar
[23] Mortemard de Boisse B, Reynaud M, Ma J, Kikkawa J, Nishimura S I, Casas-Cabanas M, Delmas C, Okubo M, Yamada A 2019 Nat. Commun. 10 2185
 Google Scholar
Google Scholar
[24] Maazaz A, Delmas C, Hagenmuller P 1983 J. Incl. Phenom. 1 45
 Google Scholar
Google Scholar
[25] Didier C, Guignard M, Denage C, Szajwaj O, Ito S, Saadoune I, Darriet J, Delmas C 2011 Electrochem. Solid-State Lett. 14 A75
 Google Scholar
Google Scholar
[26] Kobota K, Ikeuchi I, Nakayama T, Takei C, Yabuuchi N, Shiiba H, Nakayama M, Komaba S 2014 J. Phys. Chem. C 119 166
[27] Yabuuchi N, Komaba S 2014 Sci. Techn. Advan. Mater. 15 043501
 Google Scholar
Google Scholar
[28] Silván B, Gonzalo E, Djuandhi L, Sharma N, Fauth F, Saurel D 2018 J. Mater. Chem. A 6 15132
 Google Scholar
Google Scholar
[29] Yabuuchi N, Kubota K, Dahbi M, Komaba S 2014 Chem. Rev. 114 11636
 Google Scholar
Google Scholar
[30] 赵成龙 2020 博士学位论文 (北京: 中国科学院大学)
Zhao C L 2020 Ph. D. Dissertation (Beijing: University of Chinese Academy of Sciences) (in Chinese)
[31] Xu S Y, Wu X Y, Li Y M, Hu Y S, Chen L Q 2014 Chin Phys B 23
[32] Lu Z, Dahn J R 2001 J. Electrochem. Soc. 148 A1225
 Google Scholar
Google Scholar
[33] Lee D H, Xu J, Meng Y S 2013 Phys. Chem. Chem. Phys. 15 3304
 Google Scholar
Google Scholar
[34] Wang P F, Yao H R, Liu X Y, Yin Y X, Zhang J N, Wen Y, Yu X, Gu L, Guo Y G 2018 Sci. Adv. 4 eaar6018
 Google Scholar
Google Scholar
[35] Liu Q, Hu Z, Chen M, Zou C, Jin H, Wang S, Gu Q, Chou S 2019 J. Mater. Chem. A 7 9215
 Google Scholar
Google Scholar
[36] Kumakura S, Tahara Y, Kubota K, Chihara K, Komaba S 2016 Angew. Chem. Int. Ed. 55 12760
 Google Scholar
Google Scholar
[37] Rong X, Hu E, Lu Y, Meng F, Zhao C, Wang X, Zhang Q, Yu X, Gu L, Hu Y S, Li H, Huang X, Yang X, Delmas C, Chen L 2019 Joule 3 503
 Google Scholar
Google Scholar
[38] Bai X, Sathiya M, Mendoza-Sánchez B, Iadecola A, Vergnet J, Dedryvère R, Saubanère M, Abakumov A M, Rozier P, Tarascon J-M 2018 Advan. Energy Mater. 8 1802379
[39] Yabuuchi N, Hara R, Kubota K, Paulsen J, Kumakura S, Komaba S 2014 J. Mater. Chem. A 2 16851
 Google Scholar
Google Scholar
[40] Gao A, Zhang Q, Li X, Shang T, Tang Z, Lu X, Luo Y, Ding J, Kan W H, Chen H, Yin W, Wang X, Xiao D, Su D, Li H, Rong X, Yu X, Yu Q, Meng F, Nan C, Delmas C, Chen L, Hu Y, Gu L, 2021 Nat. Sustain. 5 214
 Google Scholar
Google Scholar
[41] Wang Y, Yu X, Xu S, Bai J, Xiao R, Hu Y S, Li H, Yang X Q, Chen L, Huang X 2013 Nat. Commun. 4 2365
 Google Scholar
Google Scholar
[42] Wang Y, Xiao R, Hu Y S, Avdeev M, Chen L 2015 Nat. Commun. 6 6954
 Google Scholar
Google Scholar
[43] Shanmugam R, Lai W 2014 ECS Electrochem. Lett. 3 A23
 Google Scholar
Google Scholar
[44] Yu H, Ren Y, Xiao D, Guo S, Zhu Y, Qian Y, Gu L, Zhou H 2014 Angew. Chem. Int. Ed. 53 8963
 Google Scholar
Google Scholar
[45] Guo S, Liu P, Sun Y, Zhu K, Yi J, Chen M, Ishida M, Zhou H 2015 Angew. Chem. Int. Ed. 54 11701
 Google Scholar
Google Scholar
[46] Wang P F, Yao H R, Zuo T T, Yin Y X, Guo Y G 2017 Chem. Commun. 53 1957
 Google Scholar
Google Scholar
[47] 丁飞翔, 高飞, 容晓晖, 杨凯, 陆雅翔, 胡勇胜 2019 物理化学学报 36 1904022
 Google Scholar
Google Scholar
Ding F X, Gao F, Rong X H, Yang K, Lu Y X, Hu Y S 2019 Acta Phys-Chim Sin. 36 1904022
 Google Scholar
Google Scholar
[48] BRACONNIER J J, DELMAS C, HAGENMULLER 1982 Mat. Res. Bull. 17 993
 Google Scholar
Google Scholar
[49] Parant J-P, Olazcuaga R, Devalette M, Fouassier C, Hagenmuller P 1971 J Solid State Chem 3 1
 Google Scholar
Google Scholar
[50] Ma X, Chen H, Ceder G 2011 J Electrochem Soc 158 A1307
 Google Scholar
Google Scholar
[51] Takeda Y, Nakahara K, Nishijima M, Imanishi N, Yamamoto O 1994 Mater Res Bull 29 659
 Google Scholar
Google Scholar
[52] Braconnier J J, Delmas C, Fouassier C, Hagenmuller P 1980 Mat. Res. Bull. 15 1797
 Google Scholar
Google Scholar
[53] Han M H, Gonzalo E, Casas-Cabanas M, Rojo T 2014 J Power Sources 258 266
 Google Scholar
Google Scholar
[54] Wang L, Wang J, Zhang X, Ren Y, Zuo P, Yin G, Wang J 2017 Nano Energy 34 215
 Google Scholar
Google Scholar
[55] Kim D, Lee E, Slater M, Lu W, Rood S, Johnson C S 2012 Electrochem Commun 18 66
 Google Scholar
Google Scholar
[56] Linqin M, Xinguo Q, Yongsheng H, Hong L, Liquan C, Xuejie H J E S S 2016 Energy Storage Sci Techn 5 324
 Google Scholar
Google Scholar
[57] Xie Y, Wang H, Xu G, Wang J, Sheng H, Chen Z, Ren Y, Sun C J, Wen J, Wang J, Miller D, Amine K, Ma Z 2016 Advan. Energy Mater. 6 1601306
 Google Scholar
Google Scholar
[58] Yuan D D, Wang Y X, Cao Y L, Ai X P, Yang H X 2015 ACS Appl. Mater. Interfaces 7 8585
 Google Scholar
Google Scholar
[59] Yuan D D, Wang Y X, Cao Y L, Ai X P, Yang H X 2015 Appl. Mater. Interfaces 7 8585
[60] Maletti S, Sarapulova A, Schokel A, Mikhailova D 2019 ACS Appl. Mater. Interfaces 11 33923
 Google Scholar
Google Scholar
[61] Wang P F, Yao H R, Liu X Y, Zhang J N, Gu L, Yu X Q, Yin Y X, Guo Y G 2017 Advan. Mater. 29 1700210
 Google Scholar
Google Scholar
[62] Yao H R, Wang P F, Gong Y, Zhang J, Yu X, Gu L, OuYang C, Yin Y X, Hu E, Yang X-Q, Stavitski E, Guo Y, Wan L 2017 J. Am. Chem. Soc. 139 8440
 Google Scholar
Google Scholar
[63] Wang Q, Mariyappan S, Vergnet J, Abakumov A M, Rousse G, Rabuel F, Chakir M, Tarascon J M 2019 Advan. Energy Mater. 9 1901785
 Google Scholar
Google Scholar
[64] Mariyappan S, Marchandier T, Rabuel F, Iadecola A, Rousse G, Morozov A V, Abakumov A M, Tarascon J-M 2020 Chem. Mater. 32 1657
 Google Scholar
Google Scholar
[65] Kubota K, Fujitani N, Yoda Y, Kuroki K, Tokita Y, Komaba S 2021 J Mater Chem A 9 12830
 Google Scholar
Google Scholar
[66] Ma Y, Ma Y, Wang Q, Schweidler S, Botros M, Fu T, Hahn H, Brezesinski T, Breitung B 2021 Energy Envir. Sci. 14 2883
 Google Scholar
Google Scholar
[67] Sarkar A, Velasco L, Wang D, Wang Q, Talasila G, Biasi L, Kubel C, Brezesinski T, Bhattacharya S, Hahn H, Breitung B 2018 Nat. Commun. 9 3400
 Google Scholar
Google Scholar
[68] Zhao C, Ding F, Lu Y, Chen L, Hu Y S 2020 Angew. Chem. Int. Ed. Engl. 59 264
 Google Scholar
Google Scholar
[69] Ding F, Zhao C, Zhou D, Meng Q, Xiao D, Zhang Q, Niu Y, Li Y, Rong X, Lu Y, Chen L, Hu Y S 2020 Energy Storage Mater. 30 420
[70] Zhou Q, Li Y Q, Tang F, Li K X, Rong X H, Lu Y X, Chen L Q, Hu Y S 2021 Chin. Phys. Lett. 38 076501
 Google Scholar
Google Scholar
[71] Gonzalo E, Han M H, López del Amo J M, Acebedo B, Casas-Cabanas M, Rojo T 2014 J. Mater. Chem. A 2 18523
 Google Scholar
Google Scholar
[72] Parant J P, Olazcuaga R, Devalette M, Fouassier C, Hagenmuller P 1971 J. Solid State Chem. 3 1
 Google Scholar
Google Scholar
[73] Paulsen J M, Dahn J R 1999 Solid State Ionics 126 3
 Google Scholar
Google Scholar
[74] Liu X, Zhong G, Xiao Z, Zheng B, Zuo W, Zhou K, Liu H, Liang Z, Xiang Y, Chen Z, Ortiz G, Fu R, Yang Y 2020 Nano Energy 76 104997
 Google Scholar
Google Scholar
[75] Ding F, Meng Q, Yu P, Wang H, Niu Y, Li Y, Yang Y, Rong X, Liu X, Lu Y, Chen L, Hu Y S 2021 Adv. Funct. Mater. 31 2001120
[76] Guo K S, Lu Y X, Wang H L, Ma X B, Li Z Y, Hu Y S, Dongfeng Chen 2019 Chin. Phys. B 28 68203
 Google Scholar
Google Scholar
[77] Fei Xie Y L, Liquan Chen , Hu Y S 2021 Chin. Phys. Lett. 38 118401
 Google Scholar
Google Scholar
[78] Zhao C, Yao Z, Wang Q, Li H, Wang J, Liu M, Ganapathy S, Lu Y, Cabana J, Li B, Bai X, Aspuru-Guzik A, Wagemaker M, Chen L, Hu Y S 2020 J. Am. Chem. Soc. 142 5742
 Google Scholar
Google Scholar
[79] Liang X, Yu T Y, Ryu H H, Sun Y-K 2022 Energy Storage Mater. 47 515
-
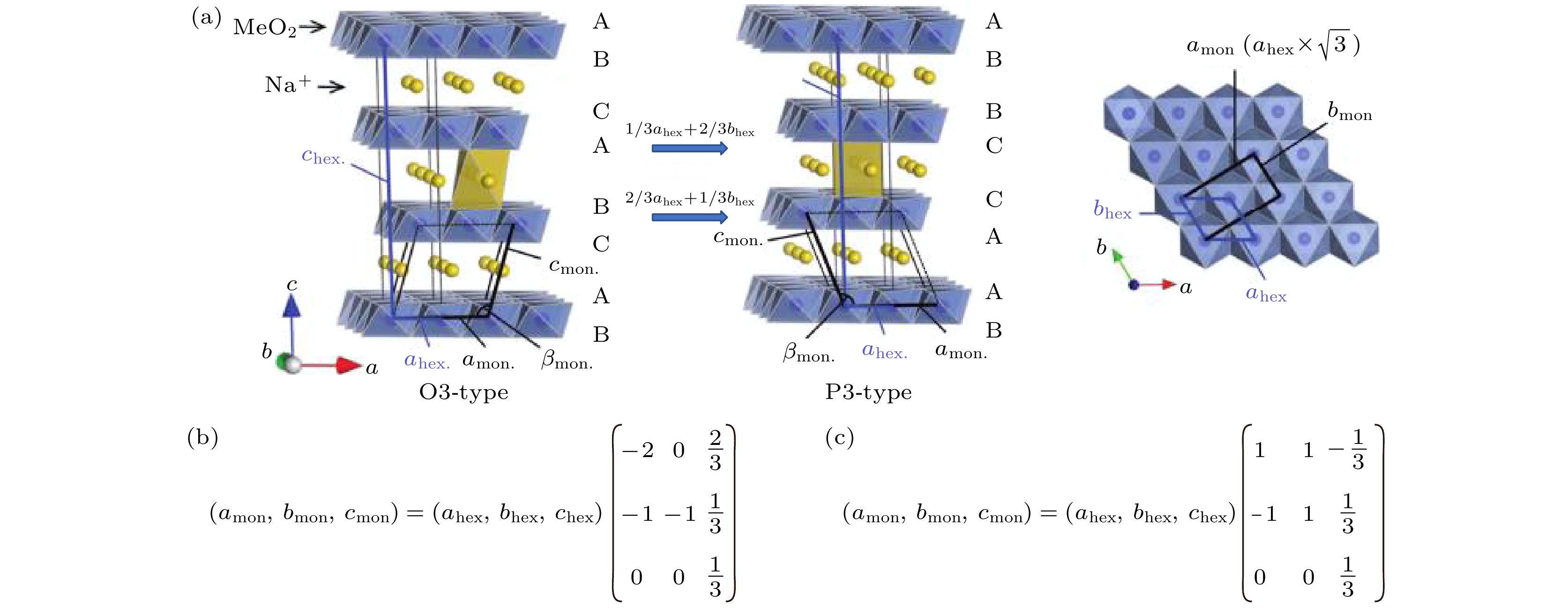
图 1 (a)—(e)常见钠离子层状材料的晶体结构示意图, 插图为层状结构中过渡金属和钠离子多面体的连接机理示意图; (f)从垂直于过渡金属层的方向观察六方和单斜结构和晶胞参数的区别和联系
Fig. 1. (a)–(e) Illustrations of crystal structures relevant to the Na+ layered oxide cathode materials, insets are the face-sharing schemes of TMO6 and NaO6 in the layered structures; (f) the view perpendicular to the layer direction highlighting the relationship between the hexagonal and monoclinic unit cells.
图 5 (a) NaNi0.5Mn0.5O2材料在2.2—3.8和2.2—4.5 V电压范围内的首周充放电曲线[20]; (b) Na1–xNi0.5Mn0.5O2电极的非原位XRD图谱[20], 星号代表集流体镍网的衍射峰; (c)NaNi0.5Mn0.5O2材料首圈在C/20C倍率下充放电过程中的原位XRD图谱[21]
Fig. 5. (a) Initial charge-discharge curves of the NaNi0.5Mn0.5O2 cell at a rate of 1/50C (4.8 mA/g) in the voltage ranges of 2.2–3.8 and 2.2–4.5 V versus sodium metal; (b) ex situ XRD patterns of the Na1–xNi0.5Mn0.5O2[20], asterisks show a nickel mesh used as a current collector; (c) in situ XRD patterns of the NaNi0.5Mn0.5O2 material at C/20 rate[21].
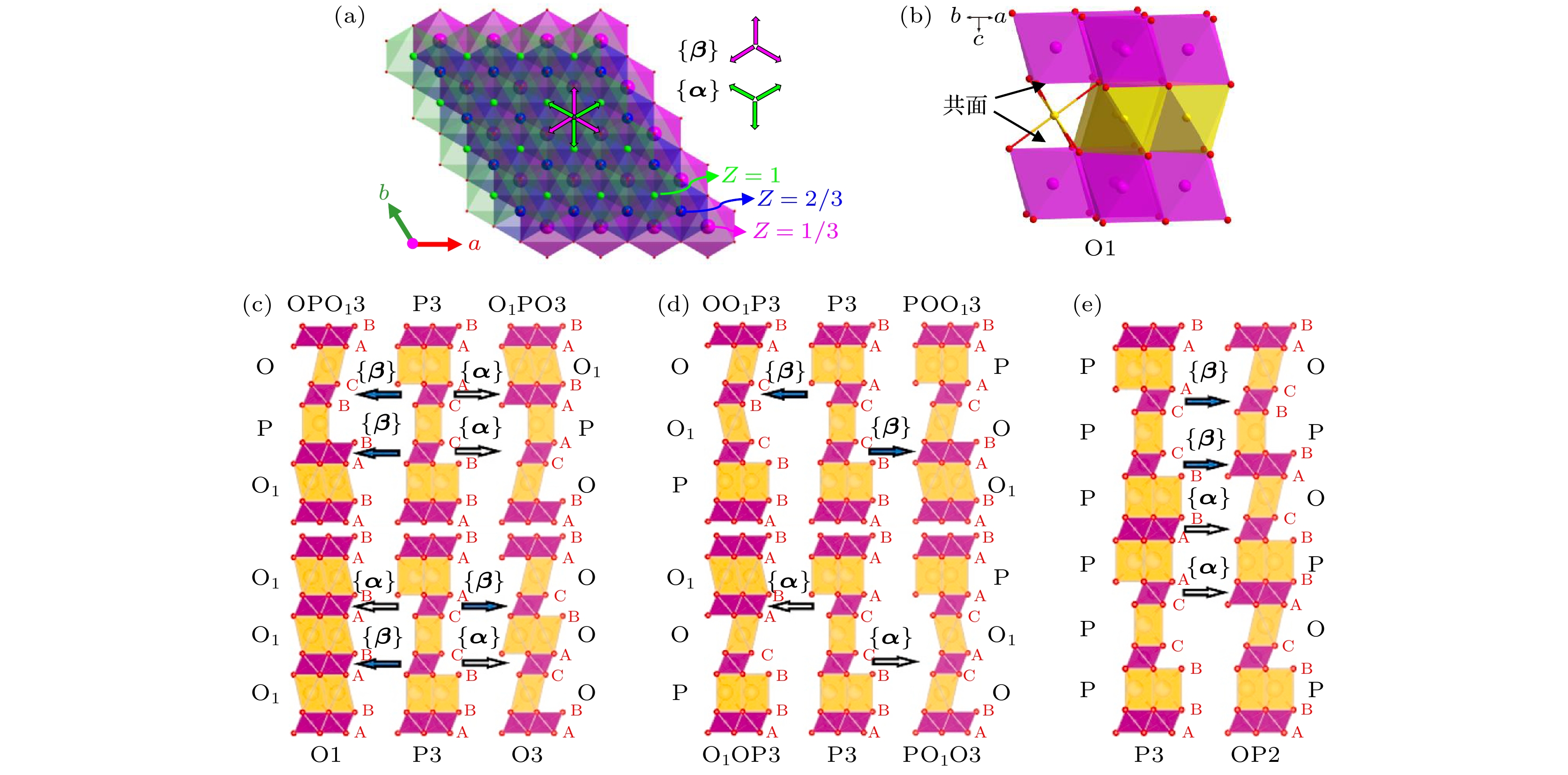
图 7 (a) P3相过渡金属层可能的滑移方向α和β; (b) O1相的晶体结构示意图; (c)通过α和β滑移P3相向O3, O1, OPO13和O1PO3相的转变示意图; (d)通过α和β滑移P3相向OO13, O1OP3, POO13和PO1O3相的转变示意图; (e)通过α和β滑移P3相向OP2相的转变示意图. 晶体结构观察方向为[100], 钠位为黄色多面体, 过渡金属位于紫色八面体, 结构示意图中没有考虑晶胞参数的变化[19]
Fig. 7. (a) P3 phase TM layer displacement vectors α and β; (b) schematic illustrations of the crystal structure of O1 phase; (c) phase transitions from P3 to O3, O1, OPO13 and O1PO3 via shifting α and β; (d) phase transitions from P3 to OO13, O1OP3, O1OP3 and POO13 via shifting α or β; (e) phase transition from P3 to OP2 via shifting α and β. All structures viewed along [100] direction, all cell parameters changes have been ignored and TM octahedra are shown in purple and all Na sites in yellow[19].
图 8 (a) Na2RuO3的原位XRD图谱和充放电曲线; (b)根据原位实验确定的随钠含量变化的相图; (c)库仑力对Na2–xRuO3材料自有序过程的机理演示[23]
Fig. 8. (a) XRD patterns tested in situ during the first cycle of Na2RuO3 with the corresponding cycling curve; (b) phase diagram as determined from the in situ experiment as a function of the sodium content; (c) coulombic forces and resultant self-ordering in Na2–xRuO3[23].
图 9 (a) O3-NaFeO2和(b) O3-NaCrO2电极在不同充电截止电压的充放电曲线[26,27]; (c)充电至高电压时Na1–xCrO2材料模拟和测试的XRD 图谱; (d) NaxFeO2材料在钠离子脱出过程的相图和铁迁移示意图[28]; (e)脱钠过程中过渡金属离子迁移机理示意图[29]
Fig. 9. Charge-discharge curves of (a) NaFeO2 and (b) NaCrO2 cathode[26,27]; (c) simulated and observed XRD patterns of Na1–xCrO2 cathode charged to high voltage; (d) scheme of phase evolution and iron migration upon sodium extraction in NaxFeO2[28]; (e) a proposed mechanism of Men+ (Metal ion) migration process on the desodiated process[29].
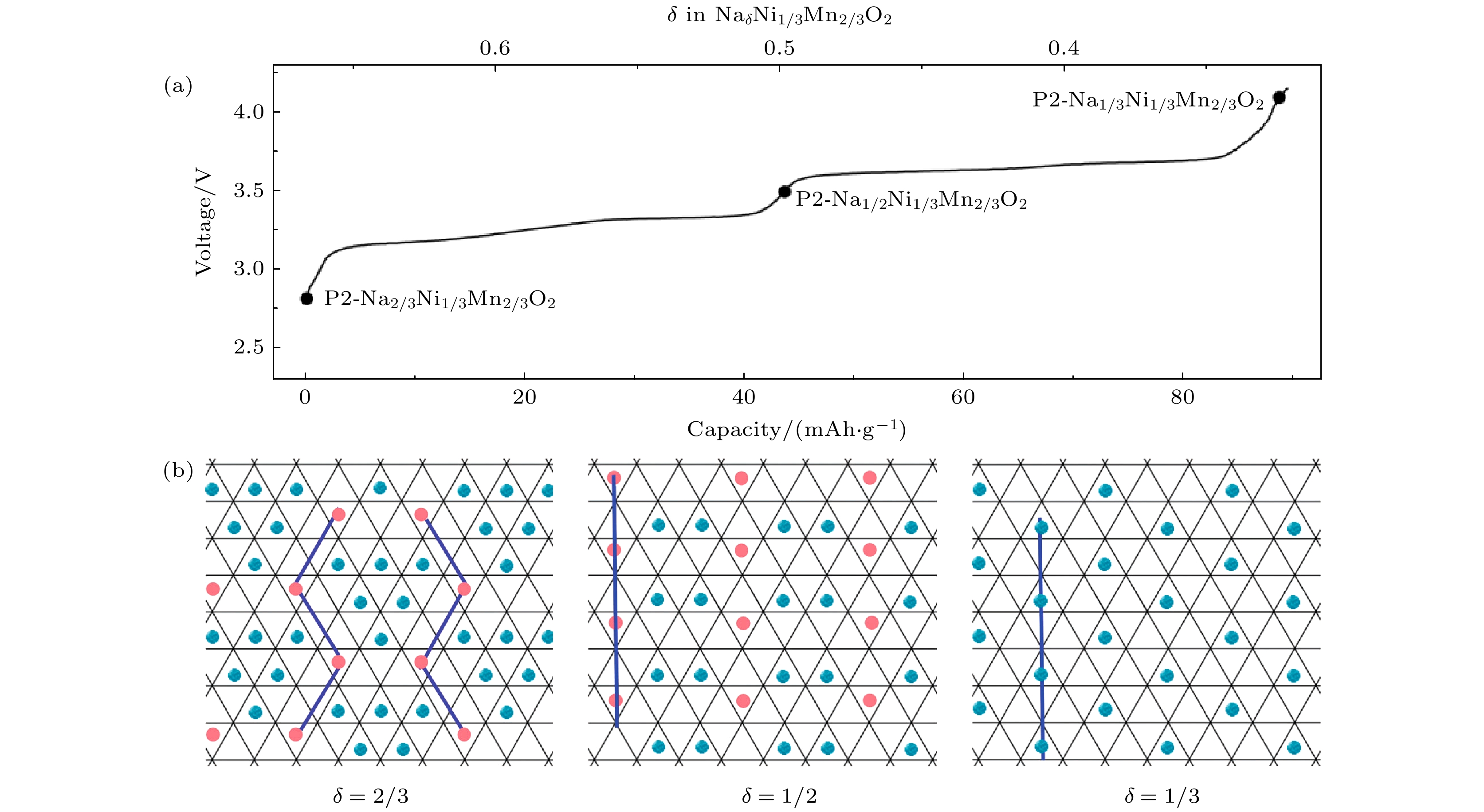
图 12 (a) P2-Na2/3Ni1/3Mn2/3O2的典型充电曲线; (b) P2-NaδNi1/3Mn2/3O2层内三棱柱位置钠离子/空位有序排布示意图(蓝色球代表占据Nae位钠离子, 粉色球代表占据Naf位钠离子)[33]
Fig. 12. (a) Typical charge profiles of P2-Na2/3Ni1/3Mn2/3O2; (b) in-plane Na+/vacancy orderings of P2-NaδNi1/3Mn2/3O2 in the triangular lattice (blue balls: Na-ions on Nae sites, pink balls: Na-ions on Naf sites) [33].
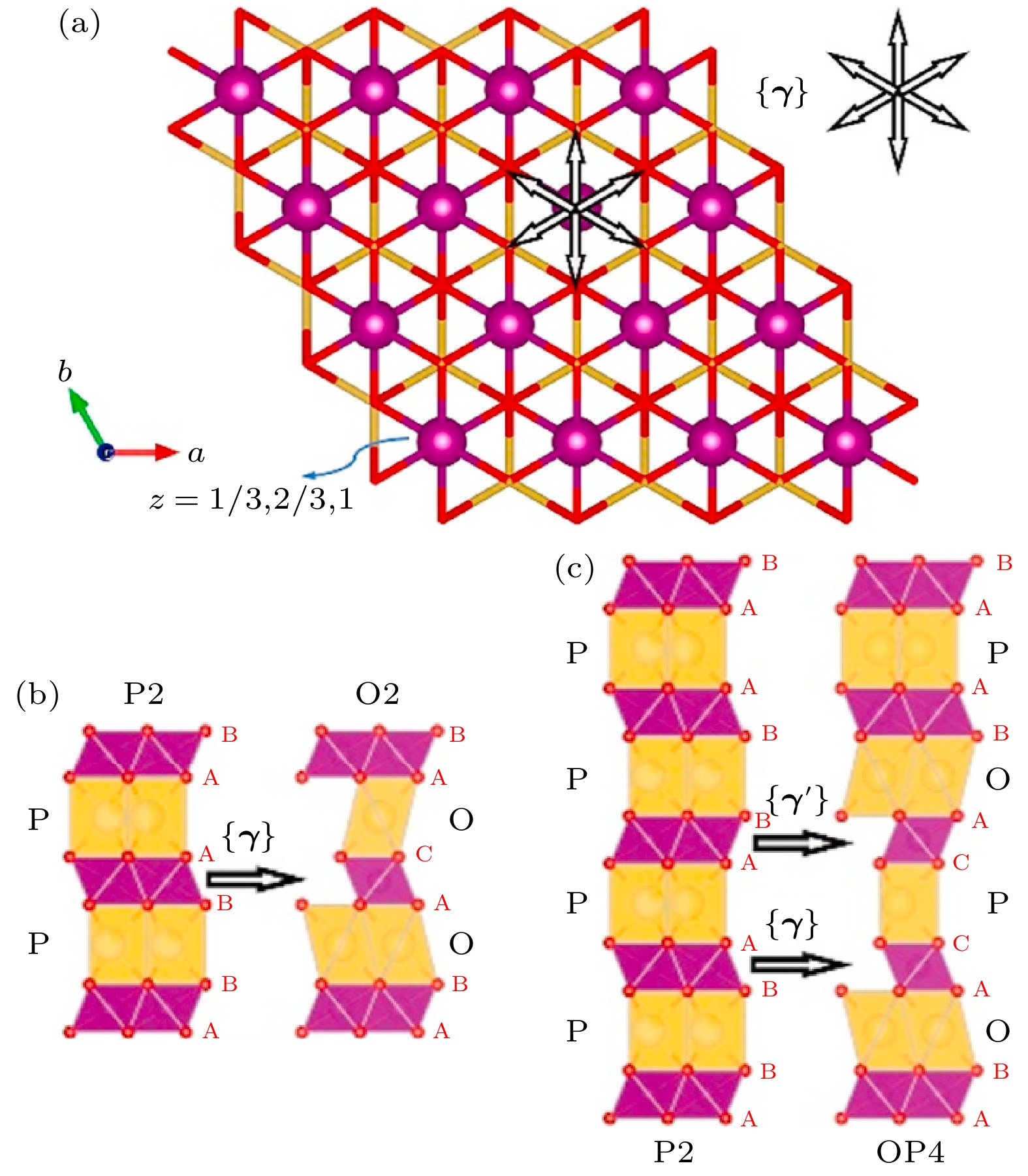
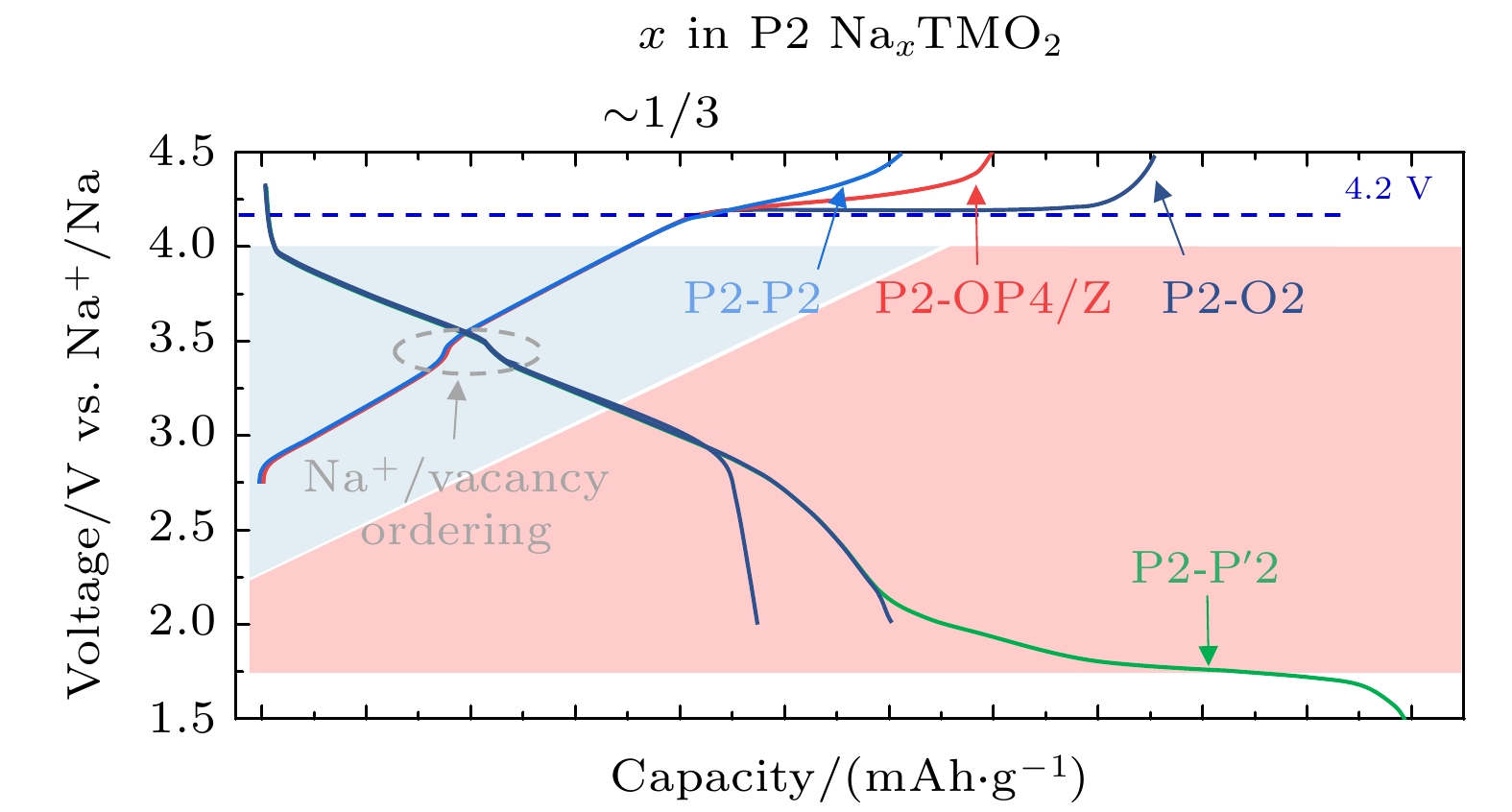
图 15 (a) P2相过渡金属层可能滑移的方向γ; (b)通过γ滑移P2相向O2相的转变示意图; (c)通过γ滑移P2相向OP4相的转变示意图. 晶体结构观察方向为[100], 钠位为黄色多面体, 过渡金属位于紫色八面体, 结构示意图中没有考虑晶胞参数的变化[19]
Fig. 15. (a) P2 phase TM layer displacement vectors γ; (b) phase transitions from P2 to O2 via shifting γ; (c) phase transitions from P2 o OP4 via shifting γ. All structures viewed along [100] direction, all cell parameters changes have been ignored and TM octahedra are shown in purple and all Na sites in yellow[19].
图 18 (a) Na0.66Li0.22Ti0.78O2电极在0.1 C倍率下0.4—2.5 V电压范围内的充放电曲线; (b) Na0.66Li0.22Ti0.78O2电极在C/7倍率下首周充放电过程中的原位XRD图谱[41]; (c) Na0.6Cr0.6Ti0.4O2电极在0.1 C倍率下0.5—2.5 V电压范围内的首周充放电曲线[42]; (d) Na0.6Cr0.6Ti0.4O2电极在C/5倍率下首周充放电过程中的原位XRD图谱[42]
Fig. 18. (a) The discharge-charge curves of Na0.66Li0.22Ti0.78O2 at a current rate of 0.1 C (10.6 mA/g) in the voltage range of 0.4–2.5 V; (b) in situ XRD patterns collected during the first discharge-charge of the Na0.66Li0.22Ti0.78O2 electrode under a current rate of C/7[41]; (c) the first discharge-charge curve of Na0.6Cr0.6Ti0.4O2 in the voltage range of 0.5–2.5 V; (d) in situ XRD patterns collected during the first discharge-charge of the Na0.6Cr0.6Ti0.4O2 electrode under a current rate of C/5[42].
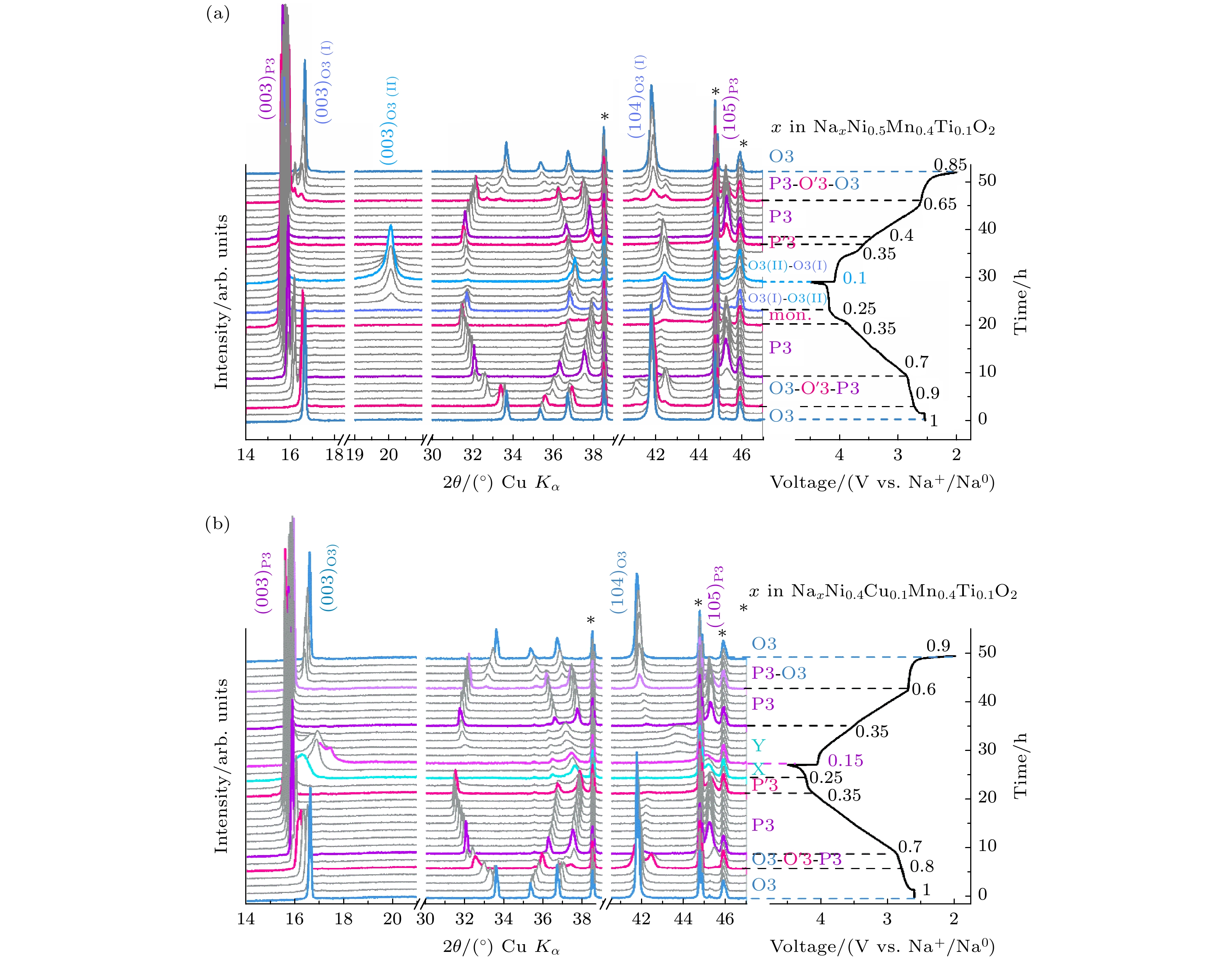
图 21 (a)高熵构型稳定O3结构的机理阐释[68]; (b) NaNi0.6Fe0.25Mn0.15O2材料在2.0—4.2 V电压范围内的原位XRD图谱和(c)结构演变示意图[69]
Fig. 21. (a) Possible mechanism of high-entropy composition in facilitating layered O3-type structure[68]; in situ XRD patterns of (b) NaNi0.6Fe0.25Mn0.15O2 electrode in the voltage range of 2.0–4.2 V and (c) schematic of structural evolution[69].
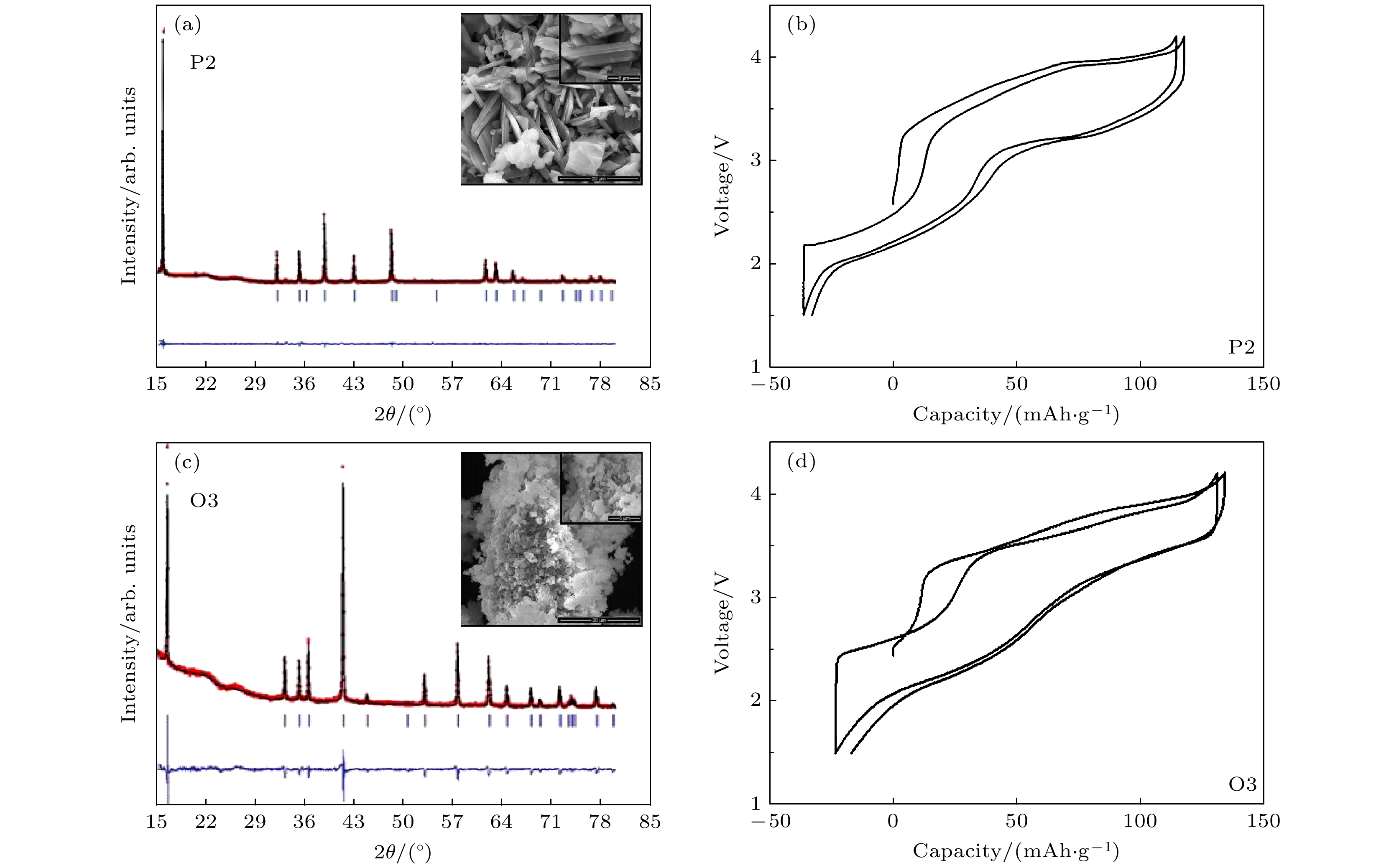
图 22 (a)P2-和(c)O3-Na2/3Fe2/3Mn1/3O2 样品的XRD 谱和SEM图片;(b) P2-和(d)O3- Na2/3Fe2/3Mn1/3O2 样品前两周在1.5—4.2 V电压范围内的充放电曲线对比[71]
Fig. 22. XRD patterns and SEM images of (a) P2- and (c) O3- Na2/3Fe2/3Mn1/3O2 samples; comparison of charge-discharge capacity of (b) P2- and (d) O3- Na2/3Fe2/3Mn1/3O2 within the 1 st and 2 nd cycles in the voltage range of 1.5–4.2 V[71].
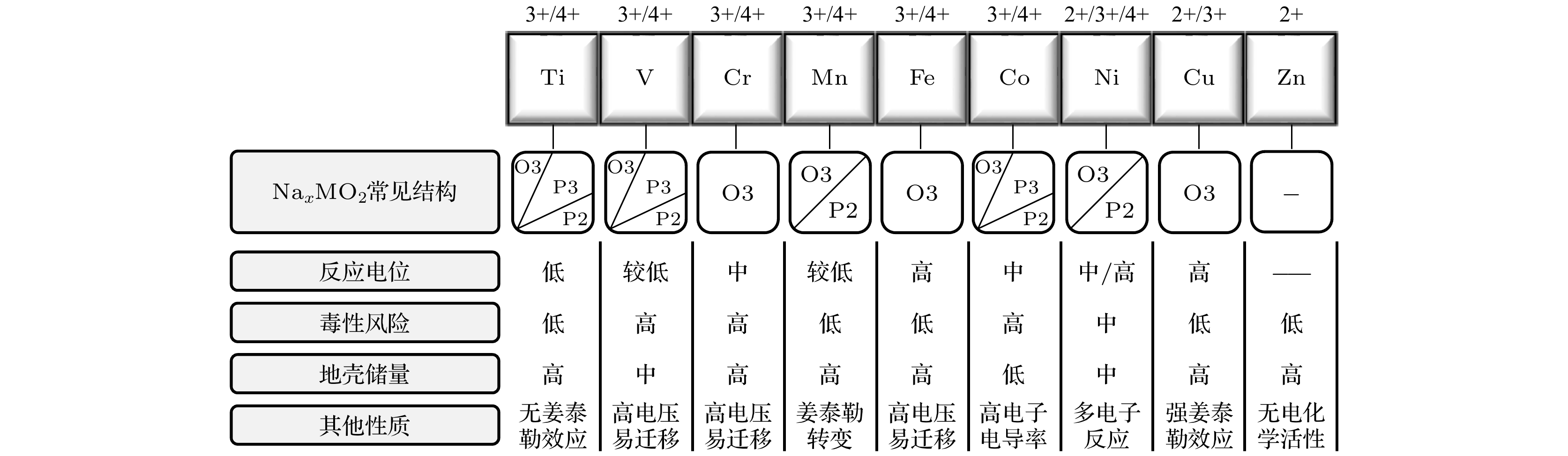
表 1 常见的结构对应的空间群和原子坐标
Table 1. The space groups and corresponding atomic positions of reported structures.
结构 空间群
(代号)原子占位 Nae Naf Na M O O3 ${R}\bar{3}{m}$
(167)— — 3b
(0, 0, 1/2)3a
(0, 0, 0)6c
(0, 0, ~0.27)O'3 C2/m
(12)— — 2d
(0, 1/2, 1/2)2a
(0, 0, 0)4i
(~0.28, 0, ~0.8)P3 R3m
(160)— — 3a
(0, 0, ~0.17)3a
(0, 0, 0)3a
(0, 0, ~0.4)P2 P63/mmc
(194)2d
(2/3, 1/3, 1/4)2b
(0, 0, 1/4)— 2a
(0, 0, 0)4f
(1/3, 2/3, ~0.09)O2 P63mc
(186)— — 2b
(1/3, 2/3, ~0.24)2b
(2/3, 1/3, 0)2b
(2/3, 1/3, ~0.39) -
[1] 陆雅翔, 赵成龙, 容晓晖, 陈立泉, 胡勇胜 2018 67 120601
 Google Scholar
Google Scholar
Lu Y X, Zhao C L, Chen L Q, Hu Y S 2018 Acta Phys. Sin. 67 120601
 Google Scholar
Google Scholar
[2] Sun Y, Guo S, Zhou H 2019 Energy Environ Sci. 12 825
 Google Scholar
Google Scholar
[3] Kubota K, Kumakura S, Yoda Y, Kuroki K, Komaba S 2018 Advan. Energy Mater. 8 1703415
 Google Scholar
Google Scholar
[4] Liu Q, Hu Z, Chen M, Zou C, Jin H, Wang S, Chou S L, Dou S X 2019 Small 0 1805381
[5] Kim S W, Seo D H, Ma X, Ceder G, Kang K 2012 Advan. Energy Mater. 2 710
 Google Scholar
Google Scholar
[6] Kim H, Park I, Lee S, Kim H, Park K Y, Park Y U, Kim H, Kim J, Lim H D, Yoon W S, Kang K 2013 Chem. Mater. 25 3614
 Google Scholar
Google Scholar
[7] Bauer A, Song J, Vail S, Pan W, Barker J, Lu Y 2018 Advan. Energy Mater. 8 1702869
 Google Scholar
Google Scholar
[8] Wang L, Lu Y, Liu J, Xu M, Cheng J, Zhang D, Goodenough J B 2013 Angew. Chem. Int. Ed. 52 1964
 Google Scholar
Google Scholar
[9] Wang S, Wang L, Zhu Z, Hu Z, Zhao Q, Chen J 2014 Angew. Chem. Int. Ed. 53 5892
 Google Scholar
Google Scholar
[10] Wang Q, Zhao C, Lu Y, Li Y, Zheng Y, Qi Y, Rong X, Jiang L, Qi X, Shao Y, Pan D, Li B, Hu Y S, Chen L 2017 Small 13 1701835
 Google Scholar
Google Scholar
[11] Wu F, Zhao C, Chen S, Lu Y, Hou Y, Hu Y S, Maier J, Yu Y 2018 Mater. Today 21 960
 Google Scholar
Google Scholar
[12] Delmas C, Fouassier C, Hagenmuller P 1980 Physica B+C 99 81
 Google Scholar
Google Scholar
[13] 胡勇胜, 陆雅翔, 陈立泉 2020 钠离子电池科学与技术 (北京: 科学出版社) 第20页
Hu Y S, Lu Y X, Chen L Q 2020 Na-ion batteries:science and technology (Beijing: Science Press) p20 (in Chinese)
[14] Mortemard de Boisse B, Cheng J H, Carlier D, Guignard M, Pan C J, Bordère S, Filimonov D, Drathen C, Suard E, Hwang B-J, Wattiaux A, Delmas C 2015 J. Mater. Chem. A 3 10976
 Google Scholar
Google Scholar
[15] Mortemard de Boisse B, Liu G, Ma J, Nishimura S I, Chung S C, Kiuchi H, Harada Y, Kikkawa J, Kobayashi Y, Okubo M, Yamada A 2016 Nat. Commun. 7 11397
 Google Scholar
Google Scholar
[16] Nanba Y, Iwao T, Boisse B M d, Zhao W, Hosono E, Asakura D, Niwa H, Kiuchi H, Miyawaki J, Harada Y, Okubo M, Yamada A 2016 Chem. Mater. 28 1058
 Google Scholar
Google Scholar
[17] Perez A J, Batuk D, Saubanère M, Rousse G, Foix D, McCalla E, Berg E J, Dugas R, H. W. van den Bos K, Doublet M L, Gonbeau D, Abakumov A, Tendeloo G, Tarascon J-M 2016 Chem. Mater. 28 8278
 Google Scholar
Google Scholar
[18] Zhao C, Wang Q, Yao Z, Wang J, Sanchez-Lengeling B, Ding F, Qi X, Lu Y, Bai X, Li B, Li H, Aspuru-Guzik A, Huang X, Delmas C, Wagemaker M, Chen L, Hu Y S 2020 Science 370 708
[19] Liu J, Kan W H, Ling C D 2021 J. Power Sources 481 229139
 Google Scholar
Google Scholar
[20] Komaba S, Yabuuchi N, Nakayama T, Ogata A, Ishikawa T, Nakai I 2012 Inorg. Chem. 51 6211
 Google Scholar
Google Scholar
[21] Sathiya M, Jacquet Q, Doublet M-L, Karakulina O M, Hadermann J, Tarascon J M 2018 Advan. Energy Mater. 8 1702599
 Google Scholar
Google Scholar
[22] Croguennec L, Pouillerie C, Mansour A N, Delmas C 2001 J. Mater. Chem. 11 131
 Google Scholar
Google Scholar
[23] Mortemard de Boisse B, Reynaud M, Ma J, Kikkawa J, Nishimura S I, Casas-Cabanas M, Delmas C, Okubo M, Yamada A 2019 Nat. Commun. 10 2185
 Google Scholar
Google Scholar
[24] Maazaz A, Delmas C, Hagenmuller P 1983 J. Incl. Phenom. 1 45
 Google Scholar
Google Scholar
[25] Didier C, Guignard M, Denage C, Szajwaj O, Ito S, Saadoune I, Darriet J, Delmas C 2011 Electrochem. Solid-State Lett. 14 A75
 Google Scholar
Google Scholar
[26] Kobota K, Ikeuchi I, Nakayama T, Takei C, Yabuuchi N, Shiiba H, Nakayama M, Komaba S 2014 J. Phys. Chem. C 119 166
[27] Yabuuchi N, Komaba S 2014 Sci. Techn. Advan. Mater. 15 043501
 Google Scholar
Google Scholar
[28] Silván B, Gonzalo E, Djuandhi L, Sharma N, Fauth F, Saurel D 2018 J. Mater. Chem. A 6 15132
 Google Scholar
Google Scholar
[29] Yabuuchi N, Kubota K, Dahbi M, Komaba S 2014 Chem. Rev. 114 11636
 Google Scholar
Google Scholar
[30] 赵成龙 2020 博士学位论文 (北京: 中国科学院大学)
Zhao C L 2020 Ph. D. Dissertation (Beijing: University of Chinese Academy of Sciences) (in Chinese)
[31] Xu S Y, Wu X Y, Li Y M, Hu Y S, Chen L Q 2014 Chin Phys B 23
[32] Lu Z, Dahn J R 2001 J. Electrochem. Soc. 148 A1225
 Google Scholar
Google Scholar
[33] Lee D H, Xu J, Meng Y S 2013 Phys. Chem. Chem. Phys. 15 3304
 Google Scholar
Google Scholar
[34] Wang P F, Yao H R, Liu X Y, Yin Y X, Zhang J N, Wen Y, Yu X, Gu L, Guo Y G 2018 Sci. Adv. 4 eaar6018
 Google Scholar
Google Scholar
[35] Liu Q, Hu Z, Chen M, Zou C, Jin H, Wang S, Gu Q, Chou S 2019 J. Mater. Chem. A 7 9215
 Google Scholar
Google Scholar
[36] Kumakura S, Tahara Y, Kubota K, Chihara K, Komaba S 2016 Angew. Chem. Int. Ed. 55 12760
 Google Scholar
Google Scholar
[37] Rong X, Hu E, Lu Y, Meng F, Zhao C, Wang X, Zhang Q, Yu X, Gu L, Hu Y S, Li H, Huang X, Yang X, Delmas C, Chen L 2019 Joule 3 503
 Google Scholar
Google Scholar
[38] Bai X, Sathiya M, Mendoza-Sánchez B, Iadecola A, Vergnet J, Dedryvère R, Saubanère M, Abakumov A M, Rozier P, Tarascon J-M 2018 Advan. Energy Mater. 8 1802379
[39] Yabuuchi N, Hara R, Kubota K, Paulsen J, Kumakura S, Komaba S 2014 J. Mater. Chem. A 2 16851
 Google Scholar
Google Scholar
[40] Gao A, Zhang Q, Li X, Shang T, Tang Z, Lu X, Luo Y, Ding J, Kan W H, Chen H, Yin W, Wang X, Xiao D, Su D, Li H, Rong X, Yu X, Yu Q, Meng F, Nan C, Delmas C, Chen L, Hu Y, Gu L, 2021 Nat. Sustain. 5 214
 Google Scholar
Google Scholar
[41] Wang Y, Yu X, Xu S, Bai J, Xiao R, Hu Y S, Li H, Yang X Q, Chen L, Huang X 2013 Nat. Commun. 4 2365
 Google Scholar
Google Scholar
[42] Wang Y, Xiao R, Hu Y S, Avdeev M, Chen L 2015 Nat. Commun. 6 6954
 Google Scholar
Google Scholar
[43] Shanmugam R, Lai W 2014 ECS Electrochem. Lett. 3 A23
 Google Scholar
Google Scholar
[44] Yu H, Ren Y, Xiao D, Guo S, Zhu Y, Qian Y, Gu L, Zhou H 2014 Angew. Chem. Int. Ed. 53 8963
 Google Scholar
Google Scholar
[45] Guo S, Liu P, Sun Y, Zhu K, Yi J, Chen M, Ishida M, Zhou H 2015 Angew. Chem. Int. Ed. 54 11701
 Google Scholar
Google Scholar
[46] Wang P F, Yao H R, Zuo T T, Yin Y X, Guo Y G 2017 Chem. Commun. 53 1957
 Google Scholar
Google Scholar
[47] 丁飞翔, 高飞, 容晓晖, 杨凯, 陆雅翔, 胡勇胜 2019 物理化学学报 36 1904022
 Google Scholar
Google Scholar
Ding F X, Gao F, Rong X H, Yang K, Lu Y X, Hu Y S 2019 Acta Phys-Chim Sin. 36 1904022
 Google Scholar
Google Scholar
[48] BRACONNIER J J, DELMAS C, HAGENMULLER 1982 Mat. Res. Bull. 17 993
 Google Scholar
Google Scholar
[49] Parant J-P, Olazcuaga R, Devalette M, Fouassier C, Hagenmuller P 1971 J Solid State Chem 3 1
 Google Scholar
Google Scholar
[50] Ma X, Chen H, Ceder G 2011 J Electrochem Soc 158 A1307
 Google Scholar
Google Scholar
[51] Takeda Y, Nakahara K, Nishijima M, Imanishi N, Yamamoto O 1994 Mater Res Bull 29 659
 Google Scholar
Google Scholar
[52] Braconnier J J, Delmas C, Fouassier C, Hagenmuller P 1980 Mat. Res. Bull. 15 1797
 Google Scholar
Google Scholar
[53] Han M H, Gonzalo E, Casas-Cabanas M, Rojo T 2014 J Power Sources 258 266
 Google Scholar
Google Scholar
[54] Wang L, Wang J, Zhang X, Ren Y, Zuo P, Yin G, Wang J 2017 Nano Energy 34 215
 Google Scholar
Google Scholar
[55] Kim D, Lee E, Slater M, Lu W, Rood S, Johnson C S 2012 Electrochem Commun 18 66
 Google Scholar
Google Scholar
[56] Linqin M, Xinguo Q, Yongsheng H, Hong L, Liquan C, Xuejie H J E S S 2016 Energy Storage Sci Techn 5 324
 Google Scholar
Google Scholar
[57] Xie Y, Wang H, Xu G, Wang J, Sheng H, Chen Z, Ren Y, Sun C J, Wen J, Wang J, Miller D, Amine K, Ma Z 2016 Advan. Energy Mater. 6 1601306
 Google Scholar
Google Scholar
[58] Yuan D D, Wang Y X, Cao Y L, Ai X P, Yang H X 2015 ACS Appl. Mater. Interfaces 7 8585
 Google Scholar
Google Scholar
[59] Yuan D D, Wang Y X, Cao Y L, Ai X P, Yang H X 2015 Appl. Mater. Interfaces 7 8585
[60] Maletti S, Sarapulova A, Schokel A, Mikhailova D 2019 ACS Appl. Mater. Interfaces 11 33923
 Google Scholar
Google Scholar
[61] Wang P F, Yao H R, Liu X Y, Zhang J N, Gu L, Yu X Q, Yin Y X, Guo Y G 2017 Advan. Mater. 29 1700210
 Google Scholar
Google Scholar
[62] Yao H R, Wang P F, Gong Y, Zhang J, Yu X, Gu L, OuYang C, Yin Y X, Hu E, Yang X-Q, Stavitski E, Guo Y, Wan L 2017 J. Am. Chem. Soc. 139 8440
 Google Scholar
Google Scholar
[63] Wang Q, Mariyappan S, Vergnet J, Abakumov A M, Rousse G, Rabuel F, Chakir M, Tarascon J M 2019 Advan. Energy Mater. 9 1901785
 Google Scholar
Google Scholar
[64] Mariyappan S, Marchandier T, Rabuel F, Iadecola A, Rousse G, Morozov A V, Abakumov A M, Tarascon J-M 2020 Chem. Mater. 32 1657
 Google Scholar
Google Scholar
[65] Kubota K, Fujitani N, Yoda Y, Kuroki K, Tokita Y, Komaba S 2021 J Mater Chem A 9 12830
 Google Scholar
Google Scholar
[66] Ma Y, Ma Y, Wang Q, Schweidler S, Botros M, Fu T, Hahn H, Brezesinski T, Breitung B 2021 Energy Envir. Sci. 14 2883
 Google Scholar
Google Scholar
[67] Sarkar A, Velasco L, Wang D, Wang Q, Talasila G, Biasi L, Kubel C, Brezesinski T, Bhattacharya S, Hahn H, Breitung B 2018 Nat. Commun. 9 3400
 Google Scholar
Google Scholar
[68] Zhao C, Ding F, Lu Y, Chen L, Hu Y S 2020 Angew. Chem. Int. Ed. Engl. 59 264
 Google Scholar
Google Scholar
[69] Ding F, Zhao C, Zhou D, Meng Q, Xiao D, Zhang Q, Niu Y, Li Y, Rong X, Lu Y, Chen L, Hu Y S 2020 Energy Storage Mater. 30 420
[70] Zhou Q, Li Y Q, Tang F, Li K X, Rong X H, Lu Y X, Chen L Q, Hu Y S 2021 Chin. Phys. Lett. 38 076501
 Google Scholar
Google Scholar
[71] Gonzalo E, Han M H, López del Amo J M, Acebedo B, Casas-Cabanas M, Rojo T 2014 J. Mater. Chem. A 2 18523
 Google Scholar
Google Scholar
[72] Parant J P, Olazcuaga R, Devalette M, Fouassier C, Hagenmuller P 1971 J. Solid State Chem. 3 1
 Google Scholar
Google Scholar
[73] Paulsen J M, Dahn J R 1999 Solid State Ionics 126 3
 Google Scholar
Google Scholar
[74] Liu X, Zhong G, Xiao Z, Zheng B, Zuo W, Zhou K, Liu H, Liang Z, Xiang Y, Chen Z, Ortiz G, Fu R, Yang Y 2020 Nano Energy 76 104997
 Google Scholar
Google Scholar
[75] Ding F, Meng Q, Yu P, Wang H, Niu Y, Li Y, Yang Y, Rong X, Liu X, Lu Y, Chen L, Hu Y S 2021 Adv. Funct. Mater. 31 2001120
[76] Guo K S, Lu Y X, Wang H L, Ma X B, Li Z Y, Hu Y S, Dongfeng Chen 2019 Chin. Phys. B 28 68203
 Google Scholar
Google Scholar
[77] Fei Xie Y L, Liquan Chen , Hu Y S 2021 Chin. Phys. Lett. 38 118401
 Google Scholar
Google Scholar
[78] Zhao C, Yao Z, Wang Q, Li H, Wang J, Liu M, Ganapathy S, Lu Y, Cabana J, Li B, Bai X, Aspuru-Guzik A, Wagemaker M, Chen L, Hu Y S 2020 J. Am. Chem. Soc. 142 5742
 Google Scholar
Google Scholar
[79] Liang X, Yu T Y, Ryu H H, Sun Y-K 2022 Energy Storage Mater. 47 515
计量
- 文章访问数: 28367
- PDF下载量: 1238
- 被引次数: 0
















 下载:
下载: